4D-Flow Cardiovascular Magnetic Resonance Sequence for Aortic Assessment: Multi-Vendor and Multi-Magnetic Field Reproducibility in Healthy Volunteers
Abstract
1. Introduction
2. Material and Methods
2.1. Study Cohort
2.2. CMR
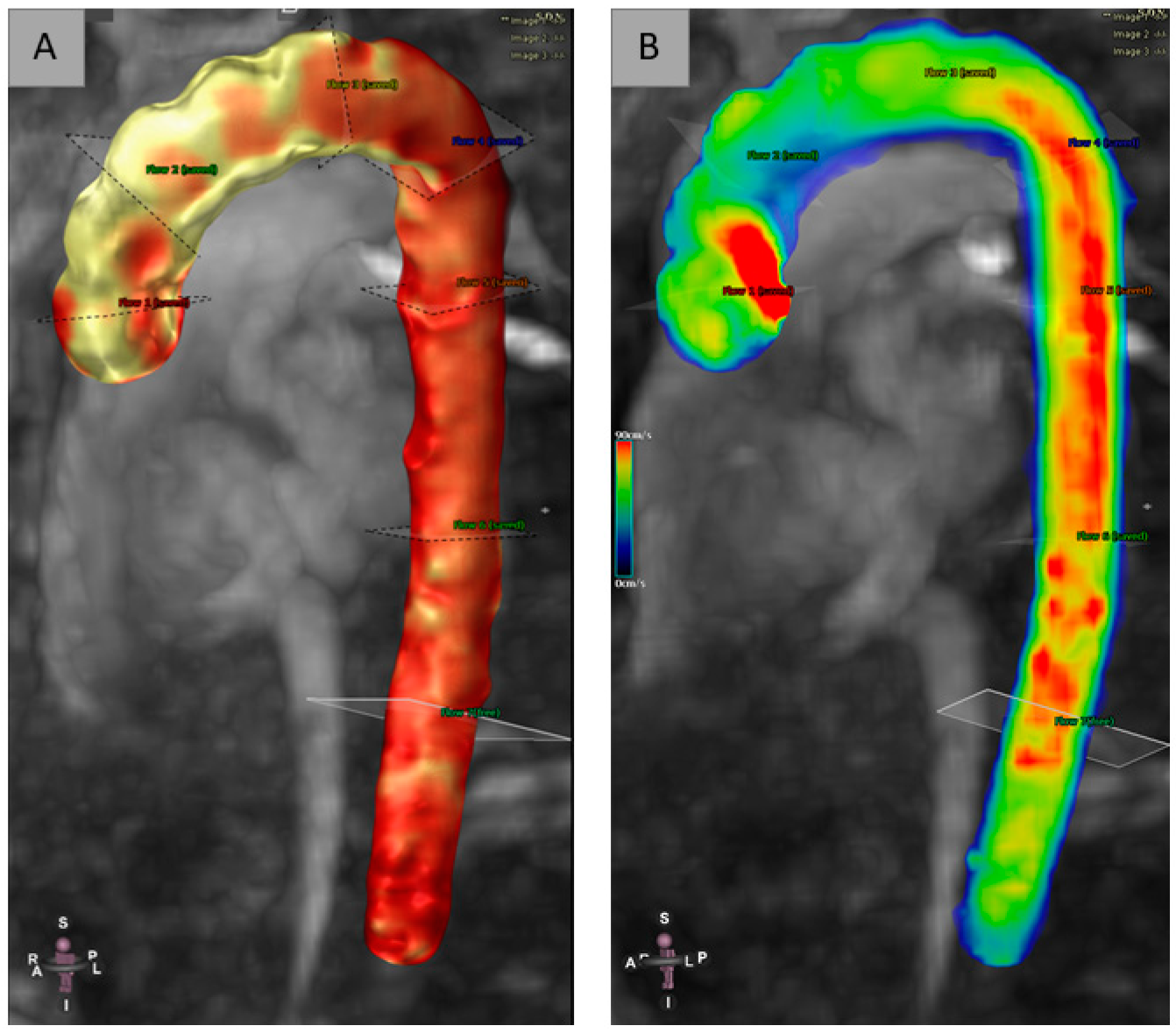
2.3. D-Flow Post-Processing
2.4. Power Analysis
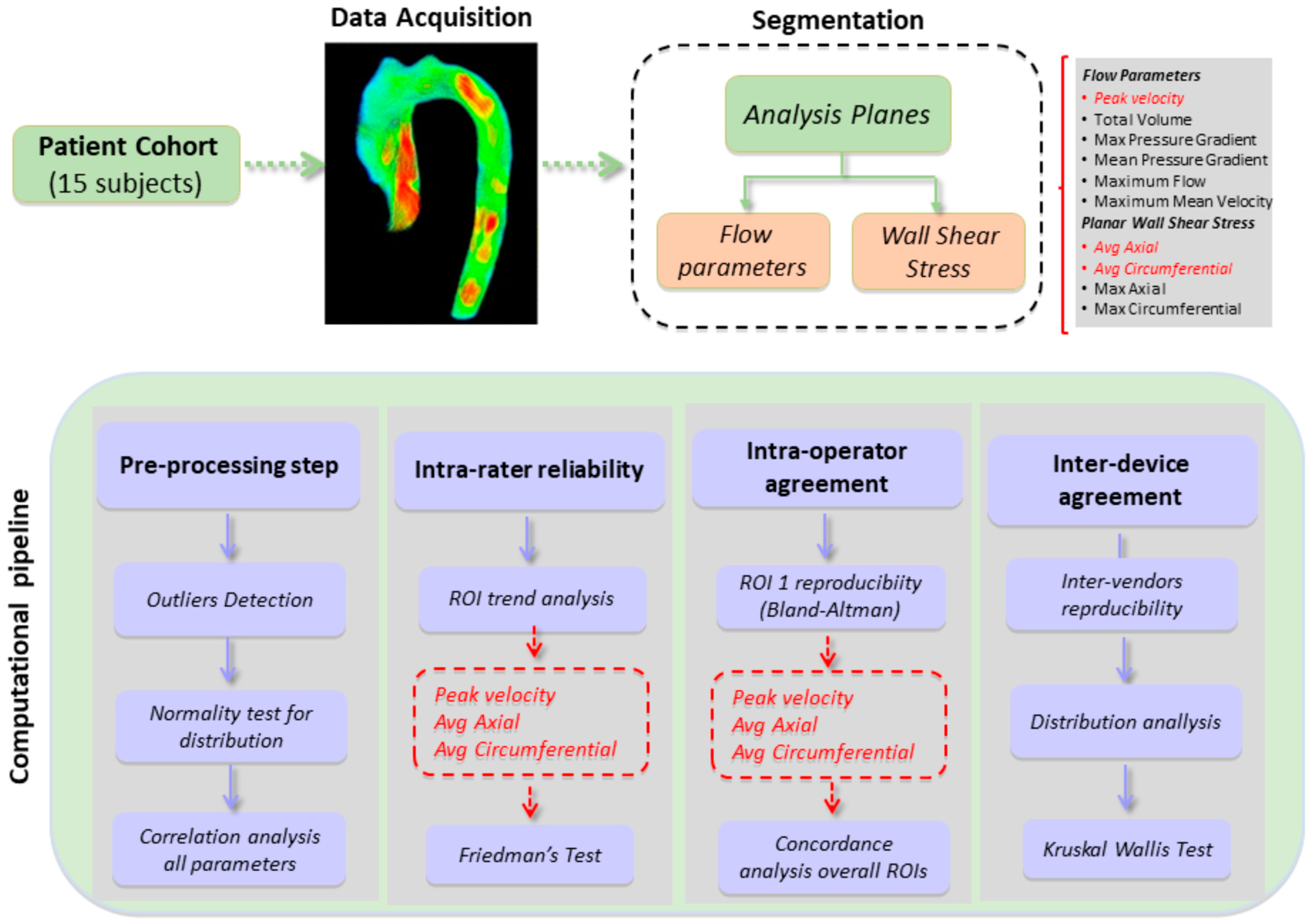
2.5. Data Analysis
3. Results
3.1. Intra-Rater Reliability
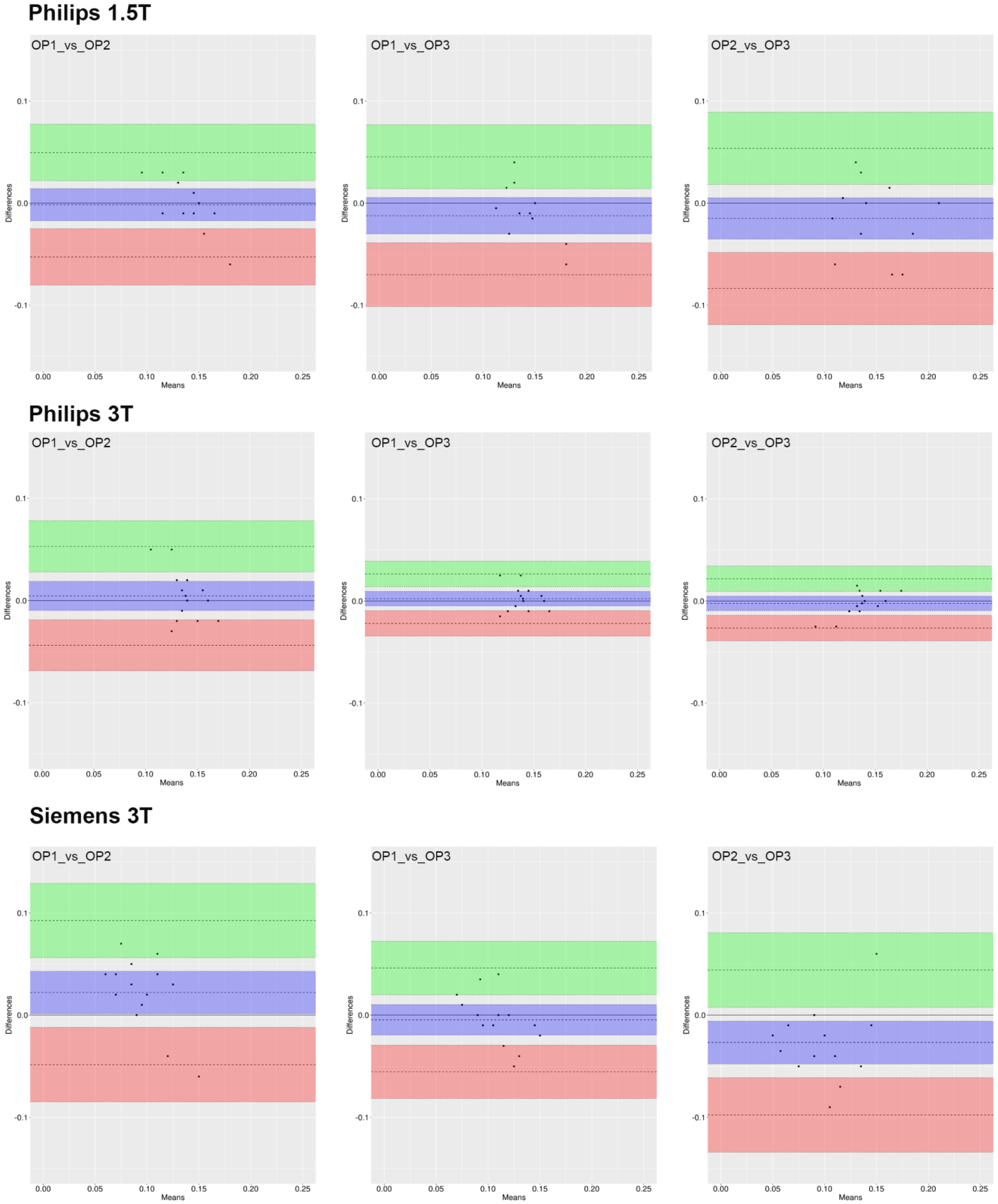
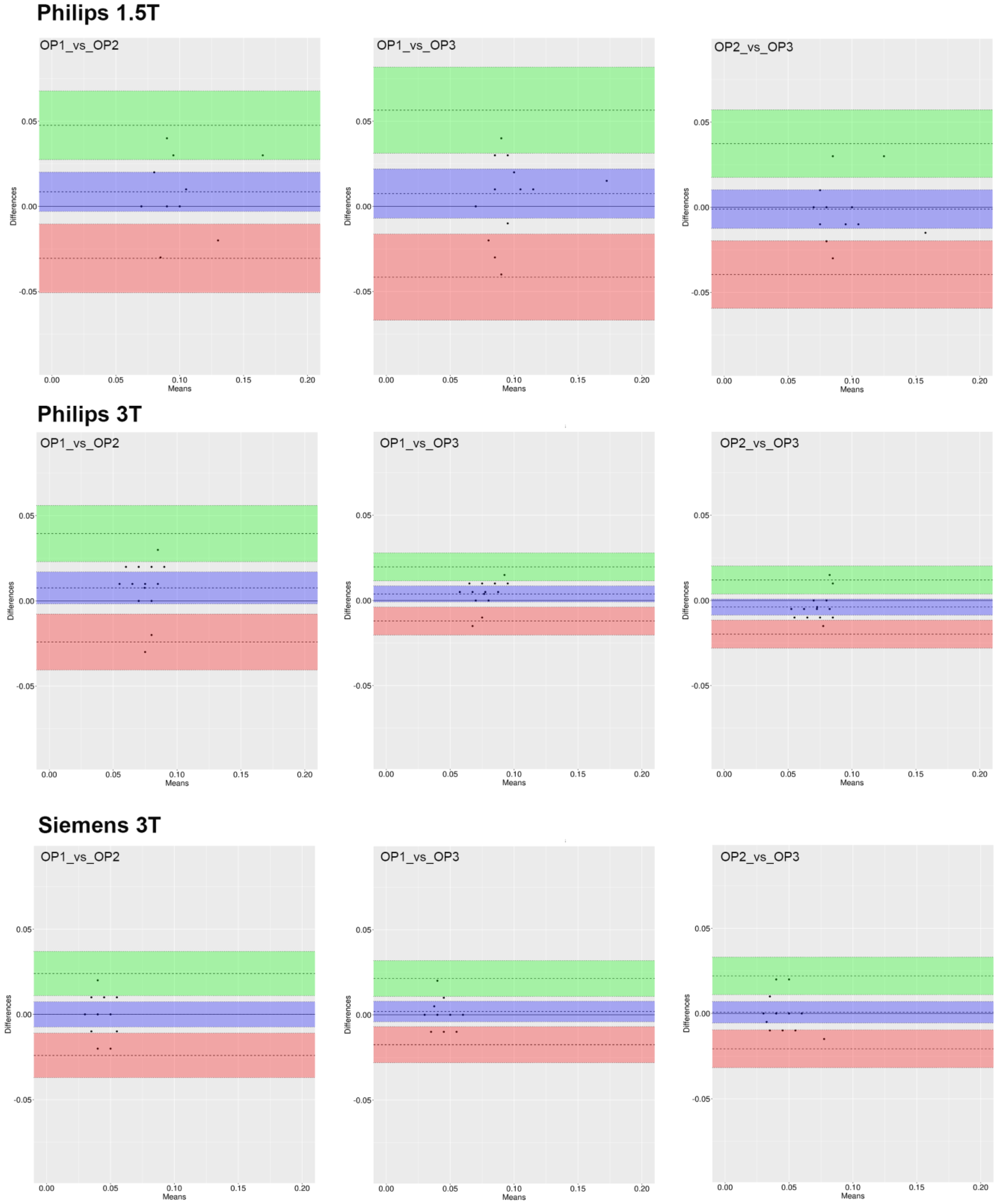
3.2. ROI_1 Reproducibility
3.3. Pre-Processing Data
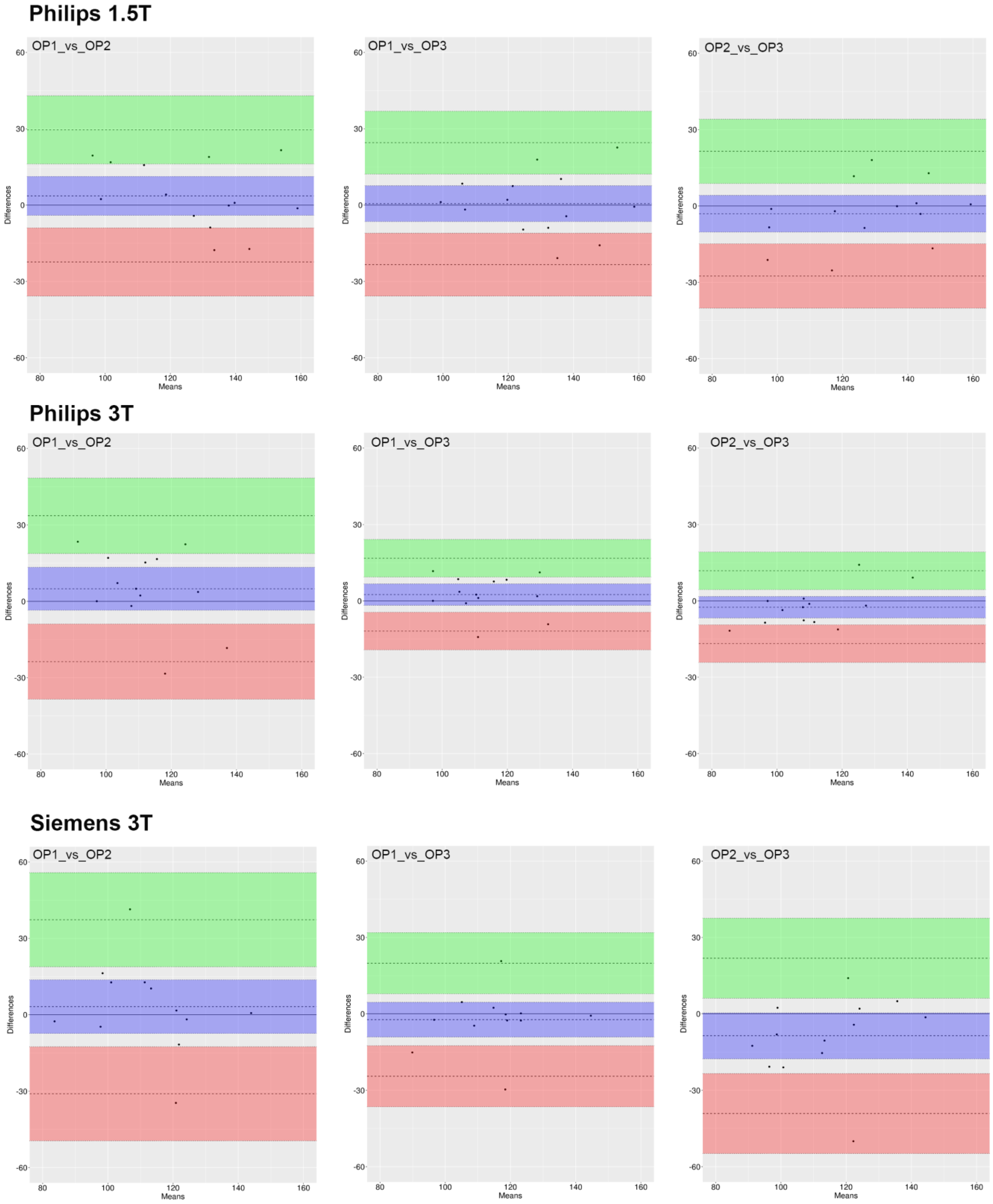
3.4. Inter-Operator Agreement
3.5. Inter-Device Agreement
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erbel, R.; Aboyans, V.; Boileaul, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926, Erratum in Eur. Heart J. 2015, 36, 2779. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Evangelista, A.; Abbara, S.; Arai, A.; Asch, F.M.; Badano, L.P.; Bolen, M.A.; Connolly, H.M.; Cuéllar-Calàbria, H.; Czerny, M.; et al. Multimodality imaging of diseases of the thoracic aorta in adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging: Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2015, 28, 119–182. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, V.; Verdini, D.; Meyersohn, N.M. Noninvasive aortic imaging. Cardiovasc. Diagn. Ther. 2018, 8 (Suppl. S1), S3–S18. [Google Scholar] [CrossRef] [PubMed]
- Myerson, S.G. Heart valve disease: Investigation by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reason. 2012, 14, 7. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.-J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reason. 2015, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.M.; Hess, A.T.; Biasiolli, L.; Glaze, S.J.; Loudon, M.; Pitcher, A.; Davis, A.; Prendergast, B.; Markl, M.; Barker, A.J.; et al. Aortic dilation in bicuspid aortic valve disease. Circ. Cardiovasc. Imaging 2013, 6, 499–507. [Google Scholar] [CrossRef]
- Schnell, S.; Smith, D.A.; Barker, A.J.; Entezari, P.; Honarmand, A.R.; Carr, M.L.; Malaisrie, S.C.; McCarthy, P.M.; Collins, J.; Carr, J.C.; et al. Altered aortic shape in bicuspid aortic valve relatives influences blood flow patterns. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1239–1247. [Google Scholar] [CrossRef]
- Bannas, P.; Lenz, A.; Petersen, J.; Sinn, M.; Adam, G.; Reichenspurner, H.; Girdauskas, E. Normalization of transvalvular flow patterns after bicuspid aortic valve repair: Insights from 4D flow cardiovascular magnetic resonance imaging. Ann. Thorac. Surg. 2018, 106, e319–e320. [Google Scholar] [CrossRef]
- Hope, M.D.; Hope, T.A.; Meadows, A.K.; Ordovas, K.G.; Urbania, T.H.; Alley, M.T.; Higgins, C.B. Bicuspid aortic valve: Four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010, 255, 53–61. [Google Scholar] [CrossRef]
- van Ooij, P.; Garcia, J.; Potters, W.V.; Malaisrie, S.C.; Collins, J.D.; Collins, J.D.; Markl, M.; Markl, M. Age related changes in aortic 3D blood flow velocities and wall shear stress: Implications for the identification of altered hemodynamics in patients with aortic valve disease. J. Magn. Reason. Imaging 2015, 43, 1239–1249. [Google Scholar] [CrossRef]
- Barker, A.J.; Markl, M.; Bürk, J.; Lorenz, R.; Bock, J.; Bauer, S.; Schulz-Menger, J.; von Knobelsdorff-Brenkenhoff, F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ. Cardiovasc. Imaging 2012, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.S.; van Ooij, P.; Planken, R.N.; Dukker, K.C.P.; de Heer, F.; Bouma, B.J.; Robbers-Visser, D.; Groenink, M.; Nederveen, A.J.; de Mol, B.A.J.M.; et al. Aortic valve stenosis and aortic diameters determine the extent of increased wall shear stress in bicuspid aortic valve disease. J. Magn. Reason. Imaging 2018, 48, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Meierhofer, C.; Schneider, E.P.; Lyko, C.; Hutter, A.; Martinoff, S.; Markl, M.; Hager, A.; Hess, J.; Stern, H.; Fratz, S.; et al. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: A prospective study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, M.Y.; Pirola, S.; Asimakopoulos, G.; Nienaber, C.; Athanasiou, T.; London Aortic Mechanobiology Working Group. Risk prediction for thoracic aortic dissection: Is it time to go with the flow? J. Thorac. Cardiovasc. Surg. 2022; ahead of print. [Google Scholar] [CrossRef]
- Lin, L. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Ebel, S.; Hübner, L.; Köhler, B.; Kropf, S.; Preim, B.; Jung, B.; Grothoff, M.; Gutberlet, M. Validation of two accelerated 4D flow MRI sequences at 3 T: A phantom study. Eur. Radiol. Exp. 2019, 3, 10. [Google Scholar] [CrossRef]
- Jamalidinan, F.; Hassanabad, A.F.; François, C.J.; Garcia, J. Four-dimensional-flow Magnetic Resonance Imaging of the Aortic Valve and Thoracic Aorta. Radiol. Clin. N. Am. 2020, 58, 753–763. [Google Scholar] [CrossRef]
- Geeraert, P.; Jamalidinan, F.; Fatehi Hassanabad, A.; Sojoudi, A.; Bristow, M.; Lydell, C.; Fedak, P.W.M.; White, J.A.; Garcia, J. Bicuspid aortic valve disease is associated with abnormal wall shear stress, viscous energy loss, and pressure drop within the ascending thoracic aorta: A cross-sectional study. Medicine 2021, 100, e26518. [Google Scholar] [CrossRef]
- Meuschke, M.; Lawonn, K.; Köhler, B.; Preim, U.; Preim, B. Clustering of aortic vortex flow in cardiac 4D PC-MRI data. In Bildverarbeitung für Die Medizin 2016; Tolxdorff, T., Deserno, T., Handels, H., Meinzer, H.P., Eds.; Informatik Aktuell; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Bock, J.; Töger, J.; Bidhult, S.; Bloch, K.M.; Arvidsson, P.; Kanski, M.; Arheden, H.; Testud, F.; Greiser, A.; Heiberg, E.; et al. Validation and reproducibility of cardiovascular 4D-flow MRI from two vendors using 2 × 2 parallel imaging acceleration in pulsatile flow phantom and in vivo with and without respiratory gating. Acta Radiol. 2018, 60, 327–337. [Google Scholar] [CrossRef]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reason. 2018, 20, 28. [Google Scholar] [CrossRef]
- Azarine, A.; Garçon, P.; Stansal, A.; Canepa, N.; Angelopoulos, G.; Silvera, S.; Sidi, D.; Marteau, V.; Zins, M. Four-dimensional Flow MRI: Principles and Cardiovascular Applications. Radiographics 2019, 39, 632–648. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punzo, B.; Ranieri, B.; Tramontano, L.; Affinito, O.; Franzese, M.; Bossone, E.; Saba, L.; Cavaliere, C.; Cademartiri, F. 4D-Flow Cardiovascular Magnetic Resonance Sequence for Aortic Assessment: Multi-Vendor and Multi-Magnetic Field Reproducibility in Healthy Volunteers. J. Clin. Med. 2023, 12, 2960. https://doi.org/10.3390/jcm12082960
Punzo B, Ranieri B, Tramontano L, Affinito O, Franzese M, Bossone E, Saba L, Cavaliere C, Cademartiri F. 4D-Flow Cardiovascular Magnetic Resonance Sequence for Aortic Assessment: Multi-Vendor and Multi-Magnetic Field Reproducibility in Healthy Volunteers. Journal of Clinical Medicine. 2023; 12(8):2960. https://doi.org/10.3390/jcm12082960
Chicago/Turabian StylePunzo, Bruna, Brigida Ranieri, Liberatore Tramontano, Ornella Affinito, Monica Franzese, Eduardo Bossone, Luca Saba, Carlo Cavaliere, and Filippo Cademartiri. 2023. "4D-Flow Cardiovascular Magnetic Resonance Sequence for Aortic Assessment: Multi-Vendor and Multi-Magnetic Field Reproducibility in Healthy Volunteers" Journal of Clinical Medicine 12, no. 8: 2960. https://doi.org/10.3390/jcm12082960
APA StylePunzo, B., Ranieri, B., Tramontano, L., Affinito, O., Franzese, M., Bossone, E., Saba, L., Cavaliere, C., & Cademartiri, F. (2023). 4D-Flow Cardiovascular Magnetic Resonance Sequence for Aortic Assessment: Multi-Vendor and Multi-Magnetic Field Reproducibility in Healthy Volunteers. Journal of Clinical Medicine, 12(8), 2960. https://doi.org/10.3390/jcm12082960








