Objective Measurement of Physical Activity and Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease: Points to Keep in Mind during Evaluations
Abstract
1. Introduction
2. The Objective Measurement of PA in COPD
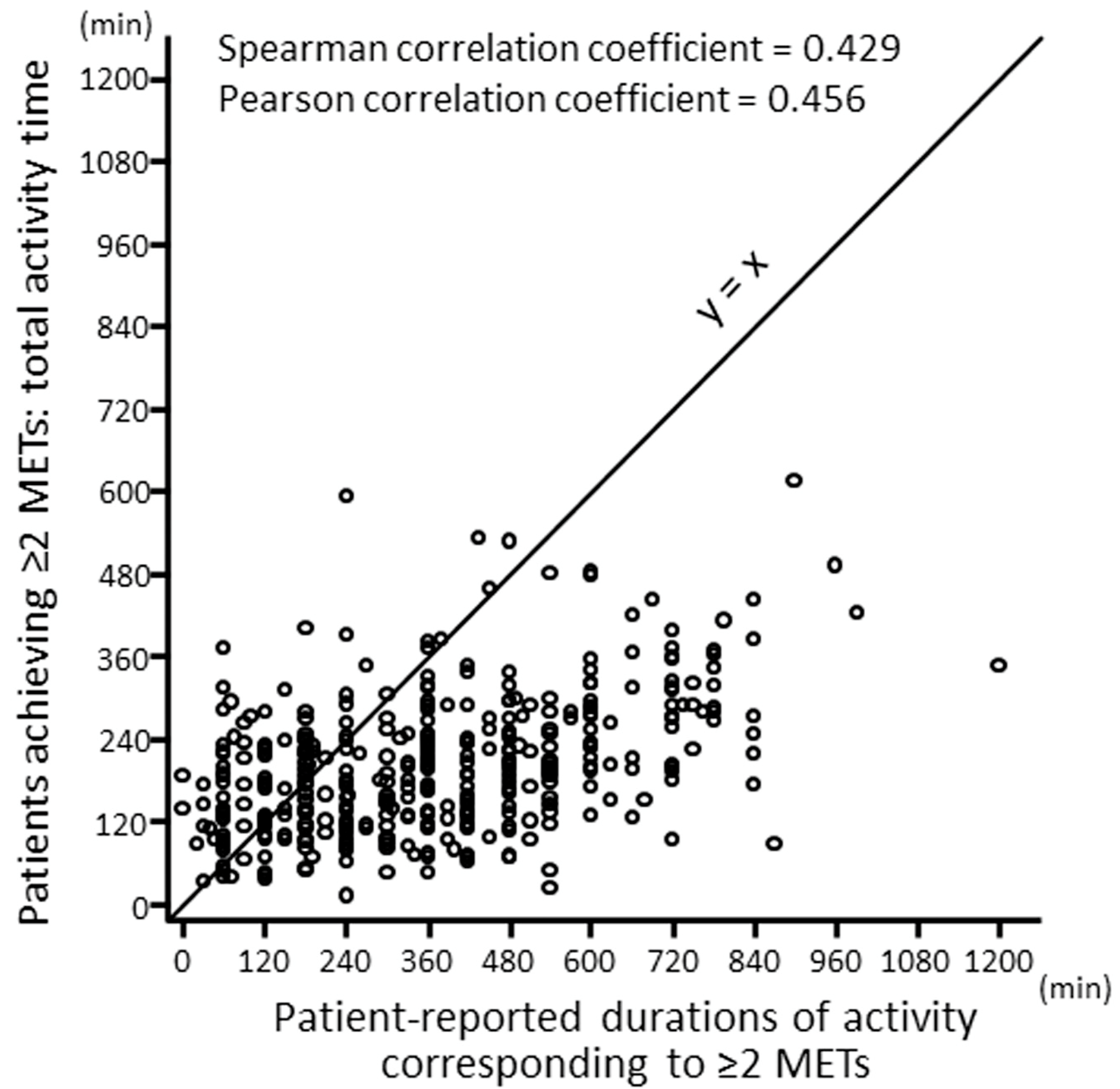
2.1. Self-Reported vs. Objectively Measured PA
2.2. Types of Accelerometry
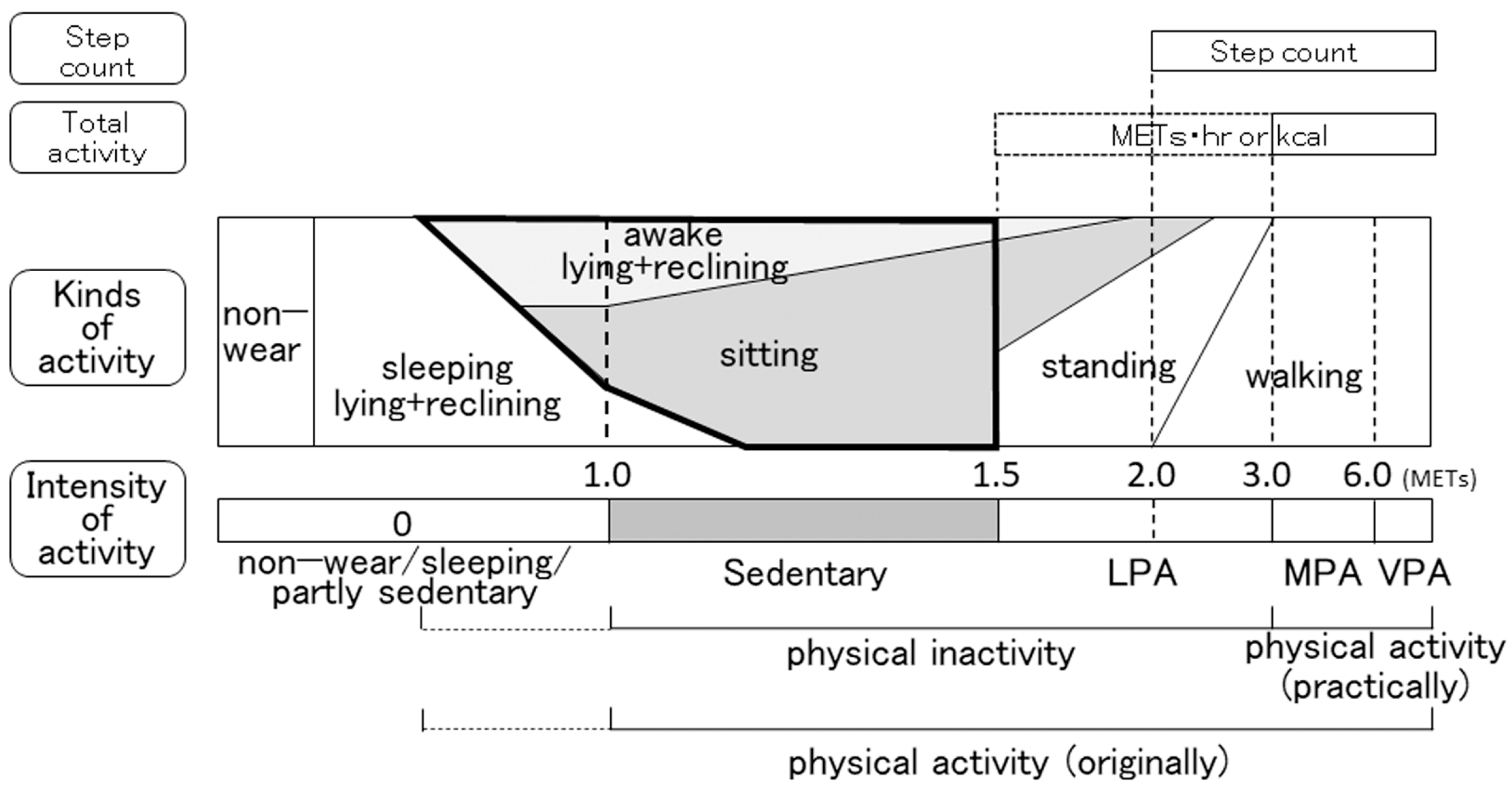
2.3. Indicators
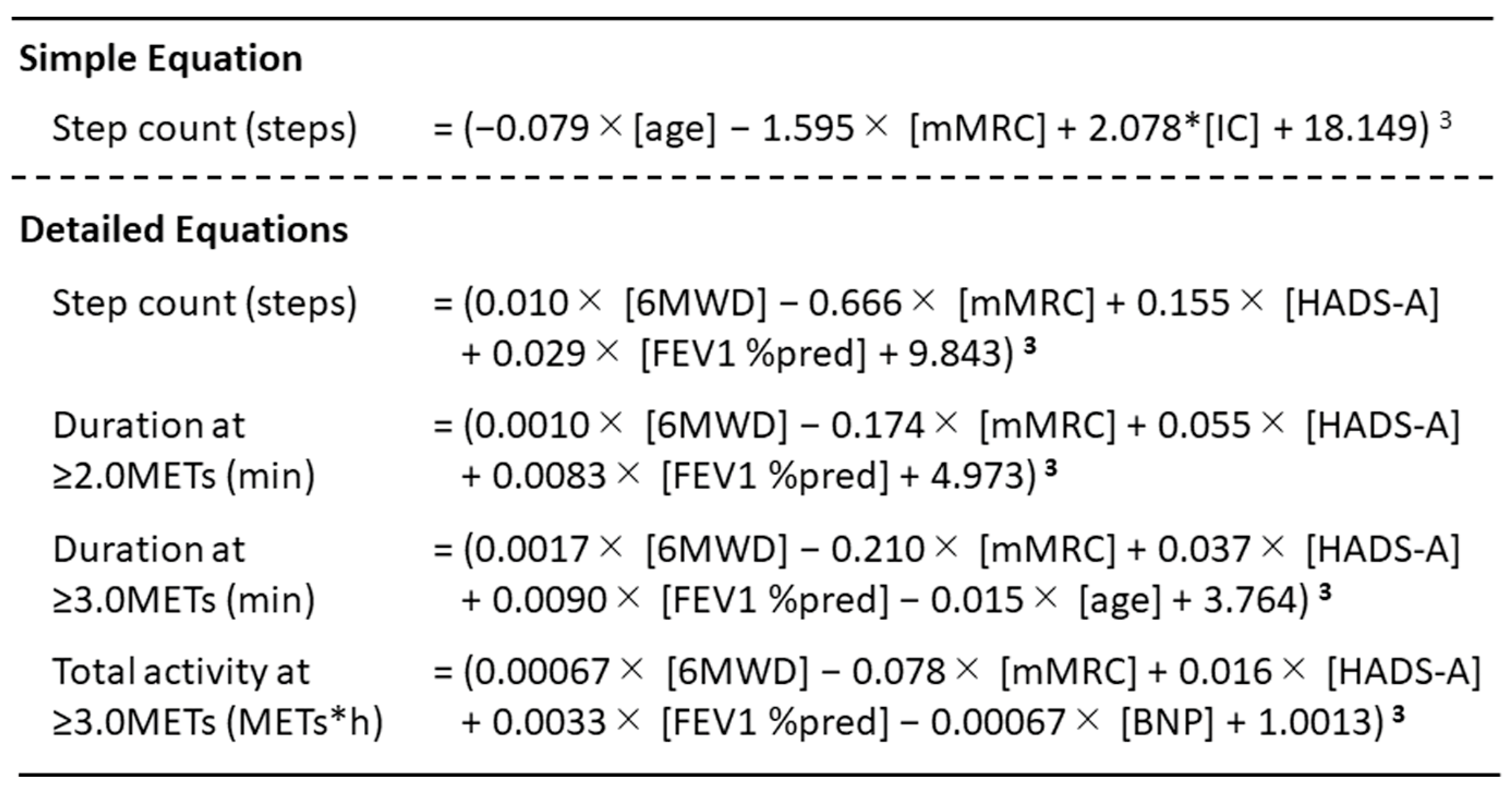
2.4. Validated Accelerometers for COPD
2.5. Environmental Factors Requiring Adjustments for Evaluations
2.5.1. Weather
2.5.2. Season
2.5.3. Day of the Week
2.5.4. Air Pollution
2.5.5. Employment Status
2.6. Methodological Factors Requiring Adjustments for Evaluations
2.6.1. Days with Uncommon Activities
2.6.2. Non-Wearing Time
2.6.3. Minimum Required Wearing Time per Day
2.6.4. Minimum Number of Valid Days Required
2.7. Patient Conditions Influencing PA
2.8. Interventions for Improving PA
2.8.1. Pharmacological Interventions
2.8.2. Non-Pharmacological Interventions
3. The Objective Measurement of SB in COPD
3.1. Sedentary Time (ST) in Subjects with Several Conditions
3.2. Objectively Measured ST and Its Problems
3.2.1. Functional Limitations of Accelerometers
3.2.2. Exclusion of Sleeping Time
3.2.3. Unification of Total Measurement Time per Day
3.2.4. Minimum Required Wearing Time per Day
3.3. Lying-Down-Time-to-Sitting-Time Ratio (LSR) in COPD Patients
3.4. Interventions for Improving ST
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef]
- Minakata, Y.; Sugino, A.; Kanda, M.; Ichikawa, T.; Akamatsu, K.; Koarai, A.; Hirano, T.; Nakanishi, M.; Sugiura, H.; Matsunaga, K.; et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2014, 52, 41–48. [Google Scholar] [CrossRef]
- Jakes, R.W.; Day, N.E.; Patel, B.; Khaw, K.T.; Oakes, S.; Luben, R.; Welch, A.; Bingham, S.; Wareham, N.J. Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation into Cancer-Norfolk Prospective Population Study. Am. J. Epidemiol. 2002, 156, 139–147. [Google Scholar] [CrossRef]
- Pelkonen, M.; Notkola, I.L.; Lakka, T.; Tukiainen, H.O.; Kivinen, P.; Nissinen, A. Delaying decline in pulmonary function with physical activity: A 25-year follow-up. Am. J. Respir. Crit. Care Med. 2003, 168, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Macera, C.A.; Addy, C.L.; Sy, F.S.; Wieland, D.; Blair, S.N. Effects of physical activity on exercise tests and respiratory function. Br. J. Sport. Med. 2003, 37, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Anto, J.M. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: A population-based cohort study. Am. J. Respir. Crit. Care Med. 2007, 175, 458–463. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Serra, I.; Schnohr, P.; Antó, J.M. Time-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: An application of the marginal structural model. Ann. Epidemiol. 2008, 18, 775–783. [Google Scholar] [CrossRef]
- Garcia-Rio, F.; Rojo, B.; Casitas, R.; Lores, V.; Madero, R.; Romero, D.; Galera, R.; Villasante, C. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012, 142, 338–346. [Google Scholar] [CrossRef]
- Moy, M.L.; Teylan, M.; Weston, N.A.; Gagnon, D.R.; Garshick, E. Daily Step Count Predicts Acute Exacerbations in a US Cohort with COPD. PLoS ONE 2013, 8, e60400. [Google Scholar] [CrossRef]
- Crook, S.; Busching, G.; Keusch, S.; Wieser, S.; Turk, A.; Frey, M.; Puhan, M.A.; Frei, A. The association between daily exacerbation symptoms and physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 2199–2206. [Google Scholar] [CrossRef]
- Alahmari, A.D.; Patel, A.R.C.; Kowlessar, B.S.; Mackay, A.J.; Singh, R.; Wedzicha, J.A.; Donaldson, G.C. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Costilla-Frias, M.; Louvaris, Z.; Gimeno-Santos, E.; Tabberer, M.; Rabinovich, R.A.; de Jong, C.; Polkey, M.I.; Hopkinson, N.S.; Karlsson, N.; et al. Both moderate and severe exacerbations accelerate physical activity decline in COPD patients. Eur. Respir. J. 2018, 51, 1702110. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Anto, J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax 2006, 61, 772–778. [Google Scholar] [CrossRef]
- Waschki, B.; Kirsten, A.; Holz, O.; Muller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Minakata, Y.; Sasaki, S. Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients. J. Clin. Med. 2020, 9, 3497. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Tremblay, M.S.; Colley, R.C.; Saunders, T.J.; Healy, G.N.; Owen, N. Physiological and health implications of a sedentary lifestyle. Appl. Physiol. Nutr. Metab. 2010, 35, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The evolving definition of “sedentary”. Exerc. Sport Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef]
- Network, S.B.R. Letter to the editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar]
- Furlanetto, K.C.; Donaria, L.; Schneider, L.P.; Lopes, J.R.; Ribeiro, M.; Fernandes, K.B.; Hernandes, N.A.; Pitta, F. Sedentary Behavior Is an Independent Predictor of Mortality in Subjects with COPD. Respir. Care 2017, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Ukawa, S.; Tamakoshi, A.; Yatsuya, H.; Yamagishi, K.; Ando, M.; Iso, H. Association between Average Daily Television Viewing Time and Chronic Obstructive Pulmonary Disease-Related Mortality: Findings from the Japan Collaborative Cohort Study. J. Epidemiol. 2015, 25, 431–436. [Google Scholar] [CrossRef]
- Cavalheri, V.; Straker, L.; Gucciardi, D.F.; Gardiner, P.A.; Hill, K. Changing physical activity and sedentary behaviour in people with COPD. Respirology 2016, 21, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.K.; Hunt, T.; Williams, M.T.; English, C.; Olds, T.S. Sedentary Behavior in People with and without a Chronic Health Condition: How Much, What and When? AIMS Public Health 2016, 3, 503–519. [Google Scholar] [CrossRef]
- McKeough, Z.; Cheng, S.W.M.; Alison, J.; Jenkins, C.; Hamer, M.; Stamatakis, E. Low leisure-based sitting time and being physically active were associated with reduced odds of death and diabetes in people with chronic obstructive pulmonary disease: A cohort study. J. Physiother. 2018, 64, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Good, J.; Buman, M.P.; Gardiner, P.A.; Copeland, J.L.; Stickland, M.K. Physical activity and sedentary time are related to clinically relevant health outcomes among adults with obstructive lung disease. BMC Pulm. Med. 2018, 18, 98. [Google Scholar] [CrossRef]
- Bernard, P.; Hains-Monfette, G.; Atoui, S.; Moullec, G. Daily Objective Physical Activity and Sedentary Time in Adults with COPD Using Spirometry Data from Canadian Measures Health Survey. Can. Respir. J. 2018, 2018, 9107435. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Minakata, Y.; Motegi, T.; Takahashi, T.; Seki, M.; Sugaya, S.; Hayashi, N.; Kuwahira, I. A Non-Interventional, Cross-Sectional Study to Evaluate Factors Relating to Daily Step Counts and Physical Activity in Japanese Patients with Chronic Obstructive Pulmonary Disease: STEP COPD. Int. J. Chron. Obs. Pulmon. Dis. 2020, 15, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Sievi, N.A.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; Kohler, M.; Clarenbach, C.F. Accelerometer- versus questionnaire-based assessment of physical activity and their changes over time in patients with COPD. Int. J. Chron. Obs. Pulmon. Dis. 2017, 12, 1113–1118. [Google Scholar] [CrossRef]
- Thyregod, M.; Bodtger, U. Coherence between self-reported and objectively measured physical activity in patients with chronic obstructive lung disease: A systematic review. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 2931–2938. [Google Scholar] [CrossRef]
- Van Remoortel, H.; Raste, Y.; Louvaris, Z.; Giavedoni, S.; Burtin, C.; Langer, D.; Wilson, F.; Rabinovich, R.; Vogiatzis, I.; Hopkinson, N.S.; et al. Validity of six activity monitors in chronic obstructive pulmonary disease: A comparison with indirect calorimetry. PLoS ONE 2012, 7, e39198. [Google Scholar] [CrossRef]
- de Groot, S.; Nieuwenhuizen, M.G. Validity and reliability of measuring activities, movement intensity and energy expenditure with the DynaPort MoveMonitor. Med. Eng. Phys. 2013, 35, 1499–1505. [Google Scholar] [CrossRef]
- Rabinovich, R.A.; Louvaris, Z.; Raste, Y.; Langer, D.; Van Remoortel, H.; Giavedoni, S.; Burtin, C.; Regueiro, E.M.; Vogiatzis, I.; Hopkinson, N.S.; et al. Validity of physical activity monitors during daily life in patients with COPD. Eur. Respir. J. 2013, 42, 1205–1215. [Google Scholar] [CrossRef]
- Farooqi, N.; Slinde, F.; Håglin, L.; Sandström, T. Validation of SenseWear Armband and ActiHeart monitors for assessments of daily energy expenditure in free-living women with chronic obstructive pulmonary disease. Physiol. Rep. 2013, 1, e00150. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.G.; Holt, L.; Belza, B.; Ferris, S.; Lakshminaryan, S.; Buchner, D.M. Quantitating physical activity in COPD using a triaxial accelerometer. Chest 2000, 117, 1359–1367. [Google Scholar] [CrossRef]
- Hunt, T.; Williams, M.T.; Olds, T.S. Reliability and validity of the multimedia activity recall in children and adults (MARCA) in people with chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e81274. [Google Scholar] [CrossRef] [PubMed]
- Sugino, A.; Minakata, Y.; Kanda, M.; Akamatsu, K.; Koarai, A.; Hirano, T.; Sugiura, H.; Matsunaga, K.; Ichinose, M. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration 2012, 83, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Minakata, Y.; Azuma, Y.; Kawabe, K.; Ono, H.; Yanagimoto, R.; Suruda, T. Verification of a Motion Sensor for Evaluating Physical Activity in COPD Patients. Can. Respir. J. 2018, 2018, 8343705. [Google Scholar] [CrossRef]
- Turner, L.J.; Houchen, L.; Williams, J.; Singh, S.J. Reliability of pedometers to measure step counts in patients with chronic respiratory disease. J. Cardiopulm. Rehabil. Prev. 2012, 32, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Luzi, S.; Murer, K.; de Bie, R.A.; de Bruin, E.D. Concurrent validity of a trunk tri-axial accelerometer system for gait analysis in older adults. Gait Posture 2009, 29, 444–448. [Google Scholar] [CrossRef]
- Harrison, S.L.; Horton, E.J.; Smith, R.; Sandland, C.J.; Steiner, M.C.; Morgan, M.D.; Singh, S.J. Physical activity monitoring: Addressing the difficulties of accurately detecting slow walking speeds. Heart Lung 2013, 42, 361–364.e1. [Google Scholar] [CrossRef]
- Feng, Y.; Wong, C.K.; Janeja, V.; Kuber, R.; Mentis, H.M. Comparison of tri-axial accelerometers step-count accuracy in slow walking conditions. Gait Posture 2017, 53, 11–16. [Google Scholar] [CrossRef]
- Oshima, Y.; Kawaguchi, K.; Tanaka, S.; Ohkawara, K.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010, 31, 370–374. [Google Scholar] [CrossRef]
- Ohkawara, K.; Oshima, Y.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I.; Tanaka, S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br. J. Nutr. 2011, 105, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, A.D.; Mackay, A.J.; Patel, A.R.; Kowlessar, B.S.; Singh, R.; Brill, S.E.; Allinson, J.P.; Wedzicha, J.A.; Donaldson, G.C. Influence of weather and atmospheric pollution on physical activity in patients with COPD. Respir. Res. 2015, 16, 71. [Google Scholar] [CrossRef]
- Balish, S.M.; Dechman, G.; Hernandez, P.; Spence, J.C.; Rhodes, R.E.; McGannon, K.; Blanchard, C. The Relationship between Weather and Objectively Measured Physical Activity among Individuals with COPD. J. Cardiopulm. Rehabil. Prev. 2017, 37, 445–449. [Google Scholar] [CrossRef]
- Vaidya, T.; Thomas-Ollivier, V.; Hug, F.; Bernady, A.; Le Blanc, C.; de Bisschop, C.; Chambellan, A. Translation and Cultural Adaptation of PROactive Instruments for COPD in French and Influence of Weather and Pollution on Its Difficulty Score. Int. J. Chron. Obs. Pulmon. Dis. 2020, 15, 471–478. [Google Scholar] [CrossRef]
- Sumukadas, D.; Witham, M.; Struthers, A.; McMurdo, M. Day length and weather conditions profoundly affect physical activity levels in older functionally impaired people. J. Epidemiol. Community Health 2009, 63, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sewell, L.; Singh, S.J.; Williams, J.E.; Morgan, M.D. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J. Cardiopulm. Rehabil. Prev. 2010, 30, 329–333. [Google Scholar] [CrossRef]
- Furlanetto, K.C.; Demeyer, H.; Sant’anna, T.; Hernandes, N.A.; Camillo, C.A.; Pons, I.S.; Gosselink, R.; Troosters, T.; Pitta, F. Physical Activity of Patients with COPD from Regions with Different Climatic Variations. COPD 2017, 14, 276–283. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Goldring, J.J.; Wedzicha, J.A. Influence of season on exacerbation characteristics in patients with COPD. Chest 2012, 141, 94–100. [Google Scholar] [CrossRef]
- Gretebeck, R.J.; Montoye, H.J. Variability of some objective measures of physical activity. Med. Sci. Sport. Exerc. 1992, 24, 1167–1172. [Google Scholar] [CrossRef]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R., Jr. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sport. Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Burkett, L.; Reis, J.P.; Ainsworth, B.E.; Macera, C.A.; Wilson, D.K. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005, 40, 293–298. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sport. Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sport. Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Ward, S.C.; Schnelle, J.F.; Buchowski, M.S. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med. Sci. Sport. Exerc. 2012, 44, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Byrom, B.; Rowe, D.A. Measuring free-living physical activity in COPD patients: Deriving methodology standards for clinical trials through a review of research studies. Contemp. Clin. Trials 2016, 47, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, H.; Burtin, C.; Van Remoortel, H.; Hornikx, M.; Langer, D.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 2014, 146, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Kantorowski, A.; Wan, E.S.; Homsy, D.; Kadri, R.; Richardson, C.R.; Moy, M.L. Determinants and outcomes of change in physical activity in COPD. ERJ Open Res. 2018, 4, 00054-2018. [Google Scholar] [CrossRef] [PubMed]
- Hains-Monfette, G.; Atoui, S.; Needham Dancause, K.; Bernard, P. Device-Assessed Physical Activity and Sedentary Behaviors in Canadians with Chronic Disease(s): Findings from the Canadian Health Measures Survey. Sports 2019, 7, 113. [Google Scholar] [CrossRef]
- Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Nakamura, S.; Anzai, T.; Hirata, K.; Ichinose, M. Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: Post hoc analysis of the VESUTO((R)) study. Int. J. Chron. Obs. Pulmon. Dis. 2019, 14, 1789–1801. [Google Scholar] [CrossRef]
- Hoaas, H.; Zanaboni, P.; Hjalmarsen, A.; Morseth, B.; Dinesen, B.; Burge, A.T.; Cox, N.S.; Holland, A.E. Seasonal variations in objectively assessed physical activity among people with COPD in two Nordic countries and Australia: A cross-sectional study. Int. J. Chron. Obs. Pulmon. Dis. 2019, 14, 1219–1228. [Google Scholar] [CrossRef]
- Paneroni, M.; Ambrosino, N.; Simonelli, C.; Bertacchini, L.; Venturelli, M.; Vitacca, M. Physical Activity in Patients with Chronic Obstructive Pulmonary Disease on Long-Term Oxygen Therapy: A Cross-Sectional Study. Int. J. Chron. Obs. Pulmon. Dis. 2019, 14, 2815–2823. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Meyer, T.; Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 2009, 33, 262–272. [Google Scholar] [CrossRef]
- Nakanishi, M.; Minakata, Y.; Tanaka, R.; Sugiura, H.; Kuroda, H.; Yoshida, M.; Yamamoto, N. Simple standard equation for daily step count in Japanese patients with chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2019, 14, 1967–1977. [Google Scholar] [CrossRef]
- Minakata, Y.; Sasaki, S.; Azuma, Y.; Kawabe, K.; Ono, H. Reference Equations for Assessing the Physical Activity of Japanese Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon. Dis. 2021, 16, 3041–3053. [Google Scholar] [CrossRef]
- Waschki, B.; Spruit, M.A.; Watz, H.; Albert, P.S.; Shrikrishna, D.; Groenen, M.; Smith, C.; Man, W.D.; Tal-Singer, R.; Edwards, L.D.; et al. Physical activity monitoring in COPD: Compliance and associations with clinical characteristics in a multicenter study. Respir. Med. 2012, 106, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Van Remoortel, H.; Hornikx, M.; Demeyer, H.; Langer, D.; Burtin, C.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013, 68, 962–963. [Google Scholar] [CrossRef]
- Demeyer, H.; Gimeno-Santos, E.; Rabinovich, R.A.; Hornikx, M.; Louvaris, Z.; de Boer, W.I.; Karlsson, N.; de Jong, C.; Van der Molen, T.; Vogiatzis, I.; et al. Physical Activity Characteristics across GOLD Quadrants Depend on the Questionnaire Used. PLoS ONE 2016, 11, e0151255. [Google Scholar] [CrossRef] [PubMed]
- Okubadejo, A.A.; O’Shea, L.; Jones, P.W.; Wedzicha, J.A. Home assessment of activities of daily living in patients with severe chronic obstructive pulmonary disease on long-term oxygen therapy. Eur. Respir. J. 1997, 10, 1572–1575. [Google Scholar] [CrossRef]
- Lahaije, A.J.; van Helvoort, H.A.; Dekhuijzen, P.N.; Vercoulen, J.H.; Heijdra, Y.F. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir. Med. 2013, 107, 834–840. [Google Scholar] [CrossRef]
- Shiue, I. Daily walking > 10 min could improve mental health in people with historical cardiovascular disease or COPD: Scottish Health Survey, 2012. Int. J. Cardiol. 2015, 179, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Arbillaga-Etxarri, A.; Gimeno-Santos, E.; Barberan-Garcia, A.; Benet, M.; Borrell, E.; Dadvand, P.; Foraster, M.; Marin, A.; Monteagudo, M.; Rodriguez-Roisin, R.; et al. Socio-environmental correlates of physical activity in patients with chronic obstructive pulmonary disease (COPD). Thorax 2017, 72, 796–802. [Google Scholar] [CrossRef]
- Tanimura, K.; Sato, S.; Fuseya, Y.; Hasegawa, K.; Uemasu, K.; Sato, A.; Oguma, T.; Hirai, T.; Mishima, M.; Muro, S. Quantitative Assessment of Erector Spinae Muscles in Patients with Chronic Obstructive Pulmonary Disease. Nov. Chest Comput. Tomogr.-Deriv. Index Prognosis. Ann. Am. Thorac. Soc. 2016, 13, 334–341. [Google Scholar]
- Shrikrishna, D.; Patel, M.; Tanner, R.J.; Seymour, J.M.; Connolly, B.A.; Puthucheary, Z.A.; Walsh, S.L.; Bloch, S.A.; Sidhu, P.S.; Hart, N.; et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur. Respir. J. 2012, 40, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, N.; Kanazawa, H.; Asai, K.; Watanabe, T.; Hirata, K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology 2015, 20, 612–617. [Google Scholar] [CrossRef]
- Tanaka, R.; Sugiura, H.; Yamada, M.; Ichikawa, T.; Koarai, A.; Fujino, N.; Yanagisawa, S.; Onodera, K.; Numakura, T.; Sato, K.; et al. Physical inactivity is associated with decreased growth differentiation factor 11 in chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 1333–1342. [Google Scholar] [CrossRef]
- Moy, M.L.; Teylan, M.; Weston, N.A.; Gagnon, D.R.; Danilack, V.A.; Garshick, E. Daily step count is associated with plasma C-reactive protein and IL-6 in a US cohort with COPD. Chest 2014, 145, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P.; Berntsen, A.; Perstrup, L.B.; Eskildsen, P.; Pedersen, B.K. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand. J. Med. Sci. Sport. 2007, 17, 580–587. [Google Scholar] [CrossRef]
- Fuertes, E.; Carsin, A.E.; Garcia-Larsen, V.; Guerra, S.; Pin, I.; Leynaert, B.; Accordini, S.; Martinez-Moratalla, J.; Anto, J.M.; Urrutia, I.; et al. The role of C-reactive protein levels on the association of physical activity with lung function in adults. PLoS ONE 2019, 14, e0222578. [Google Scholar] [CrossRef] [PubMed]
- Taka, C.; Hayashi, R.; Shimokawa, K.; Tokui, K.; Okazawa, S.; Kambara, K.; Inomata, M.; Yamada, T.; Matsui, S.; Tobe, K. SIRT1 and FOXO1 mRNA expression in PBMC correlates to physical activity in COPD patients. Int. J. Chron. Obs. Pulmon. Dis. 2017, 12, 3237–3244. [Google Scholar] [CrossRef] [PubMed]
- Burge, A.T.; Cox, N.S.; Abramson, M.J.; Holland, A.E. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2020, 4, Cd012626. [Google Scholar] [CrossRef]
- Hataji, O.; Naito, M.; Ito, K.; Watanabe, F.; Gabazza, E.C.; Taguchi, O. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2013, 8, 1–5. [Google Scholar]
- Watz, H.; Krippner, F.; Kirsten, A.; Magnussen, H.; Vogelmeier, C. Indacaterol improves lung hyperinflation and physical activity in patients with moderate chronic obstructive pulmonary disease--a randomized, multicenter, double-blind, placebo-controlled study. BMC Pulm. Med. 2014, 14, 158. [Google Scholar] [CrossRef]
- Minakata, Y.; Morishita, Y.; Ichikawa, T.; Akamatsu, K.; Hirano, T.; Nakanishi, M.; Matsunaga, K.; Ichinose, M. Effects of pharmacologic treatment based on airflow limitation and breathlessness on daily physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 1275–1282. [Google Scholar] [CrossRef]
- Watz, H.; Troosters, T.; Beeh, K.M.; Garcia-Aymerich, J.; Paggiaro, P.; Molins, E.; Notari, M.; Zapata, A.; Jarreta, D.; Garcia Gil, E. ACTIVATE: The effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int. J. Chron. Obs. Pulmon. Dis. 2017, 12, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Kamei, T.; Nakamura, H.D.; Nanki, N.D.; Minakata, Y.D.; Matsunaga, K.D.; Mori, Y.D. Clinical benefit of two-times-per-day aclidinium bromide compared with once-a-day tiotropium bromide hydrate in COPD: A multicentre, open-label, randomised study. BMJ Open 2019, 9, e024114. [Google Scholar] [CrossRef]
- Hirano, T.; Matsunaga, K.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Oishi, K.; Asami, M.; Edakuni, N.; Ogawa, H.; et al. Combination of assist use of short-acting beta-2 agonists inhalation and guidance based on patient-specific restrictions in daily behavior: Impact on physical activity of Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2019, 57, 133–139. [Google Scholar] [CrossRef]
- Tsujimura, Y.; Hiramatsu, T.; Kojima, E.; Tabira, K. Effect of pulmonary rehabilitation with assistive use of short-acting β2 agonist in COPD patients using long-acting bronchodilators. Physiother. Theory Pract. 2019, 37, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Beeh, K.M.; Watz, H.; Puente-Maestu, L.; de Teresa, L.; Jarreta, D.; Caracta, C.; Gil, E.G.; Magnussen, H. Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: A randomized, placebo-controlled, crossover trial. BMC Pulm. Med. 2014, 14, 209. [Google Scholar] [CrossRef]
- Nishijima, Y.; Minami, S.; Yamamoto, S.; Ogata, Y.; Koba, T.; Futami, S.; Komuta, K. Influence of indacaterol on daily physical activity in patients with untreated chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 439–444. [Google Scholar]
- Watz, H.; Mailander, C.; Baier, M.; Kirsten, A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: A randomised, placebo-controlled, crossover study (The MOVE Study). BMC Pulm. Med. 2016, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Casaburi, R.; Vincken, W.; Puente-Maestu, L.; Swales, J.; Lawrence, D.; Kramer, B. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir. Med. 2011, 105, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Sciurba, F.C.; Decramer, M.; Siafakas, N.M.; Klioze, S.S.; Sutradhar, S.C.; Weisman, I.M.; Yunis, C. Tiotropium in patients with moderate COPD naive to maintenance therapy: A randomised placebo-controlled trial. NPJ Prim. Care Respir. Med. 2014, 24, 14003. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, M.; Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Seki, T.; Anzai, T.; Nakamura, S.; Hirata, K. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 1407–1419. [Google Scholar] [CrossRef]
- Troosters, T.; Maltais, F.; Leidy, N.; Lavoie, K.L.; Sedeno, M.; Janssens, W.; Garcia-Aymerich, J.; Erzen, D.; De Sousa, D.; Korducki, L.; et al. Effect of Bronchodilation, Exercise Training, and Behavior Modification on Symptoms and Physical Activity in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1021–1032. [Google Scholar] [CrossRef]
- Miravitlles, M.; García-Rivero, J.L.; Ribera, X.; Galera, J.; García, A.; Palomino, R.; Pomares, X. Exercise capacity and physical activity in COPD patients treated with a LAMA/LABA combination: A systematic review and meta-analysis. Respir. Res. 2022, 23, 347. [Google Scholar] [CrossRef]
- Shioya, T.; Sato, S.; Iwakura, M.; Takahashi, H.; Terui, Y.; Uemura, S.; Satake, M. Improvement of physical activity in chronic obstructive pulmonary disease by pulmonary rehabilitation and pharmacological treatment. Respir. Investig. 2018, 56, 292–306. [Google Scholar] [CrossRef]
- Locke, E.A.; Latham, G.P. A Theory of Goal Setting & Task Performance; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1990. [Google Scholar]
- Altenburg, W.A.; ten Hacken, N.H.; Bossenbroek, L.; Kerstjens, H.A.; de Greef, M.H.; Wempe, J.B. Short- and long-term effects of a physical activity counselling programme in COPD: A randomized controlled trial. Respir. Med. 2015, 109, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Moy, M.L.; Collins, R.J.; Martinez, C.H.; Kadri, R.; Roman, P.; Holleman, R.G.; Kim, H.M.; Nguyen, H.Q.; Cohen, M.D.; Goodrich, D.E.; et al. An Internet-Mediated Pedometer-Based Program Improves Health-Related Quality-of-Life Domains and Daily Step Counts in COPD: A Randomized Controlled Trial. Chest 2015, 148, 128–137. [Google Scholar] [CrossRef]
- Wan, E.S.; Kantorowski, A.; Homsy, D.; Teylan, M.; Kadri, R.; Richardson, C.R.; Gagnon, D.R.; Garshick, E.; Moy, M.L. Promoting physical activity in COPD: Insights from a randomized trial of a web-based intervention and pedometer use. Respir. Med. 2017, 130, 102–110. [Google Scholar] [CrossRef]
- Moy, M.L.; Martinez, C.H.; Kadri, R.; Roman, P.; Holleman, R.G.; Kim, H.M.; Nguyen, H.Q.; Cohen, M.D.; Goodrich, D.E.; Giardino, N.D.; et al. Long-Term Effects of an Internet-Mediated Pedometer-Based Walking Program for Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. J. Med. Internet Res. 2016, 18, e215. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.A.; Wan, E.S.; Shimada, S.L.; Richardson, C.R.; Moy, M.L. Age and Attitudes Towards an Internet-Mediated, Pedometer-Based Physical Activity Intervention for Chronic Obstructive Pulmonary Disease: Secondary Analysis. JMIR Aging 2020, 3, e19527. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Minakata, Y.; Azuma, Y.; Kaki, T.; Kawabe, K.; Ono, H. Effects of individualized target setting on step count in Japanese patients with chronic obstructive pulmonary disease: A pilot study. Adv. Respir. Med. 2022, 90, 1–8. [Google Scholar] [CrossRef]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Lee, H.; Cardinal, B.J. Evidence to support including lifestyle light-intensity recommendations in physical activity guidelines for older adults. Am. J. Health Promot. 2015, 29, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, l4570. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Diaz, K.M.; Duran, A.T.; Colabianchi, N.; Judd, S.E.; Howard, V.J.; Hooker, S.P. Potential Effects on Mortality of Replacing Sedentary Time with Short Sedentary Bouts or Physical Activity: A National Cohort Study. Am. J. Epidemiol. 2019, 188, 537–544. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sport. Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef]
- Grontved, A.; Hu, F.B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A meta-analysis. JAMA 2011, 305, 2448–2455. [Google Scholar] [CrossRef]
- Young, D.R.; Hivert, M.F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.T.; Lewis, C.E.; Owen, N.; Perry, C.K.; Siddique, J.; et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory from the American Heart Association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Howard, B.; Healy, G.N.; Owen, N. Too much sitting—A health hazard. Diabetes Res. Clin. Pract. 2012, 97, 368–376. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; McNamara, E.; Tainio, M.; de Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sport. Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Owen, N.; Biddle, S.J.; Dunstan, D.W. Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Curr. Diabetes Rep. 2014, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Minakata, Y.; Kaki, T.; Sasaki, S.; Kawabe, K.; Ono, H. Time spent by COPD patients lying down during sedentary behavior. Health Educ. Public Health 2021, 4, 415–420. [Google Scholar]
- Takahashi, K.; Uchida, M.; Kato, G.; Takamori, A.; Kinoshita, T.; Yoshida, M.; Tajiri, R.; Kojima, K.; Inoue, H.; Kobayashi, H.; et al. First-Line Treatment with Tiotropium/Olodaterol Improves Physical Activity in Patients with Treatment-Naïve Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon. Dis. 2020, 15, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.; Weedon, A.; Esliger, D.; Saukko, P.; Morgan, M.; Steiner, M.; Downey, J.; Singh, S.; Sherar, L. Study protocol for Chronic Obstructive Pulmonary Disease-Sitting and ExacerbAtions Trial (COPD-SEAT): A randomised controlled feasibility trial of a home-based self-monitoring sedentary behaviour intervention. BMJ Open 2016, 6, e013014. [Google Scholar] [CrossRef]
- Geidl, W.; Carl, J.; Cassar, S.; Lehbert, N.; Mino, E.; Wittmann, M.; Wagner, R.; Schultz, K.; Pfeifer, K. Physical Activity and Sedentary Behaviour Patterns in 326 Persons with COPD before Starting a Pulmonary Rehabilitation: A Cluster Analysis. J. Clin. Med. 2019, 8, 1346. [Google Scholar] [CrossRef]
- Wootton, S.L.; Hill, K.; Alison, J.A.; Ng, L.W.C.; Jenkins, S.; Eastwood, P.R.; Hillman, D.R.; Jenkins, C.; Spencer, L.; Cecins, N.; et al. Effects of ground-based walking training on daily physical activity in people with COPD: A randomised controlled trial. Respir. Med. 2017, 132, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.; Meijer, K.; Pitta, F.; Azcuna, H.; Goertz, Y.M.J.; Essers, J.M.N.; Wouters, E.F.M.; Spruit, M.A. Changes in physical activity and sedentary behaviour following pulmonary rehabilitation in patients with COPD. Respir. Med. 2017, 126, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.W.; Steiner, M.C.; Morgan, M.D.; Kingsnorth, A.P.; Esliger, D.W.; Singh, S.J.; Sherar, L.B. 24-hour accelerometry in COPD: Exploring physical activity, sedentary behavior, sleep and clinical characteristics. Int. J. Chron. Obs. Pulmon. Dis. 2019, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.W.M.; Alison, J.; Stamatakis, E.; Dennis, S.; McNamara, R.; Spencer, L.; McKeough, Z. Six-week behaviour change intervention to reduce sedentary behaviour in people with chronic obstructive pulmonary disease: A randomised controlled trial. Thorax 2022, 77, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tashiro, H.; Tajiri, R.; Takamori, A.; Uchida, M.; Kato, G.; Kurihara, Y.; Sadamatsu, H.; Kinoshita, T.; Yoshida, M.; et al. Factors Associated with Reduction of Sedentary Time Following Tiotropium/Olodaterol Therapy in Treatment-Naïve Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon. Dis. 2021, 16, 3297–3307. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minakata, Y.; Azuma, Y.; Sasaki, S.; Murakami, Y. Objective Measurement of Physical Activity and Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease: Points to Keep in Mind during Evaluations. J. Clin. Med. 2023, 12, 3254. https://doi.org/10.3390/jcm12093254
Minakata Y, Azuma Y, Sasaki S, Murakami Y. Objective Measurement of Physical Activity and Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease: Points to Keep in Mind during Evaluations. Journal of Clinical Medicine. 2023; 12(9):3254. https://doi.org/10.3390/jcm12093254
Chicago/Turabian StyleMinakata, Yoshiaki, Yuichiro Azuma, Seigo Sasaki, and Yusuke Murakami. 2023. "Objective Measurement of Physical Activity and Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease: Points to Keep in Mind during Evaluations" Journal of Clinical Medicine 12, no. 9: 3254. https://doi.org/10.3390/jcm12093254
APA StyleMinakata, Y., Azuma, Y., Sasaki, S., & Murakami, Y. (2023). Objective Measurement of Physical Activity and Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease: Points to Keep in Mind during Evaluations. Journal of Clinical Medicine, 12(9), 3254. https://doi.org/10.3390/jcm12093254






