Lung Injury in COVID-19 Has Pulmonary Edema as an Important Component and Treatment with Furosemide and Negative Fluid Balance (NEGBAL) Decreases Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Setting
2.3. Data Compilation
2.4. Negative Fluid Balance (NEGBAL) Approach
2.5. Statistical Analysis Plan
2.5.1. Data Source and Description
2.5.2. Baseline Data
2.5.3. Primary Analysis
- An intrinsic analysis via paired differences t-tests regarding the evolution of the variables PaFiO2, creatinine and hematocrit at the moment of admission, days 4 and 8 and discharge and CT SCORE and VSC (superior vena cava) previous to the treatment, at the moment of admission, and at days 4, 8 and 12 for the same group;
- A comparative analysis via unpaired differences t-tests regarding the same variables as above (as well as the variable leukocytes at the moment of admission) but comparing for each day the values of both groups, together with a comparison (again using unpaired difference t-tests) of the variables accumulated hydric balance (ACC HYD BAL) and hospitalization days between groups and a Pearson’s χ2 test for the result;
- A simple regression model to assess the correlation between PaFiO2 and ACC HYD BAL in both groups.
- A multivariate regression model to assess the correlation between PaFiO2 and ACC HYD BAL and vaccination (VAC) for the whole population.
- Paired differences t-tests comparing the values of the variable ACC HYD BAL for survivors and non survivors within each group and for the whole population.
2.5.4. Results
3. Results
3.1. Population Analysis
3.2. Primary Outcome
3.3. Secondary Outcomes
3.4. Complementary Analysis
3.5. Analysis Variable Security
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Gattarello, S.; Steinberg, I.; Busana, M.; Palermo, P.; Lazzari, S.; Romitti, F.; Quintel, M.; Meissner, K.; Marini, J.J.; et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 2021, 30, 210138. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B.; et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients with Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 1937–1942. [Google Scholar] [CrossRef]
- Amaratunga, E.A.; Corwin, D.S.; Moran, L.; Snyder, R. Bradycardia in Patients with COVID-19: A Calm Before the Storm? Cureus 2020, 12, e8599. [Google Scholar] [CrossRef]
- Tao, Z.; Xu, J.; Chen, W.; Yang, Z.; Xu, X.; Liu, L.; Chen, R.; Xie, J.; Liu, M.; Wu, J.; et al. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2021, 93, 1478–1488. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Som, A.; Carey, D.; Reid, N.; Mendoza, D.P.; Flores, E.J.; Li, M.D.; Shepard, J.-A.O.; Little, B.P. Pulmonary Vascular Manifestations of COVID-19 Pneumonia. Radiol. Cardiothorac. Imaging 2020, 2, e200277. [Google Scholar] [CrossRef] [PubMed]
- Ansems, K.; Grundeis, F.; Dahms, K.; Mikolajewska, A.; Thieme, V.; Piechotta, V.; Metzendorf, M.-I.; Stegemann, M.; Benstoem, C.; Fichtner, F. Remdesivir for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 8, CD014962. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Pratx, L.D.B.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef]
- Lan, S.H.; Lai, C.C.; Huang, H.T.; Chang, S.P.; Lu, L.C.; Hsueh, P.R. Tocilizumab for severe COVID-19: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2020, 56, 106103. [Google Scholar] [CrossRef]
- Huang, E.; Jordan, S.C. Tocilizumab for COVID-19—The Ongoing Search for Effective Therapies. N. Engl. J. Med. 2020, 383, 2387–2388. [Google Scholar] [CrossRef]
- Davidson, M.; Menon, S.; Chaimani, A.; Evrenoglou, T.; Ghosn, L.; Graña, C.; Henschke, N.; Cogo, E.; Villanueva, G.; Ferrand, G.; et al. Interleukin-1 blocking agents for treating COVID-19. Cochrane Database Syst. Rev. 2022, 1, CD015308. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target Ther. 2020, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.D.; Grund, B.; Barkauskas, C.E.; Holland, T.L.; Gottlieb, R.L.; Sandkovsky, U.; Brown, S.M.; Knowlton, K.U.; Self, W.H.; Files, D.C.; et al. A Neutralizing Monoclonal Antibody for Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Molla, M.M.A.; Saif-Ur-Rahman, K.M. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf. Health 2021, 3, 87–91. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef]
- Group, R.C. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Rothlin, R.P.; Duarte, M.; Pelorosso, F.G.; Nicolosi, L.; Salgado, M.V.; Vetulli, H.M.; Spitzer, E. Angiotensin Receptor Blockers for COVID-19: Pathophysiological and Pharmacological Considerations About Ongoing and Future Prospective Clinical Trials. Front. Pharmacol. 2021, 12, 603736. [Google Scholar] [CrossRef]
- Rasch, S.; Schmidle, P.; Sancak, S.; Herner, A.; Huberle, C.; Schulz, D.; Mayr, U.; Schneider, J.; Spinner, C.D.; Geisler, F.; et al. Increased extravascular lung water index (EVLWI) reflects rapid non-cardiogenic oedema and mortality in COVID-19 associated ARDS. Sci. Rep. 2021, 11, 11524. [Google Scholar] [CrossRef]
- Shi, R.; Lai, C.; Teboul, J.-L.; Dres, M.; Moretto, F.; De Vita, N.; Pham, T.; Bonny, V.; Mayaux, J.; Vaschetto, R.; et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: The PiCCOVID study. Crit. Care 2021, 25, 186. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Chen, W.; Zhou, H.; Gong, Y.; Zhu, B.; Lv, X.; Guo, H.; Duan, J.; Zhou, J.; Marcon, E.; et al. Pulmonary Edema in COVID-19 Patients: Mechanisms and Treatment Potential. Front. Pharmacol. 2021, 12, 664349. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.F.; Zanardi, P.; Alo, V.; Rodriguez, M.; Magdaleno, F.; De Langhe, V.; Dos Santos, V.; Murialdo, G.; Villoldo, A.; Coria, M.; et al. Pulmonary Edema in COVID-19 Treated with Furosemide and Negative Fluid Balance (NEGBAL): A Different and Promising Approach. J. Clin. Med. 2021, 10, 5599. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhao, Y.-B.; Wang, Q.; Li, J.-Y.; Zhou, Z.-J.; Liao, C.-H.; Ge, X.-Y. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020, 22, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, M.E.; Hemmati, R.; Tashakor, A.; Homaei, A.; Yousefzadeh, M.; Hemati, K.; Hosseinkhani, S. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed. Pharmacother. 2021, 137, 111363. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Choi, M.; Aiello, E.A.; Ennis, I.L.; Villa-Abrille, M.C. The RAAS and SARS-CoV-2: A riddle to solve. Hipertens Riesgo Vasc. 2020, 37, 169–175. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Rysz, S.; Al-Saadi, J.; Sjöström, A.; Farm, M.; Jalde, F.C.; Plattén, M.; Eriksson, H.; Klein, M.; Vargas-Paris, R.; Nyrén, S.; et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 2021, 12, 2417. [Google Scholar] [CrossRef]
- Duarte, M.; Pelorosso, F.; Nicolosi, L.N.; Salgado, M.V.; Vetulli, H.; Aquieri, A.; Azzato, F.; Castro, M.; Coyle, J.; Davolos, I.; et al. Telmisartan for treatment of COVID-19 patients: An open multicenter randomized clinical trial. EClinicalMedicine 2021, 37, 100962. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, R.; Zhang, C.; Ren, W.; Yu, A.; Zhou, X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care 2020, 24, 290. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Ferreira, A.J.; Simões ESilva, A.C. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp. Physiol. 2008, 93, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, J.L.; Berger, B.A.; Pulvino, C.; Lavie, C.J.; Lippi, G.; Benoit, S.W. Coronavirus disease 2019 is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J. Med. Virol. 2021, 93, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Villard, O.; Morquin, D.; Molinari, N.; Raingeard, I.; Nagot, N.; Cristol, J.-P.; Jung, B.; Roubille, C.; Foulongne, V.; Fesler, P.; et al. The Plasmatic Aldosterone and C-Reactive Protein Levels, and the Severity of COVID-19: The Dyhor-19 Study. J. Clin. Med. 2020, 9, 2315. [Google Scholar] [CrossRef]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef]
- Sibbald, W.J.; Short, A.K.; Warshawski, F.J.; Cunningham, D.G.; Cheung, H. Thermal dye measurements of extravascular lung water in critically ill patients. Intravascular Starling forces and extravascular lung water in the adult respiratory distress syndrome. Chest 1985, 87, 585–592. [Google Scholar] [CrossRef]
- Casey, J.D.; Semler, M.W.; Rice, T.W. Fluid Management in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 57–65. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. Clinical practice. Acute pulmonary edema. N. Engl. J. Med. 2005, 353, 2788–2796. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Rossi, S. COVID-19 pneumonia: ARDS or not? Crit. Care 2020, 24, 154. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Thoughts on the alveolar phase of COVID-19. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L115–L120. [Google Scholar] [CrossRef] [PubMed]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Umeh, C.; Giberson, C.; Kumar, S.; Aseri, M.; Barve, P. A Multicenter Retrospective Analysis on the Etiology of Bradycardia in COVID-19 Patients. Cureus 2022, 14, e21294. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus. Apher. Sci. 2020, 59, 102926. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Zinellu, A.; Scano, V.; Mulas, G.; De Riu, G.; Pascale, R.M.; Arru, L.B.; Carru, C.; Pirina, P.; Mangoni, A.A.; et al. Laboratory test alterations in patients with COVID-19 and non COVID-19 interstitial pneumonia: A preliminary report. J. Infect. Dev. Ctries 2020, 14, 685–690. [Google Scholar] [CrossRef]
- Eslami, V.; Abrishami, A.; Zarei, E.; Khalili, N.; Baharvand, Z.; Sanei-Taheri, M. The Association of CT-measured Cardiac Indices with Lung Involvement and Clinical Outcome in Patients with COVID-19. Acad. Radiol. 2021, 28, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Argulian, E.; Sud, K.; Vogel, B.; Bohra, C.; Garg, V.P.; Talebi, S.; Lerakis, S.; Narula, J. Right Ventricular Dilation in Hospitalized Patients with COVID-19 Infection. JACC Cardiovasc. Imaging 2020, 13, 2459–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Zheng, J.-B.; Jin, Y.; Tang, R.; Li, M.; Xiu, C.-H.; Dai, Q.-Q.; Zuo, S.; Wang, H.-Q.; Wang, H.-L.; et al. Acute right ventricular dysfunction in severe COVID-19 pneumonia. Rev. Cardiovasc. Med. 2020, 21, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Schuller, D.; Calandrino, F.S.; Schuster, D.P. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am. Rev. Respir. Dis. 1992, 145, 990–998. [Google Scholar] [CrossRef]
- Martin, G.S.; Mangialardi, R.J.; Wheeler, A.P.; Dupont, W.D.; Morris, J.A.; Bernard, G.R. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit. Care Med. 2002, 30, 2175–2182. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Effectiveness of mRNA COVID-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021, 385, e90. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; Deboisblanc, B.; Connors, A.F.J.; Hite, R.D.; Harabin, A.L. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Noble, M.I. The Frank—Starling curve. Clin. Sci. Mol. Med. 1978, 54, 1–7. [Google Scholar] [CrossRef]
- García-Ayllón, M.; Moreno-Pérez, O.; García-Arriaza, J.; Ramos-Rincón, J.; Cortés-Gómez, M.; Brinkmalm, G.; Andrés, M.; León-Ramírez, J.; Boix, V.; Gil, J.; et al. Plasma ACE2 species are differentially altered in COVID-19 patients. FASEB J. 2021, 35, e21745. [Google Scholar] [CrossRef] [PubMed]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Alsous, F.; Khamiees, M.; DeGirolamo, A.; Amoateng-Adjepong, Y.; Manthous, C.A. Negative fluid balance predicts survival in patients with septic shock: A retrospective pilot study. Chest 2000, 117, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, W.; Hodgins, S.; McGee, W.T. Tissue Edema, Fluid Balance, and Patient Outcomes in Severe Sepsis: An Organ Systems Review. J. Intensive Care Med. 2018, 33, 502–509. [Google Scholar] [CrossRef]
| Variable | Total Population | CONTROL Group n = 58 | NEGBAL Group n = 58 | p Value |
|---|---|---|---|---|
| Demographics—Pathological antecedents Variables | ||||
| Age | 59.6 ± 13.6 | 59.45 ± 12.04 | 59.86 ± 15.14 (100) | 0.87 (−5.44, 4.61) |
| Obesity | 59 (50.8%) | 30 (51.7) | 29 | 1 |
| BMI | 30.1 ± 5.16 | 30.25 ± 5.46 | 29.93 ± 4.89 | 0.74 (−1.58, 2.22) |
| AHT | 47 (40.5%) | 24 | 23 | 1 |
| DBT | 30 (25.8%) | 17 | 13 | 0.52 |
| COPD | 20 (17.3%) | 10 | 10 | 0.52 |
| COVID-19 Day | 8.41 ± 3 | 8.93 ± 2.7 | 8 ± 3.2 | 0.75 |
| VAC-1 | 52 (44.8%) | 18 | 34 | 0.01 |
| VAC-2 | 27 (22.4%) | 5 | 22 | 0.01 |
| Clinical variables | ||||
| Heart Rate | 83.5 ± 15 | 84.55 ± 14.9 | 82.5 ± 15.19 | 0.46 (−3.48, 7.58) |
| Systolic BP | 127 ± 19.2 | 127.2 ± 20.77 | 128.3 ± 17.75 | 0.75 (−3.48, 7.58) |
| Diastolic BP | 77.4 ± 12.4 | 76.79 ± 12.47 | 78.14 ± 12.4 | 0.56 (−5.92, 3.23) |
| Temperature | 36.5 ± 0.86 | 36.63 ± 0.88 | 36.43 ± 0.84 | 0.20 (−0.11, 0.52) |
| Resp. Rate | 24.9 ± 4.58 | 25.34 ± 4.61 | 24.47 ± 4.56 | 0.30 (−0.80, 2.56) |
| APACHE II | 8.01 ± 3.26 | 7.6 ± 3.04 | 8.43 ± 3.44 | 0.17 (−2.02, 0.36) |
| Biochemical variables | ||||
| Po2 | 73.3.± 18.8 | 73.99 ± 21.55 | 72.8 ± 15.97 | 0.73 (−5.79, 8.17) |
| Pafio2 | 164 ± 70.2 | 164.81 ± 73.64 | 163.6 ± 67.26 | 0.92 (−24.6, 27.1) |
| Pco2 | 33.28 ± 5.15 | 33.75 ± 5.40 | 32.81 ± 4.90 | 0.32 (−0.95, 2.83) |
| HCO3 | 21.8 ± 3.06 | 21.64 ± 3.01 | 23.14 ± 3.12 | 0.38 (−1.62, 0.63) |
| Creatinine | 9.01 ± 2.22 | 9.41 ± 2.38 | 8.75 ± 2.01 | 0.11 (−0.15, 1.47) |
| Hematocrit | 39.7 ± 4.9 | 40.38 ± 5.22 | 39.20 ± 4.54 | 0.19 (−0.62, 2.97) |
| Lymphocytes | 959 ± 665 | 963 ± 773 | 955 ± 540 | 0.42 (−345, 145) |
| Troponina | 10.4 ± 8.35 | 10.84 ± 8.53 | 9.99 ± 8.25 | 0.63 (−2.73, 4.43) |
| proBNP | 224 ± 235 | 277 ± 246.19 | 188.80 ± 222.86 | 0.11 (−22.4,198) |
| GOT | 49.9 ± 37.7 | 53.84 ± 38.85 | 45.15 ± 36.39 | 0.26 (−6.56, 23.9) |
| GPT | 57.2 ± 68 | 60.69 ± 64.60 | 53.38 ± 71.96 | 0.58 (−20.18, 35.3) |
| Chest Tomography Score and Diameter Superior Vena Cava variables | ||||
| CT Score | 15.7 ± 4.63 | 15.8 ± 4.14 | 15.6 ± 5.08 | 0.80 (−1.51,1.94) |
| Ø SVC | 17.7 ± 2.97 | 18.1 ± 2.55 | 17.3 ± 3.29 | 0.14 (−0.28,1.93) |
| Variable | CONTROL Group n = 58 | NEGBAL Group n = 58 | p Value |
|---|---|---|---|
| Biochemical variables | |||
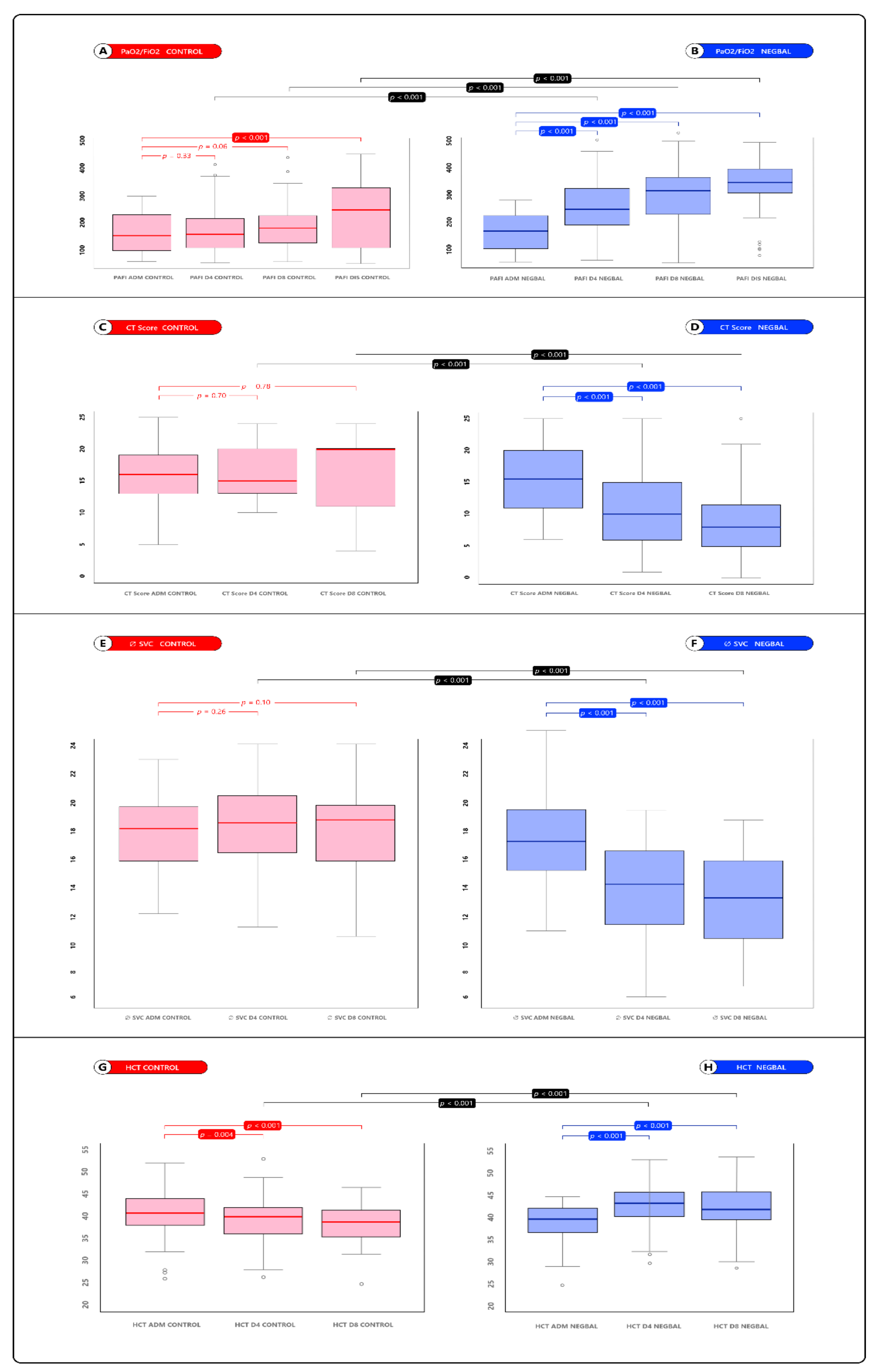
| PaPafio2 day 4 | 175 ± 90.5 | 249 ± 100 | <0.001 (−109, −37.8) |
| PaPafio2 day 8 | 183 ± 87 | 290 ± 101 | <0.001 (−146, −67.2) |
| PaPafio2 day dis. | 235 ± 116 | 344 ± 97.6 | <0.001 (−141, −61) |
| HCT day 4 | 39.1 ± 5.21 | 43.1 ± 5.21 | <0.001 (−5.90, −2) |
| HCT day 8 | 38.5 ± 4.67 | 42.5 ± 5.23 | <0.001 (−6.38, −2.05) |
| LYMP day 4 | 697 ± 402 | 1056 ± 735 | 0.002 (−586, −130) |
| CREAT day dis. | 13.1 ± 9.8 | 11.6 ± 8.2 | 0.37 (−1.84, 4.85) |
| Chest Tomography Score and Diameter Superior Vena Cava | |||
| CT Score day 4 | 16.4 ± 4.31 | 10.6 ± 5.96 | <0.001 (3.31, 6.8) |
| CT Score day 8 | 16.3 ± 5.79 | 8.77 ± 5.57 | <0.001 (4.18, 10.9) |
| Ø SVC day 4 | 18.9 ± 3.1 | 13.8 ± 4.09 | <0.001 (3.29, 6.8) |
| Ø SVC day 8 | 17.8 ± 3.42 | 12.7 ± 3.43 | <0.001 (3.14, 7.21) |
| Accumulated Fluid Balance | |||
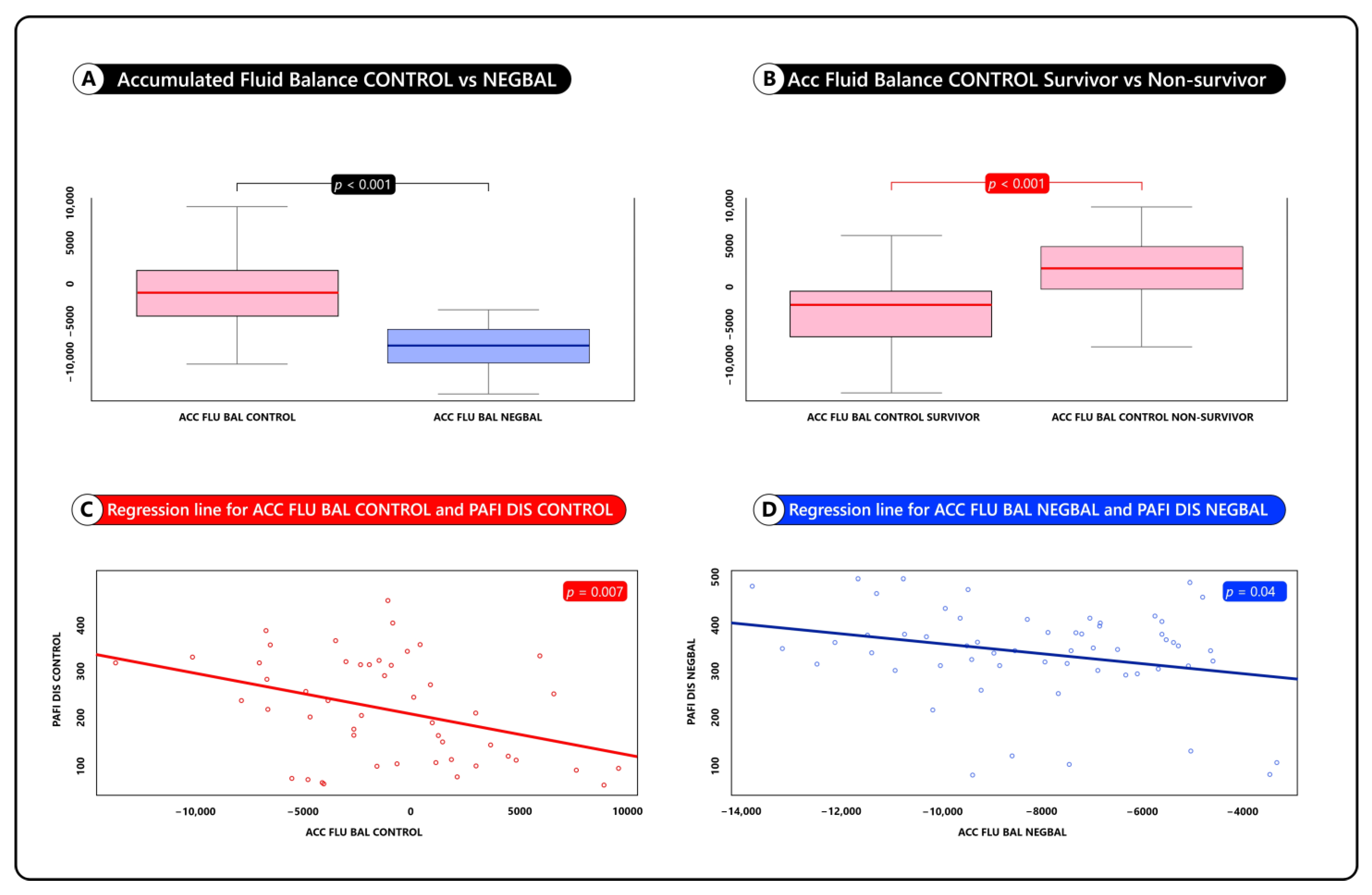
| ACC FLU BAL | −685 ± 1000 | −7898 ± 1000 | <0.001 (5419, 8453) |
| Hospital stay, ICU stay and IMV days | |||
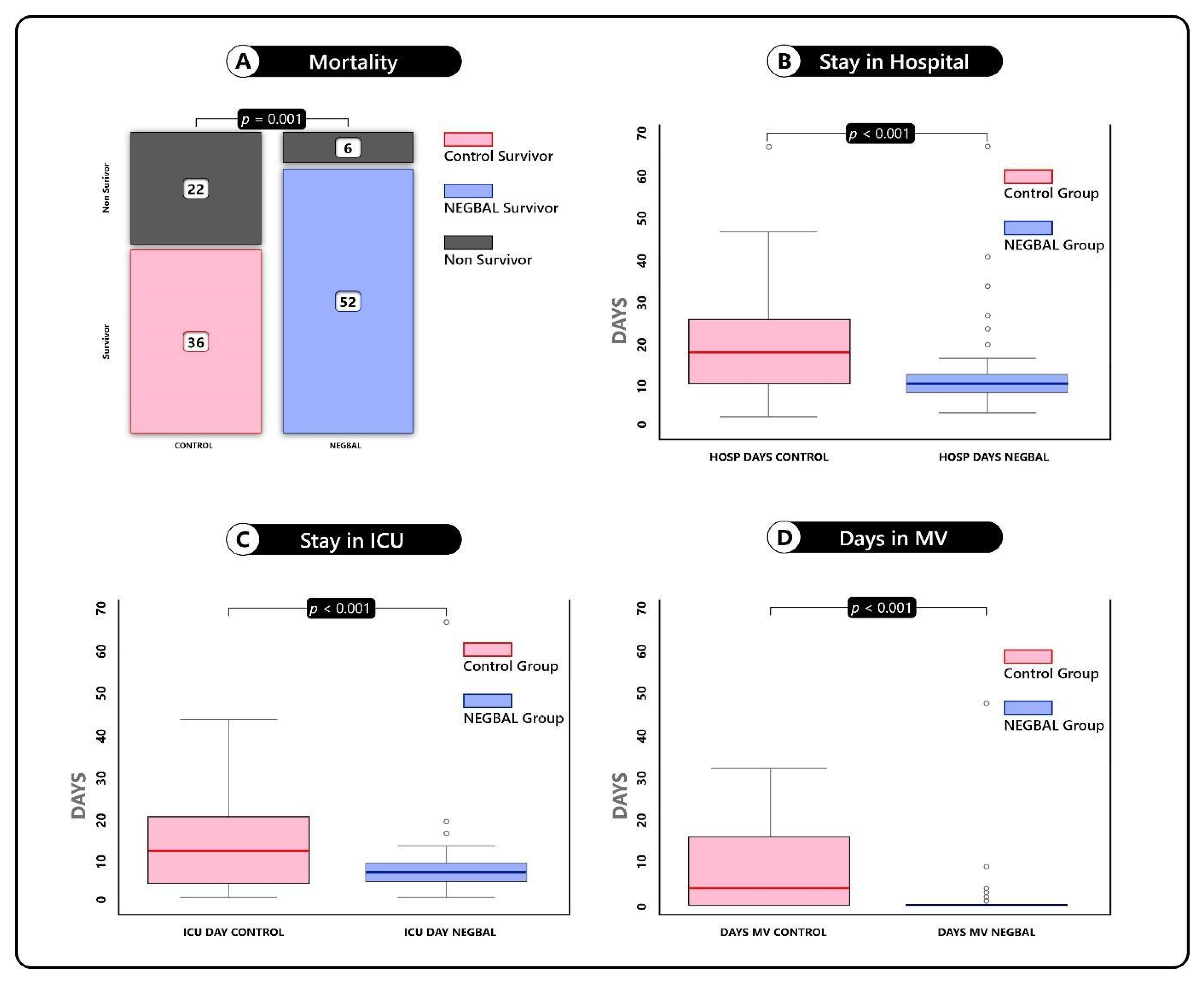
| Hospital Stay | 20.5 ± 13.2 | 13 ± 13.2 | <0.001 (3.15, 11.8) |
| ICU Stay | 15.7 ± 13 | 9 ± 8.91 | <0.001 (2.77, 10.3) |
| Days of IMV use | 4 ± 10.2 | 1 ± 6.4 | <0.001 (4.20, 10.5) |
| Patients IMV use | 31 | 9 | <0.001 |
| Mortality | |||
| Mortality | 22 | 6 | 0.001 |
| Accumulated Fluid Balance CONTROL group | |||
| Control Group | SURVIVORS Control group | NON SURVIVORS Control group | p value |
| ACC FLU BAL | −3685 ± 1000 | +5898 ± 1000 | <0.001 (−7648,−2688) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.L.F.; Zanardi, P.; Alo, V.; Dos Santos, V.; Bovone, L.; Rodriguez, M.; Magdaleno, F.; De Langhe, V.; Villoldo, A.; Martinez Souvielle, R.; et al. Lung Injury in COVID-19 Has Pulmonary Edema as an Important Component and Treatment with Furosemide and Negative Fluid Balance (NEGBAL) Decreases Mortality. J. Clin. Med. 2023, 12, 1542. https://doi.org/10.3390/jcm12041542
Santos JLF, Zanardi P, Alo V, Dos Santos V, Bovone L, Rodriguez M, Magdaleno F, De Langhe V, Villoldo A, Martinez Souvielle R, et al. Lung Injury in COVID-19 Has Pulmonary Edema as an Important Component and Treatment with Furosemide and Negative Fluid Balance (NEGBAL) Decreases Mortality. Journal of Clinical Medicine. 2023; 12(4):1542. https://doi.org/10.3390/jcm12041542
Chicago/Turabian StyleSantos, Jose L. Francisco, Patricio Zanardi, Veronica Alo, Vanina Dos Santos, Leonardo Bovone, Marcelo Rodriguez, Federico Magdaleno, Virginia De Langhe, Andrea Villoldo, Romina Martinez Souvielle, and et al. 2023. "Lung Injury in COVID-19 Has Pulmonary Edema as an Important Component and Treatment with Furosemide and Negative Fluid Balance (NEGBAL) Decreases Mortality" Journal of Clinical Medicine 12, no. 4: 1542. https://doi.org/10.3390/jcm12041542
APA StyleSantos, J. L. F., Zanardi, P., Alo, V., Dos Santos, V., Bovone, L., Rodriguez, M., Magdaleno, F., De Langhe, V., Villoldo, A., Martinez Souvielle, R., Alconcher, J., Quiros, D., Milicchio, C., & Garcia Saiz, E. (2023). Lung Injury in COVID-19 Has Pulmonary Edema as an Important Component and Treatment with Furosemide and Negative Fluid Balance (NEGBAL) Decreases Mortality. Journal of Clinical Medicine, 12(4), 1542. https://doi.org/10.3390/jcm12041542






