Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CT Scanning Protocol
Qualitative Analysis
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics on the Basis of Kidney Attenuation at Chest CT
3.2. Clinical Characteristics on the Basis of Perirenal Fat Stranding at Chest CT
3.3. Determinants of AKI
3.4. Kidney Imaging According with Kidney Function as Predictor of Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Situation Report-51 SITUATION IN NUMBERS Total and New Cases in Last 24 Hours; World Health Organization: Geneva, Switzerland, 2020.
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA—J. Am. Med. Assoc. 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Pan, X.W.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.-H.; Cui, X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Esposito, P.; Taramasso, L.; Magnasco, L.; Saio, M.; Briano, F.; Russo, C.; Dettori, S.; Vena, A.; Di Biagio, A.; et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J. Nephrol. 2021, 34, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rojas, M.A.; Vega-Vega, O.; Bobadilla, N.A. Is the kidney a target of SARS-CoV-2? Am. J. Physiol. Physiol. 2020, 318, F1454–F1462. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020, 16, 308–310. [Google Scholar] [CrossRef]
- Jafari-Oori, M.; Fiorentino, M.; Castellano, G.; Ebadi, A.; Rahimi-Bashar, F.; Guest, P.C.; Vahedian-Azimi, A.; Sahebkar, A. Acute Kidney Injury and Covid-19: A Scoping Review and Meta-Analysis. Adv. Exp. Med. Biol. 2021, 1321, 309–324. [Google Scholar] [CrossRef]
- Behzad, S.; Aghaghazvini, L.; Radmard, A.R.; Gholamrezanezhad, A. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin. Imaging 2020, 66, 35–41. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Urraro, F.; Grassi, R.; Giacobbe, G.; Patelli, G.; Cappabianca, S.; Reginelli, A. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol. Medica 2020, 125, 500–504. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Investig. Radiol. 2020, 55, 327–331. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=covid-19+and+Chest+CT (accessed on 4 January 2022).
- Khatami, F.; Saatchi, M.; Zadeh, S.S.T.; Aghamir, Z.S.; Shabestari, A.N.; Reis, L.O.; Aghamir, S.M.K. A meta-analysis of accuracy and sensitivity of chest CT and RT-PCR in COVID-19 diagnosis. Sci. Rep. 2020, 10, 22402. [Google Scholar] [CrossRef]
- Idilman, I.S.; Dizman, G.T.; Duzgun, S.A.; Irmak, I.; Karcaaltincaba, M.; Inkaya, A.C.; Demirkazik, F.; Durhan, G.; Akpinar, M.G.; Ariyurek, O.M.; et al. Lung and kidney perfusion deficits diagnosed by dual-energy computed tomography in patients with COVID-19-related systemic microangiopathy. Eur. Radiol. 2020, 31, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C.; Novakovic, V.A.; Xie, R.; Shi, J. Circulating Microparticles in the Pathogenesis and Early Anticoagulation of Thrombosis in COVID-19 With Kidney Injury. Front. Cell Dev. Biol. 2022, 9, 784505. [Google Scholar] [CrossRef]
- Hectors, S.J.; Riyahi, S.; Dev, H.; Krishnan, K.; Margolis, D.J.A.; Prince, M.R. Multivariate analysis of CT imaging, laboratory, and demographical features for prediction of acute kidney injury in COVID-19 patients: A Bi-centric analysis. Abdom. Radiol. 2020, 46, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Kistner, A.; Tamm, C.; Svensson, A.M.; Beckman, M.O.; Strand, F.; Sköld, M.; Nyrén, S. Negative effects of iodine-based contrast agent on renal function in patients with moderate reduced renal function hospitalized for COVID-19. BMC Nephrol. 2021, 22, 297. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Lyu, S.; Liang, W.; Yang, R.; Zhang, R.; Xiao, W.; Liu, J.; Yan, S.; Zheng, L.; et al. COVID-19 associated kidney impairment in adult: Qualitative and quantitative analyses with non-enhanced CT on admission. Eur. J. Radiol. 2020, 131, 109240. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- KDIGO Clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–141. [CrossRef]
- Xu, X.; Yu, C.; Qu, J.; Zhang, L.; Jiang, S.; Huang, D.; Chen, B.; Zhang, Z.; Guan, W.; Ling, Z.; et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients with COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J. Am. Soc. Nephrol. 2020, 31, 1157–1165. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Naicker, S.; Yang, C.-W.; Hwang, S.-J.; Liu, B.-C.; Chen, J.-H.; Jha, V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020, 97, 824–828. [Google Scholar] [CrossRef]
- Santoriello, D.; Khairallah, P.; Bomback, A.S.; Xu, K.; Kudose, S.; Batal, I.; Barasch, J.; Radhakrishnan, J.; D’Agati, V.; Markowitz, G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Kudose, S.; Batal, I.; Santoriello, D.; Xu, K.; Barasch, J.; Peleg, Y.; Canetta, P.; Ratner, L.E.; Marasa, M.; Gharavi, A.G.; et al. Kidney Biopsy Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Khatibi, S.M.H.; Soofiyani, S.R.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Vahed, S.Z. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2020, 31, e2176. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-W.; Lee, J.N.; Park, K.M.; Byeon, K.H.; Cheon, H.; Ha, Y.-S.; Choi, S.H.; Kim, B.S.; Kim, T.-H.; Yoo, E.S.; et al. Prognostic impact of perirenal fat stranding on oncologic outcomes in ureteral urothelial carcinoma. Investig. Clin. Urol. 2021, 62, 23–31. [Google Scholar] [CrossRef]
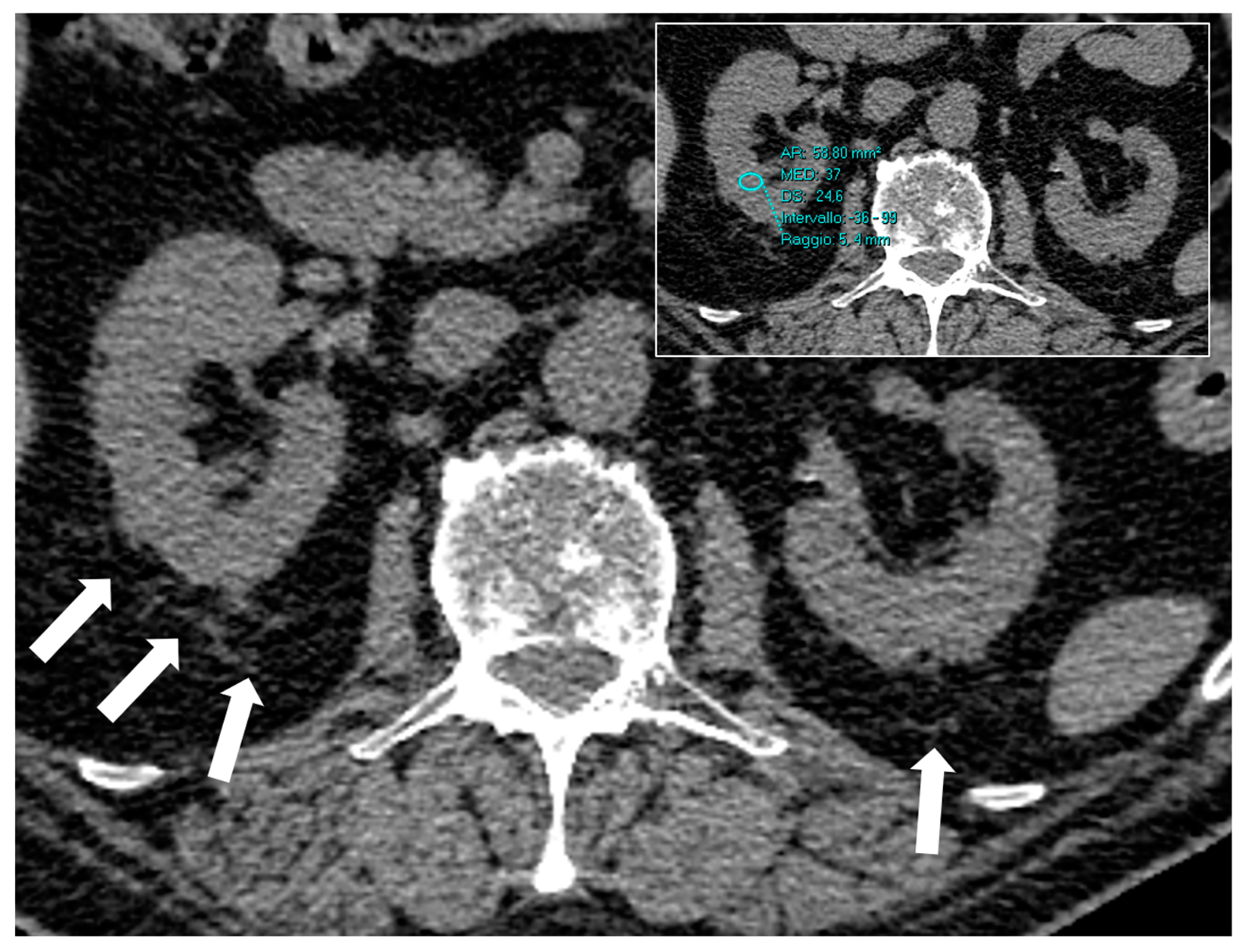
| All | Q1 (<24) | Q2-Q3-Q4 (≥24) | p Value | |
|---|---|---|---|---|
| Variables | ||||
| N | 86 | 23 | 63 | |
| RPA, HU | 31.9 ± 23.1 | 16.4 ± 6.5 | 37.6 ± 24.3 | <0.001 |
| Demographic Characteristics | ||||
| Age, years | 68 ± 13 | 65 ± 12 | 68 ± 13 | 0.295 |
| Male gender, % | 74.4 | 69.6 | 76.2 | 0.533 |
| Hypertension, % | 42.3 | 47.8 | 40.3 | 0.534 |
| Diabetes, % | 12.9 | 8.7 | 14.5 | 0.478 |
| Chalson score | 1.4 ± 2.1 | 1.3 ± 2.0 | 1.4 ± 2.1 | 0.871 |
| Systolic blood pressure, mmHg | 133 ± 23 | 131 ± 26 | 134 ± 22 | 0.673 |
| Diastolic blood pressure, mmHg | 74 ± 14 | 74 ± 15 | 75 ± 14 | 0.846 |
| Heart rate, beats per min | 88 ± 17 | 90 ± 19 | 87 ± 18 | 0.546 |
| Respiratory rate, n/min | 21 ± 7 | 21 ± 7 | 21 ± 7 | 0.772 |
| Glasgow coma scale | 14.1 ± 2.7 | 13.6 ± 3.7 | 14.3 ± 2.3 | 0.271 |
| O2 saturation, % | 91 ± 9 | 92 ± 8 | 90 ± 10 | 0.570 |
| PaO2/FiO2 ratio | 250 ± 94 | 222 ± 87 | 258 ± 98 | 0.277 |
| Temperature, °C | 37.6 ± 1.1 | 37.4 ± 0.8 | 37.6 ± 1.1 | 0.456 |
| Laboratory Characteristics | ||||
| White blood cell count, x109/L | 8865 ± 6447 | 9153 ± 5700 | 8760 ± 6739 | 0.804 |
| Hemoglobin, g/dL | 13.1 ± 2.1 | 13.2 ±2.3 | 13.1 ± 2.1 | 0.889 |
| Platelets count, ×109/L | 215 ± 101 | 224 ± 121 | 212 ± 93 | 0.606 |
| Creatinine, mg/dL | 1.0 (4.0) | 0.9 (0.1) | 1.0 (0.5) | 0.111 |
| eGFR, ml/min/1.73 m2 | 71.2 (37.7) | 73.3 (31.8) | 68.7 (38.3) | 0.100 |
| Urea, mg/dL | 58 ± 42 | 47 ± 25 | 62 ± 46 | 0.156 |
| Alanine aminotransferase, U/L | 41 ± 30 | 43 ± 31 | 40 ± 29 | 0.733 |
| D-dimer, mg/L | 1067 (959) | 1312 (574) | 1939 (448) | 0.159 |
| Creatine kinase, U/L | 98 (119) | 130 (118) | 89 (84) | 0.723 |
| Lactate dehydrogenase, U/L | 285 (201) | 317 (261) | 280 (207) | 0.545 |
| C-reactive protein, mg/L | 89 (112) | 101 (179) | 83 (109) | 0.149 |
| Interleukin-6, pg/mL | 54 (90) | 57 (89) | 49 (76) | 0.084 |
| Procalcitonin, ng/mL | 1.08 ± 5.39 | 0.66 ± 1.02 | 1.25 ± 6.39 | 0.665 |
| Potassium, mEq/L | 3.8 ± 0.6 | 3.6 ± 0.5 | 3.9 ± 0.6 | 0.080 |
| Kidney Status | ||||
| Chronic Kidney Disease, % | 20.9 | 4.3 | 27.0 | 0.022 |
| Acute Kidney Injury, % | 38.4 | 52.2 | 33.3 | 0.014 |
| Acute Kidney Injury stages 1-2-3, % | 13-9-16 | 9-9-35 | 14-9-9 | 0.046 |
| Teraphy | ||||
| Hydroxychloroquine, % | 94.4 | 95.2 | 94.1 | 0.850 |
| Tocilizumab, % | 48.3 | 67.7 | 41.9 | 0.111 |
| Corticosteroids, % | 98.0 | 100.0 | 97.5 | 0.596 |
| Radiologic Findings in Chest CT | ||||
| Perirenal stranding, % | 69.4 | 54.5 | 74.6 | 0.079 |
| Bilateral pulmonary infiltration, % | 43.0 | 47.8 | 41.3 | 0.587 |
| Ground glass opacities, % | 76.4 | 91.3 | 71.4 | 0.053 |
| Honeycombing opacities, % | 9.4 | 9.1 | 9.5 | 0.952 |
| Outcomes | ||||
| Hospitalization, days | 22 ±15 | 26 ±12 | 21 ±16 | 0.219 |
| Intra-hospital mortality | 37.2 | 43.5 | 34.9 | 0.467 |
| Follow up, days | 242 ±174 | 213 ±179 | 255 ±172 | 0.350 |
| 9 months-mortality, % | 41.9 | 52.2 | 38.1 | 0.241 |
| All | NO PFS | PFS | p Value | |
|---|---|---|---|---|
| Variables | ||||
| N | 86 | 27 | 59 | |
| Demographic Characteristic | ||||
| Age, years | 68 ± 13 | 61 ± 15 | 70 ± 11 | 0.003 |
| Male gender, % | 74.4 | 57.7 | 83.0 | 0.012 |
| Hypertension, % | 42.9 | 23.1 | 51.7 | 0.014 |
| Diabetes, % | 12.8 | 3.8 | 17.2 | 0.092 |
| Charlson score | 1.4 ± 2.1 | 1.1 ± 2.2 | 1.5 ± 2.1 | 0.469 |
| Systolic blood pressure, mmHg | 133 ± 23 | 129 ± 19 | 134 ±24 | 0.426 |
| O2 saturation, % | 91 ± 9 | 90 ± 8 | 91 ± 10 | 0.698 |
| PaO2/FiO2 ratio | 250 ± 94 | 249 ± 108 | 251 ± 96 | 0.948 |
| Temperature, °C | 37.6 ± 1.1 | 37.6 ± 1.1 | 37.5 ± 1.0 | 0.636 |
| Laboratory Characteristics | ||||
| Creatinine, mg/dL | 1.0 (4.0) | 0.9 (0.4) | 1.1 (0.5) | 0.027 |
| eGFR, ml/min/1.73 m2 | 71 (38) | 82 (30) | 65 (38) | <0.001 |
| Urea, mg/dL | 58 ± 42 | 43.2 ± 23.2 | 64.2 ± 45.6 | 0.039 |
| C-reactive protein, mg/L | 89 (112) | 120 (181) | 83 (101) | 0.352 |
| Interleukin-6, pg/mL | 54 (90) | 56 (84) | 52 (83) | 0.167 |
| Procalcitonin, ng/mL | 1.08 ± 5.39 | 0.51 ± 0.74 | 1.35 ± 6.52 | 0.192 |
| Kidney Status | ||||
| Chronic Kidney Disease, % | 20.9 | 11.5 | 25.4 | 0.149 |
| Acute Kidney Injury, % | 38.4 | 34.6 | 40.7 | 0.597 |
| Acute Kidney Injury stages 1-2-3, % | 13-9-16 | 8-11-15 | 15-8-17 | 0.774 |
| Radiologic Findings in Chest CT | ||||
| Renal parenchymal attenuation, HU | 31.9 ± 23.1 | 29.3 ± 17.6 | 33.3 ± 25.3 | 0.459 |
| Bilateral pulmonary infiltration, % | 43.0 | 34.6 | 47.5 | 0.271 |
| Ground glass opacities, % | 76.4 | 73.1 | 78.0 | 0.624 |
| Honeycombing opacities, % | 9.4 | 8.0 | 10.1 | 0.757 |
| Outcomes | ||||
| Hospitalization, days | 22 ± 15 | 22 ± 17 | 22 ± 15 | 0.986 |
| Intra-hospital mortality | 37.2 | 15.4 | 45.8 | 0.007 |
| Follow up, days | 242 ± 174 | 325 ± 131 | 209 ± 179 | 0.004 |
| 9 months-mortality, % | 41.9 | 19.2 | 50.8 | 0.006 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p | Odds Ratio | 95% CI | p | |
| Age, years * | 1.00 | 0.97–1.03 | 0.960 | 0.96 | 0.91–1.01 | 0.146 |
| Male gender * | 0.87 | 0.32–2.33 | 0.777 | 0.26 | 0.05–1.23 | 0.090 |
| Charlson index * | 1.11 | 0.90–1.38 | 0.325 | 1.16 | 0.89–1.53 | 0.266 |
| Hypertension | 2.79 | 1.13–6.88 | 0.025 | 1.25 | 0.30–5.30 | 0.759 |
| Diabetes | 0.56 | 0.14–2.29 | 0.422 | |||
| Heart failure | 1.19 | 0.25–5.70 | 0.828 | |||
| Creatinine, mg/dl | 4.84 | 1.23–29.13 | 0.026 | 50.7 | 4.62–556.01 | 0.001 |
| RPA <24 HU | 2.22 | 0.86–5.74 | 0.100 | 4.56 | 1.27–16.44 | 0.020 |
| Perirenal stranding | 1.29 | 0.50–3.38 | 0.593 | |||
| O2 Saturation, % | 0.98 | 0.93–1.03 | 0.479 | |||
| PaO2/FiO2 ratio | 0.99 | 0.99–1.00 | 0.071 | |||
| Chest radiographic abnormality * | 0.66 | 0.21–2.03 | 0.469 | |||
| Cancer history * | 0.81 | 0.40–1.66 | 0.565 | |||
| Lactate dehydrogenase, U/L * | 1.00 | 0.99–1.00 | 0.114 | |||
| Direct bilirubin, * | 2.40 | 0.61–9.40 | 0.207 | |||
| L/N ratio * | 0.69 | 0.21–2.26 | 0.545 | |||
| Dyspnea * | 1.02 | 0.42–2.48 | 0.966 | |||
| Risk Factors | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age, years | 1.04 | 1.01–1.06 | 0.003 | 1.00 | 0.96–1.04 | 0.944 |
| Male gender | 2.74 | 1.16–6.46 | 0.021 | 1.78 | 0.68–4.59 | 0.266 |
| Charlson index | 1.11 | 0.95–1.30 | 0.168 | |||
| Hypertension | 1.71 | 0.85–3.42 | 0.130 | |||
| Diabetes | 1.52 | 0.58–3.95 | 0.390 | |||
| Chronic kidney disease | 1.19 | 0.52–2.77 | 0.677 | 1.28 | 0.42–3.94 | 0.661 |
| Heart failure | 2.88 | 1.10–7.57 | 0.031 | 2.80 | 0.88–8.88 | 0.080 |
| Creatinine, mg/dl | 1.46 | 1.18–1.80 | <0.0001 | 1.10 | 0.73–1.66 | 0.647 |
| Acute kidney injury | 2.00 | 1.03–3.89 | 0.042 | 2.71 | 1.21–6.08 | 0.015 |
| RPA ≤24 (Q1) | 1.52 | 0.74–3.12 | 0.249 | 1.44 | 0.63–3.29 | 0.381 |
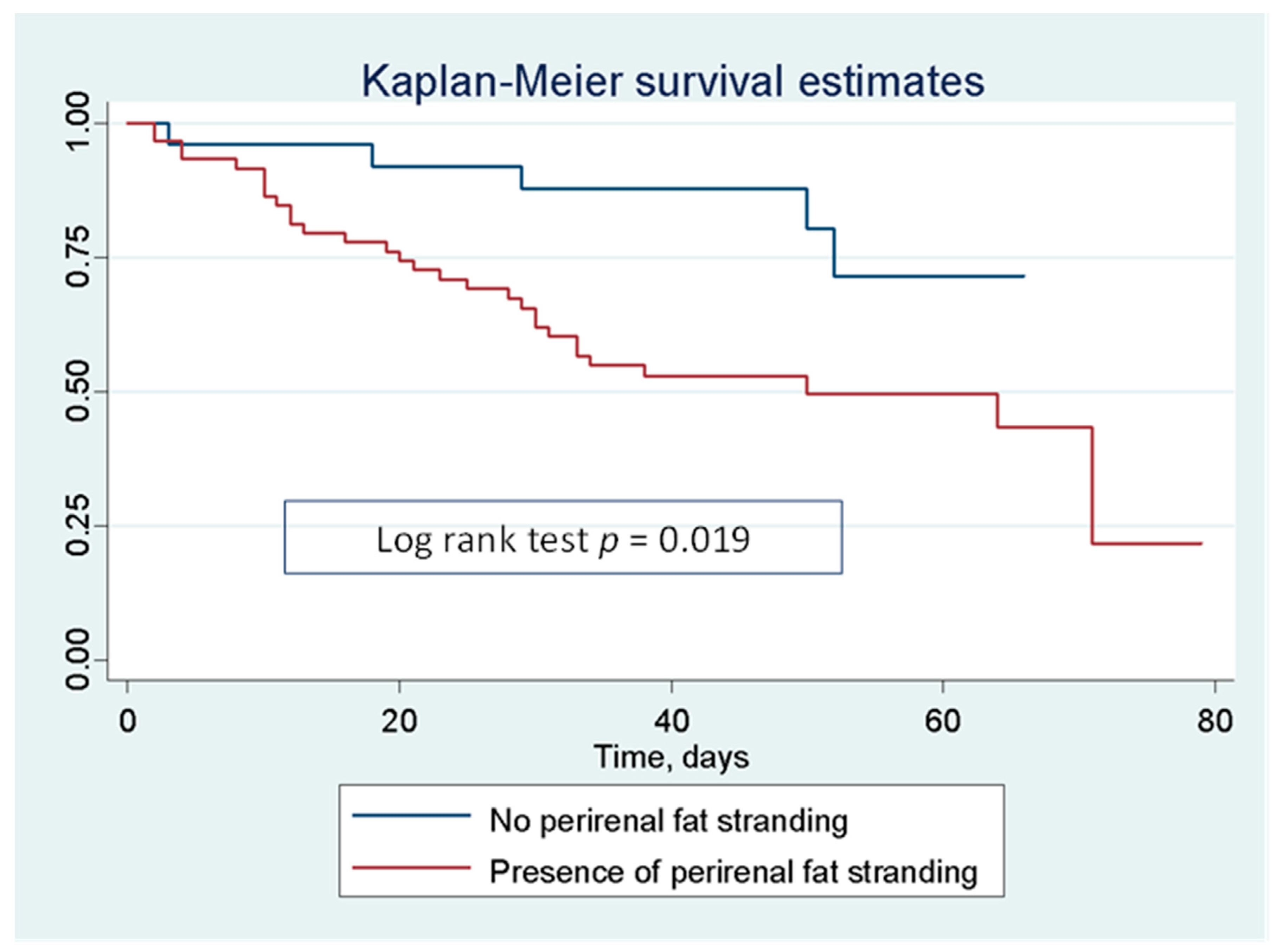
| Perirenal fat stranding | 2.95 | 1.14–7.63 | 0.026 | 4.14 | 1.16–14.79 | 0.029 |
| O2 Saturation, % | 0.97 | 0.94–1.01 | 0.210 | |||
| PaO2/FiO2 ratio | 1.00 | 0.99–1.01 | 0.178 | |||
| Chest radiographic abnormality * | 1.23 | 0.48–3.18 | 0.668 | |||
| Cancer history * | 1.53 | 1.01–2.33 | 0.043 | 1.40 | 0.86–2.27 | 0.176 |
| Lactate dehydrogenase, U/L * | 1.00 | 1.00–1.00 | 0.094 | |||
| Direct bilirubin, * | 1.79 | 0.70–4.58 | 0.225 | |||
| L/N ratio * | 0.84 | 0.44–1.62 | 0.611 | |||
| Dyspnea * | 0.94 | 0.48–1.85 | 0.867 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Tagliafico, A.S.; Derchi, L.; Bignotti, B.; Tosto, S.; Martinoli, C.; Signori, A.; Brigati, F.; Viazzi, F. Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19. J. Clin. Med. 2023, 12, 929. https://doi.org/10.3390/jcm12030929
Russo E, Tagliafico AS, Derchi L, Bignotti B, Tosto S, Martinoli C, Signori A, Brigati F, Viazzi F. Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19. Journal of Clinical Medicine. 2023; 12(3):929. https://doi.org/10.3390/jcm12030929
Chicago/Turabian StyleRusso, Elisa, Alberto Stefano Tagliafico, Lorenzo Derchi, Bianca Bignotti, Simona Tosto, Carlo Martinoli, Alessio Signori, Francesca Brigati, and Francesca Viazzi. 2023. "Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19" Journal of Clinical Medicine 12, no. 3: 929. https://doi.org/10.3390/jcm12030929
APA StyleRusso, E., Tagliafico, A. S., Derchi, L., Bignotti, B., Tosto, S., Martinoli, C., Signori, A., Brigati, F., & Viazzi, F. (2023). Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19. Journal of Clinical Medicine, 12(3), 929. https://doi.org/10.3390/jcm12030929






