Enoxaparin Posology According to Prothrombotic Status and Bleeding Risk in Hospitalized Patients with SARS-CoV-2 Pneumonia
Abstract
1. Introduction
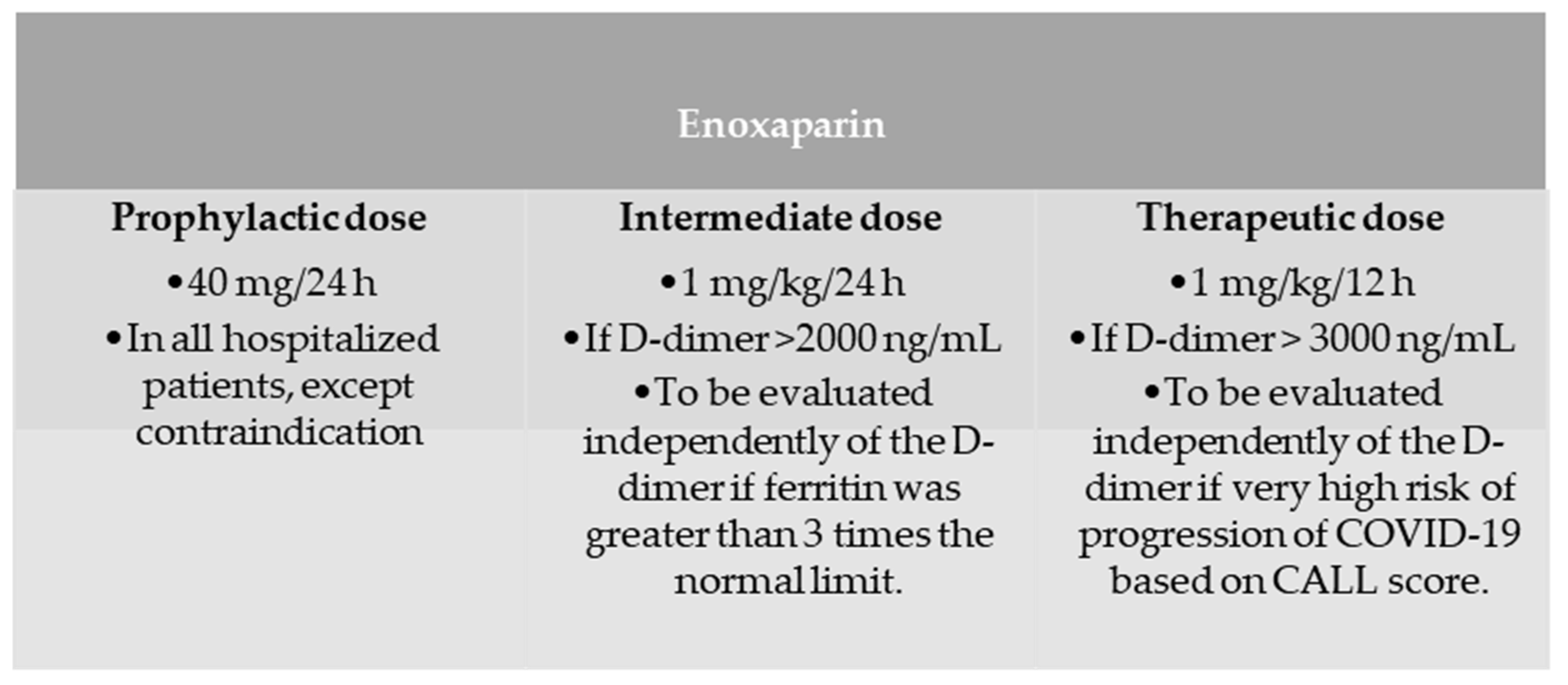
2. Materials and Methods
2.1. Study Design
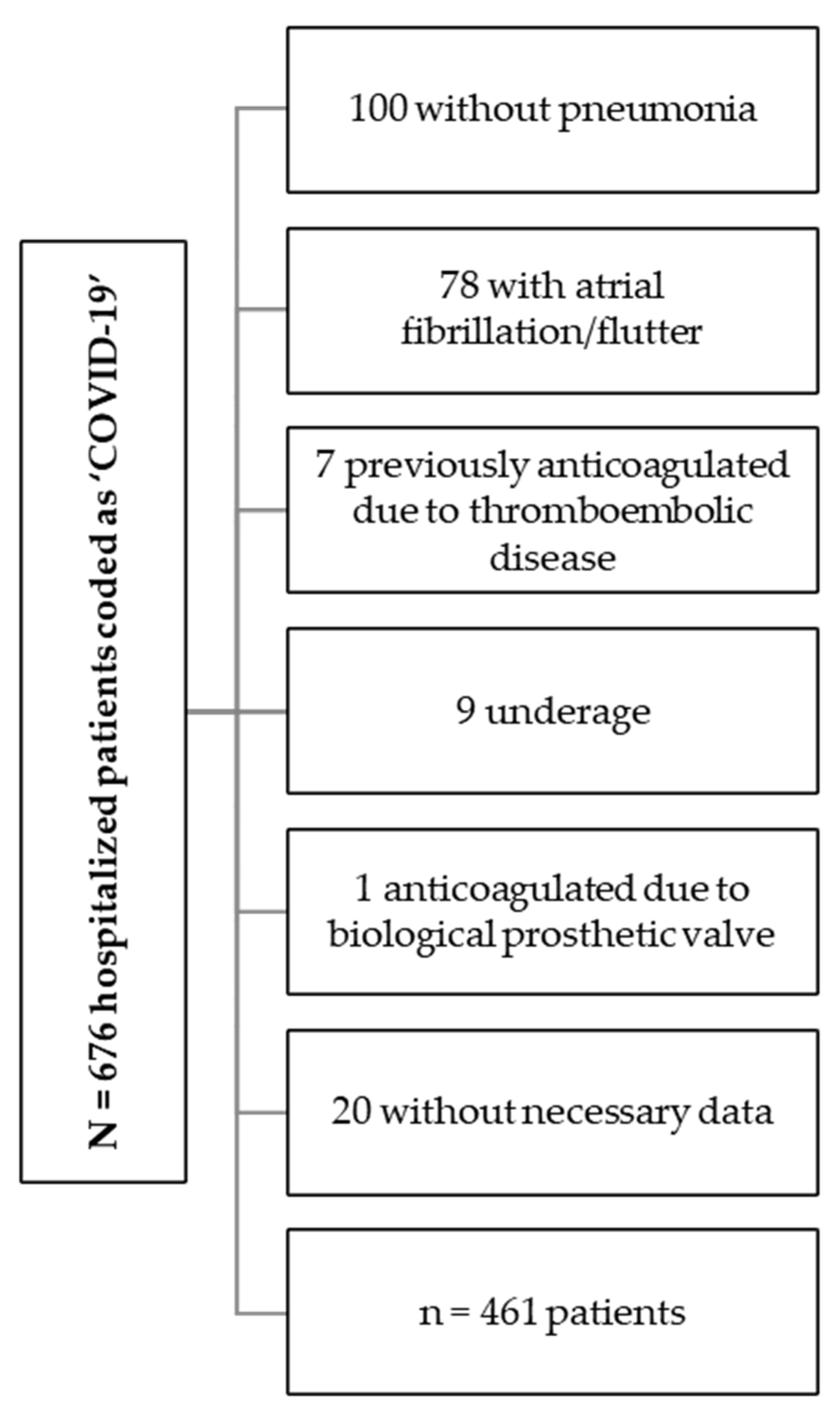
2.2. Patients
2.3. Selection of Thrombotic and Hemorrhagic Risk Scales
2.4. Variables
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ACEI | Angiotensin-converting enzyme inhibitor |
| CALL score | comorbidity, age, lymphocyte, and lactate dehydrogenase score |
| CKD-EPI-09 | Chronic kidney disease epidemiology collaboration equation (2009) |
| CT | Computed tomography |
| COPD | Chronic obstructive pulmonary disease |
| DPP-4 | Dipeptidyl-peptidase 4 |
| DVT | Deep vein thrombosis |
| ICU | Intensive care unit |
| IL-6 | Interleukin 6 |
| ISTH | International Society on Thrombosis and Haemostasis |
| LMWH | Low molecular weight heparin |
| OSAHS | Obstructive sleep apnea/hypopnea syndrome |
| PCR | Polymerase chain reaction |
| PPS | Padua prediction score |
| RALE score | Radiographic Assessment of Lung Edema score |
| SpO2/FiO2 | Relationship between peripheral oxygen saturation and fractional inspired oxygen |
| VTE | Venous thromboembolism |
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ogawa, H. COVID-19-Associated Coagulopathy and Disseminated Intravascular Coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.M.; Johnson Moore, K.L.; Moore, J.S. Coagulopathy during COVID-19 Infection: A Brief Review. Clin. Exp. Med. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Qiao, M.; Sohail, M.; Zhang, X. Non-Anticoagulant Heparin Derivatives for COVID-19 Treatment. Int. J. Biol. Macromol. 2023, 226, 974–981. [Google Scholar] [CrossRef]
- Abo Elmaaty, A.; Eldehna, W.M.; Khattab, M.; Kutkat, O.; Alnajjar, R.; El-Taweel, A.N.; Al-Rashood, S.T.; Abourehab, M.A.S.; Binjubair, F.A.; Saleh, M.A.; et al. Anticoagulants as Potential SARS-CoV-2 M(pro) Inhibitors for COVID-19 Patients: In Vitro, Molecular Docking, Molecular Dynamics, DFT, and SAR Studies. Int. J. Mol. Sci. 2022, 23, 12235. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of Heparin and Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Spike Glycoprotein Binding Interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant Treatment Is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Kosanovic, D.; Yaroshetskiy, A.I.; Tsareva, N.A.; Merzhoeva, Z.M.; Trushenko, N.V.; Nekludova, G.V.; Schermuly, R.T.; Avdeev, S.N. Recombinant Tissue Plasminogen Activator Treatment for COVID-19 Associated ARDS and Acute Cor Pulmonale. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 104, 108–110. [Google Scholar] [CrossRef]
- Rentsch, C.T.; Beckman, J.A.; Tomlinson, L.; Gellad, W.F.; Alcorn, C.; Kidwai-Khan, F.; Skanderson, M.; Brittain, E.; King, J.T.J.; Ho, Y.-L.; et al. Early Initiation of Prophylactic Anticoagulation for Prevention of Coronavirus Disease 2019 Mortality in Patients Admitted to Hospital in the United States: Cohort Study. BMJ 2021, 372, n311. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Antinori, A.; Berselli, N.; Blandi, L.; Bonaccio, M.; Cauda, R.; Guaraldi, G.; Menicanti, L.; Mennuni, M.; et al. Heparin in COVID-19 Patients Is Associated with Reduced In-Hospital Mortality: The Multicenter Italian CORIST Study. Thromb. Haemost. 2021, 121, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rivas, N.; Aibar, J.; Gabara-Xancó, C.; Trueba-Vicente, Á.; Urbelz-Pérez, A.; Gómez-Del Olmo, V.; Demelo-Rodríguez, P.; Rivera-Gallego, A.; Bosch-Nicolau, P.; Perez-Pinar, M.; et al. Efficacy and Safety of Tinzaparin in Prophylactic, Intermediate and Therapeutic Doses in Non-Critically Ill Patients Hospitalized with COVID-19: The PROTHROMCOVID Randomized Controlled Trial. J. Clin. Med. 2022, 11, 5632. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Furtado, R.H.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; de Aquino Martins, P.; de Oliveira, A.L.; et al. Therapeutic versus Prophylactic Anticoagulation for Patients Admitted to Hospital with COVID-19 and Elevated D-Dimer Concentration (ACTION): An Open-Label, Multicentre, Randomised, Controlled Trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard Prophylactic versus Intermediate Dose Enoxaparin in Adults with Severe COVID-19: A Multi-Center, Open-Label, Randomized Controlled Trial. J. Thromb. Haemost. 2021, 19, 2225–2234. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; Moghadam, K.G.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clini. JAMA 2021, 325, 1620–1630. [Google Scholar] [CrossRef]
- Lemos, A.C.B.; do Espírito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus Prophylactic Anticoagulation for Severe COVID-19: A Randomized Phase II Clinical Trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; Carrier, M.; Rosenson, R.S.; Reynolds, H.R.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of Therapeutic Heparin versus Prophylactic Heparin on Death, Mechanical Ventilation, or Intensive Care Unit Admission in Moderately Ill Patients with COVID-19 Admitted to Hospital: RAPID Randomised Clinical Trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-Risk Hospitalized Patients with COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 Guidelines on the Use of Anticoagulation for Thromboprophylaxis in Patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef]
- Garofalo, E.; Cammarota, G.; Neri, G.; Macheda, S.; Biamonte, E.; Pasqua, P.; Guzzo, M.L.; Longhini, F.; Bruni, A. Bivalirudin vs. Enoxaparin in Intubated COVID-19 Patients: A Pilot Multicenter Randomized Controlled Trial. J. Clin. Med. 2022, 11, 5992. [Google Scholar] [CrossRef]
- Grifoni, E.; Valoriani, A.; Cei, F.; Vannucchi, V.; Moroni, F.; Pelagatti, L.; Tarquini, R.; Landini, G.; Masotti, L. The CALL Score for Predicting Outcomes in Patients with COVID-19. Clin. Infect. Dis. 2021, 72, 182–183. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The Unique Characteristics of COVID-19 Coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Caprini, J.A.; Arcelus, J.I.; Hasty, J.H.; Tamhane, A.C.; Fabrega, F. Clinical Assessment of Venous Thromboembolic Risk in Surgical Patients. Semin. Thromb. Hemost. 1991, 17 (Suppl. S3), 304–312. [Google Scholar] [PubMed]
- Cronin, M.; Dengler, N.; Krauss, E.S.; Segal, A.; Wei, N.; Daly, M.; Mota, F.; Caprini, J.A. Completion of the Updated Caprini Risk Assessment Model (2013 Version). Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2019, 25, 1076029619838052. [Google Scholar] [CrossRef]
- Tsaplin, S.; Schastlivtsev, I.; Zhuravlev, S.; Barinov, V.; Lobastov, K.; Caprini, J.A. The Original and Modified Caprini Score Equally Predicts Venous Thromboembolism in COVID-19 Patients. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1371–1381.e4. [Google Scholar] [CrossRef] [PubMed]
- Barbar, S.; Noventa, F.; Rossetto, V.; Ferrari, A.; Brandolin, B.; Perlati, M.; De Bon, E.; Tormene, D.; Pagnan, A.; Prandoni, P. A Risk Assessment Model for the Identification of Hospitalized Medical Patients at Risk for Venous Thromboembolism: The Padua Prediction Score. J. Thromb. Haemost. 2010, 8, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.X.; Xu, J.L.; Mao, Q.X.; Liu, R.; Zhang, W.Y.; Qian, H.Y.; Xu, L. Association of Padua Prediction Score with In-Hospital Prognosis in COVID-19 Patients. QJM 2020, 113, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Medrano Ortega, F.; Navarro Puerto, A.; Vidal Serrano, S.; Alonso Ortiz del Río, C.; Gutiérrez Tous, R.; Marín León, I. Guía PRETEMED 2007 Sobre Prevención de Enfermedad Tromboembólica Venosa En Patología Médica; Sademi: Córdoba, Argentina, 2007. [Google Scholar]
- Rosenberg, D.; Eichorn, A.; Alarcon, M.; McCullagh, L.; McGinn, T.; Spyropoulos, A.C. External Validation of the Risk Assessment Model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for Medical Patients in a Tertiary Health System. J. Am. Heart Assoc. 2014, 3, e001152. [Google Scholar] [CrossRef]
- Helmy, M.A.; Milad, L.M.; Hasanin, A.; Elsayed, E.A.; Kamel, O.Y.; Mostafa, M.; Fathy, S.; Elsayad, M. Bleeding and Thrombotic Complications in Patients with Severe COVID-19: A Prospective Observational Study. Health Sci. Rep. 2022, 5, e736. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, L.; Li, F.; Liu, J.; Zhang, L.; Li, Q.; Gu, J.; Liang, S.; Zhao, Q.; Liu, J.; et al. Risk Assessment of Venous Thromboembolism and Bleeding in COVID-19 Patients. Clin. Respir. J. 2022, 16, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zimatore, C.; Pisani, L.; Lippolis, V.; Warren, M.A.; Calfee, C.S.; Ware, L.B.; Algera, A.G.; Smit, M.R.; Grasso, S.; Schultz, M.J. Accuracy of the Radiographic Assessment of Lung Edema Score for the Diagnosis of ARDS. Front. Physiol. 2021, 12, 672823. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Angerås, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W. Definition of Major Bleeding in Clinical Investigations of Antihemostatic Medicinal Products in Surgical Patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Clinical Characteristics of Patients Hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic Factors for Mortality, Intensive Care Unit and Hospital Admission Due to SARS-CoV-2: A Systematic Review and Meta-Analysis of Cohort Studies in Europe. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2022, 31, 220098. [Google Scholar] [CrossRef]
- Uruma, Y.; Manabe, T.; Fujikura, Y.; Iikura, M.; Hojo, M.; Kudo, K. Effect of Asthma, COPD, and ACO on COVID-19: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0276774. [Google Scholar] [CrossRef]
- Rosero, P.A.; Realpe, J.S.; Farinango, C.D.; Restrepo, D.S.; Salazar-Cabrera, R.; Lopez, D.M. Risk Factors for COVID-19: A Systematic Mapping Study. Stud. Health Technol. Inform. 2022, 299, 63–74. [Google Scholar] [CrossRef]
- Sayad, B.; Rahimi, Z. Blood Coagulation Parameters in Patients with Severe COVID-19 from Kermanshah Province, Islamic Republic of Iran. East. Mediterr. Health J. 2020, 26, 999–1004. [Google Scholar] [CrossRef]
- Tekle, E.; Gelaw, Y.; Dagnew, M.; Gelaw, A.; Negash, M.; Kassa, E.; Bizuneh, S.; Wudineh, D.; Asrie, F. Risk Stratification and Prognostic Value of Prothrombin Time and Activated Partial Thromboplastin Time among COVID-19 Patients. PLoS ONE 2022, 17, e0272216. [Google Scholar] [CrossRef]
- Duc, V.T.; Thuy, T.T.M.; Nam, N.H.; Tram, H.T.B.; Thao, T.T.P.; Doan, L.T.; Hy, L.N.G.; Quynh, L.N.D.; Hong Duc, N.; Thang, L.M.; et al. Correlation of Chest X-ray Scores in SARS-CoV-2 Patients with the Clinical Severity Classification and the Quick COVID-19 Severity Index. Cureus 2022, 14, e24864. [Google Scholar] [CrossRef] [PubMed]
- López Arévalo, R.J. Uso Del “Score RALE Modificado” En Radiografía Simple de Tórax (AP) Como Medio Predictor En Pacientes COVID-19 Críticos. Octubre 2020–Marzo 2021, Universidad Nacional de San Martín (Doctoral Thesis). Fondo Editorial. 2021. Available online: https://repositorio.unsm.edu.pe/handle/11458/4232 (accessed on 24 January 2023).
- Erturk Sengel, B.; Tukenmez Tigen, E.; Ilgin, C.; Basari, T.; Bedir, M.; Odabasi, Z.; Korten, V. Application of CALL Score for Prediction of Progression Risk in Patients with COVID-19 at University Hospital in Turkey. Int. J. Clin. Pract. 2021, 75, e14642. [Google Scholar] [CrossRef]
- Golemi, I.; Adum, J.P.S.; Tafur, A.; Caprini, J. Venous Thromboembolism Prophylaxis Using the Caprini Score. Dis. Mon. 2019, 65, 249–298. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.J.; Press, A.; Fishbein, J.; Lesser, M.; McCullagh, L.; McGinn, T.; Spyropoulos, A.C. External Validation of the IMPROVE Bleeding Risk Assessment Model in Medical Patients. Thromb. Haemost. 2016, 116, 530–536. [Google Scholar] [CrossRef]
- Remap-Cap Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Godolphin, P.J.; Fisher, D.J.; Berry, L.R.; Derde, L.P.G.; Diaz, J.V.; Gordon, A.C.; Lorenzi, E.; Marshall, J.C.; Murthy, S.; Shankar-Hari, M.; et al. Association between Tocilizumab, Sarilumab and All-Cause Mortality at 28 Days in Hospitalised Patients with COVID-19: A Network Meta-Analysis. PLoS ONE 2022, 17, e0270668. [Google Scholar] [CrossRef] [PubMed]
- Corral-Gudino, L.; Cusacovich, I.; Martín-González, J.I.; Muela-Molinero, A.; Abadía-Otero, J.; González-Fuentes, R.; Ruíz-de-Temiño, Á.; Tapia-Moral, E.; Cuadrado-Medina, F.; Martín-Asenjo, M.; et al. Effect of Intravenous Pulses of Methylprednisolone 250 Mg versus Dexamethasone 6 Mg in Hospitalised Adults with Severe COVID-19 Pneumonia: An Open-Label Randomised Trial. Eur. J. Clin. Investig. 2022, 53, e13881. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Davoli, C.; Borrazzo, C.; Olivadese, V.; Ceccarelli, G.; Fusco, P.; Lazzaro, A.; Lionello, R.; Ricchio, M.; Serapide, F.; et al. Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone. Biomedicines 2022, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Rein, L.; Calero, K.; Shah, R.; Ojielo, C.; Hudock, K.M.; Lodhi, S.; Sadaka, F.; Bellam, S.; Palma, C.; Hager, D.N.; et al. Randomized Phase 3 Trial of Ruxolitinib for COVID-19-Associated Acute Respiratory Distress Syndrome. Crit. Care Med. 2022, 50, 1701–1713. [Google Scholar] [CrossRef]
- NICE. COVID-19 Rapid Guideline: Managing COVID-19; NICE: London, UK, 2022. [Google Scholar]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Iaccarino, J.; Jimenez, D.; LeGal, G.; et al. Thromboprophylaxis in Patients with COVID-19: A Brief Update to the CHEST Guideline and Expert Panel Report. Chest 2022, 162, 213–225. [Google Scholar] [CrossRef]
- Schulman, S.; Sholzberg, M.; Spyropoulos, A.C.; Zarychanski, R.; Resnick, H.E.; Bradbury, C.A.; Broxmeyer, L.; Connors, J.M.; Falanga, A.; Iba, T.; et al. ISTH Guidelines for Antithrombotic Treatment in COVID-19. J. Thromb. Haemost. 2022, 20, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
| n = 461 | Prophylaxis (n = 369) | Intermediate (n = 27) | Therapeutic (n = 65) | p Value |
|---|---|---|---|---|
| Age, median (Q1–Q3) (years) | 66 (55–78) | 64 (54–75) | 76 (63–82) | <0.001 |
| Men, n (%) | 214 (58%) | 16 (59.3%) | 34 (52.3%) | 0.67 |
| Comorbidities | ||||
| Arterial hypertension, n (%) | 224 (60.7%) | 13 (48.1%) | 44 (67.7%) | 0.211 |
| Diabetes mellitus, n (%) | 129 (35%) | 8 (29.6%) | 35 (53.8%) | 0.01 |
| Dyslipidemia, n (%) | 137 (37.1%) | 9 (33.3%) | 37 (56.9%) | 0.009 |
| Obesity, n (%) | 72 (19.5%) | 13 (48.1%) | 17 (26.1%) | 0.117 |
| Smoking, n (%) Active Previous | 27 (7.5%) 84 (23.3%) | 2 (8%) 6 (24%) | 3 (4.9%) 16 (26.2%) | 0.951 |
| Chronic obstructive pulmonary disease, n (%) | 22 (6%) | 4 (14.8%) | 2 (3.1%) | 0.098 |
| Sleep apnea/hypopnea syndrome, n (%) | 21 (5.7%) | 2 (7.4%) | 6 (9.2%) | 0.539 |
| Asthma, n (%) | 19 (5.1%) | 1 (3.7%) | 6 (9.2%) | 0.381 |
| Chronic kidney disease, n (%) G1 (n = 257) (55.8%) G2 (n = 119) (25.8%) G3a (n = 39) (8.5%) G3b (n = 27) (5.8%) G4 (n = 12) (2.6%) G5 (n = 7) (1.5%) | 219 (59.3%) 85 (23%) 30 (8.1%) 20 (5.4%) 8 (2.2%) 7 (1.9%) | 17 (63%) 7 (25.9%) 2 (7.4%) 0 1 (3.7%) 0 | 21 (32.3%) 27 (41.5%) 7 (10.8%) 7 (10.8%) 3 (4.6%) 0 | 0.011 |
| Respiratory severity | ||||
| SpO2/FiO2, median (Q1–Q3) | 375.7 (320–452) | 323.8 (198–448) | 247.9 (119–419) | <0.001 |
| Radiological severity | ||||
| RALE score, median (Q1–Q3) | 5 (4–6) | 6 (4–7) | 6 (5–7) | <0.001 |
| Scales | ||||
| CALL score, median (Q1–Q3) | 10 (8–12) | 10 (8–12) | 11 (10–12) | <0.001 |
| PRETEMED, median (Q1–Q3) | 5.26 (3–7) | 6.19 (4–8) | 7.35 (6–9) | <0.001 |
| Padua, median (Q1–Q3) | 3.3 (1–5) | 4.07 (2–6) | 5.28 (4–7) | <0.001 |
| Caprini, median (Q1–Q3) | 4.86 (3–6) | 5.63 (4–7) | 5.91 (5–7) | <0.001 |
| IMPROVE bleeding risk, median (Q1–Q3) | 2.74 (1–3.5) | 3.61 (2–5.5) | 3.73 (2–5.5) | <0.001 |
| Laboratory test | ||||
| Peak D-dimer, median (Q1–Q3) (ng/mL) | 4452.9 (788–2109) | 3308.8 (931–5200) | 17,938.8 (4144.5–23,338.5) | <0.001 |
| Peak activated thromboplastin time, median (Q1–Q3) (ratio) | 1.34 (0.94–1.1) | 1.19 (0.97–1.15) | 1.76 (0.98–1.17) | <0.001 |
| Nadir prothrombin time, median (Q1–Q3) (%) | 77.8 (70–86) | 70.7 (61–79) | 73.1 (64.5–81) | <0.001 |
| Nadir lymphocytes, median (Q1–Q3) (cels/mm3) | 959.8 (500–1140) | 760 (450–1020) | 655.3 (370–795) | <0.001 |
| Peak lactate dehydrogenase, median (Q1–Q3) (U/L) | 491.6 (284–485) | 430 (312–525) | 504.3 (345–651) | <0.001 |
| Peak C-reactive protein, median (Q1–Q3) (mg/L) | 154.8 (74.2–201.7) | 158.9 (78–208.3) | 203.8 (104.6–250.4) | <0.001 |
| Peak procalcitonin, median (Q1–Q3) (ng/mL) | 1.34 (0.06–0.31) | 0.58 (0.05–0.16) | 6.86 (0.1–0.69) | 0.001 |
| Peak ferritin, median (Q1–Q3) (ng/mL) | 1456.7 (361.4–1498.4) | 2835.3 (682.8–2687.5) | 1582.8 (415.6–1915.9) | <0.001 |
| COVID-19 treatment | ||||
| Steroids, n (%) Dexamethasone 6 mg Dexamethasone 24 mg Methylprednisolone 250 mg | 190 (51.5%) 98 (26.6%) 81 (22%) | 14 (51.9%) 11 (40.7%) 2 (7.4%) | 26 (40%) 25 (38.5%) 14 (21.5%) | 0.089 |
| Tocilizumab, n (%) | 20 (5.4%) | 1 (3.7%) | 5 (7.7%) | 0.691 |
| Anakinra, n (%) | 12 (3.3%) | 3 (11.1%) | 4 (4.6%) | 0.12 |
| Ruxolitinib, n (%) | 81 (22%) | 10 (37%) | 11 (16.9%) | 0.105 |
| Remdesivir, n (%) | 18 (4.9%) | 2 (7.4%) | 3 (4.6%) | 0.834 |
| Relative Risk | 95% Confidence Interval | p Value | |
|---|---|---|---|
| Age | 1.077 | 1.028–1.128 | 0.002 |
| Sex (men) | 2.164 | 0.566–8.278 | 0.259 |
| Comorbidities | |||
| Arterial hypertension | 5.304 | 1.421–19.798 | 0.013 |
| Diabetes mellitus | 4.792 | 1.360–16.877 | 0.015 |
| Dyslipidemia | 5.989 | 1.578–22.734 | 0.009 |
| Obesity | 4.320 | 1.204–15.505 | 0.025 |
| Smoking | |||
| Active | 2.248 | 0.464–10.895 | 0.315 |
| Previous | 9.619 | 0.673–137.438 | 0.095 |
| Asthma | 1.620 | 0.051–51.053 | 0.784 |
| Sleep apnea/hypopnea syndrome | 5.123 | 1.072–24.495 | 0.041 |
| Chronic obstructive pulmonary disease | 31.623 | 2.979–335.625 | 0.004 |
| Chronic kidney disease | 0.157 | ||
| G2 | 2.937 | 0.666–12.942 | 0.155 |
| G3a | 15.736 | 2.103–117.761 | 0.007 |
| G3b | 4.914 | 0.463–52.103 | 0.186 |
| G4 | 9.036 | 0.583–140.001 | 0.115 |
| G5 | 9.642 | 0.187–496.062 | 0.260 |
| Respiratory severity | |||
| SpO2/FiO2 | 0.995 | 0.990–1 | 0.32 |
| Radiological severity | |||
| RALE score | 2.078 | 1.242–3.477 | 0.005 |
| Scales | |||
| CALL score | 1.338 | 0.840–2.131 | 0.22 |
| PRETEMED | 0.764 | 0.361–1.618 | 0.482 |
| Padua | 1.218 | 0.633–2.343 | 0.555 |
| Caprini | 2.754 | 1.011–7.501 | 0.048 |
| IMPROVE bleeding risk | 0.976 | 0.662–1.438 | 0.901 |
| Laboratory test | |||
| Peak D-dimer(ng/mL) | 1 | 1 | 0.06 |
| Peak activated thromboplastin time(ratio) | 0.976 | 0.814–1.171 | 0.797 |
| Nadir prothrombin time (%) | 0.939 | 0.894-.985 | 0.01 |
| Nadir lymphocytes (cels/mm3) | 1 | 0.999–1 | 0.177 |
| Peak lactate dehydrogenase (U/L) | 1.002 | 1–1.005 | 0.081 |
| Peak C-reactive protein (mg/L) | 1.004 | 0.999–1 | 0.117 |
| Peak procalcitonin (ng/mL) | 1.047 | 0.978–1.120 | 0.187 |
| Peak ferritin (ng/mL) | 1 | 1–1.001 | 0.058 |
| COVID-19 treatment | |||
| Steroids (dexamethasone 6 mg) Dexamethasone 24 mg Methylprednisolone 250 mg | 1.823 0.432 | 0.522–6.36 0.071–2.635 | 0.346 0.363 |
| Low molecular weight heparin (prophylactic) Intermediate Therapeutic | 0.108 0.173 | 0.011–1.033 0.038–0.8 | 0.53 0.025 |
| Tocilizumab | 0.056 | 0.006–0.545 | 0.013 |
| Anakinra | 6.273 | 0.552–71.301 | 0.139 |
| Ruxolitinib | 0.971 | 0.281–3.351 | 0.963 |
| Remdesivir | 6.804 | 0.671–68.995 | 0.105 |
| Bleeding | n of Events | Relative Risk | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|
| Minor | |||||
| PRETEMED | 1.05 | 0.787–1.401 | 0.741 | ||
| Padua | 1.029 | 0.738–1.434 | 0.868 | ||
| Caprini | 1.079 | 0.689–1.687 | 0.741 | ||
| IMPROVE bleeding | 1.263 | 1.105–1.573 | 0.037 | ||
| Low molecular weight heparin | |||||
| Prophylactic | 16 | 0.702 | 0.235–2.093 | 0.525 | |
| Intermediate | 1 | 0.422 | 0.044–4.022 | 0.453 | |
| Therapeutic | 5 | 0.652 | 0.026–3.652 | 0.657 | |
| Major | |||||
| PRETEMED | 1.046 | 0.487–2.246 | 0.909 | ||
| Padua | 0.865 | 0.379–1.974 | 0.730 | ||
| Caprini | 1.259 | 0.401–3.955 | 0.693 | ||
| IMPROVE bleeding | 1.511 | 0.837–2.728 | 0.171 | ||
| Low molecular weight heparin | |||||
| Prophylactic | 0 | * | * | * | |
| Intermediate | 0 | * | * | * | |
| Therapeutic | 4 | * | * | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Delgado, J.; Lojo-Cruz, C.; Rubio Marín, P.; Menor Campos, E.M.; Michán-Doña, A. Enoxaparin Posology According to Prothrombotic Status and Bleeding Risk in Hospitalized Patients with SARS-CoV-2 Pneumonia. J. Clin. Med. 2023, 12, 928. https://doi.org/10.3390/jcm12030928
Mora-Delgado J, Lojo-Cruz C, Rubio Marín P, Menor Campos EM, Michán-Doña A. Enoxaparin Posology According to Prothrombotic Status and Bleeding Risk in Hospitalized Patients with SARS-CoV-2 Pneumonia. Journal of Clinical Medicine. 2023; 12(3):928. https://doi.org/10.3390/jcm12030928
Chicago/Turabian StyleMora-Delgado, Juan, Cristina Lojo-Cruz, Patricia Rubio Marín, Eva María Menor Campos, and Alfredo Michán-Doña. 2023. "Enoxaparin Posology According to Prothrombotic Status and Bleeding Risk in Hospitalized Patients with SARS-CoV-2 Pneumonia" Journal of Clinical Medicine 12, no. 3: 928. https://doi.org/10.3390/jcm12030928
APA StyleMora-Delgado, J., Lojo-Cruz, C., Rubio Marín, P., Menor Campos, E. M., & Michán-Doña, A. (2023). Enoxaparin Posology According to Prothrombotic Status and Bleeding Risk in Hospitalized Patients with SARS-CoV-2 Pneumonia. Journal of Clinical Medicine, 12(3), 928. https://doi.org/10.3390/jcm12030928






