Intra- and Inter-Rater Reliability and Agreement of Ultrasound Imaging of Muscle Architecture and Patellar Tendon in Post-COVID-19 Patients Who Had Experienced Moderate or Severe COVID-19 Infection †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
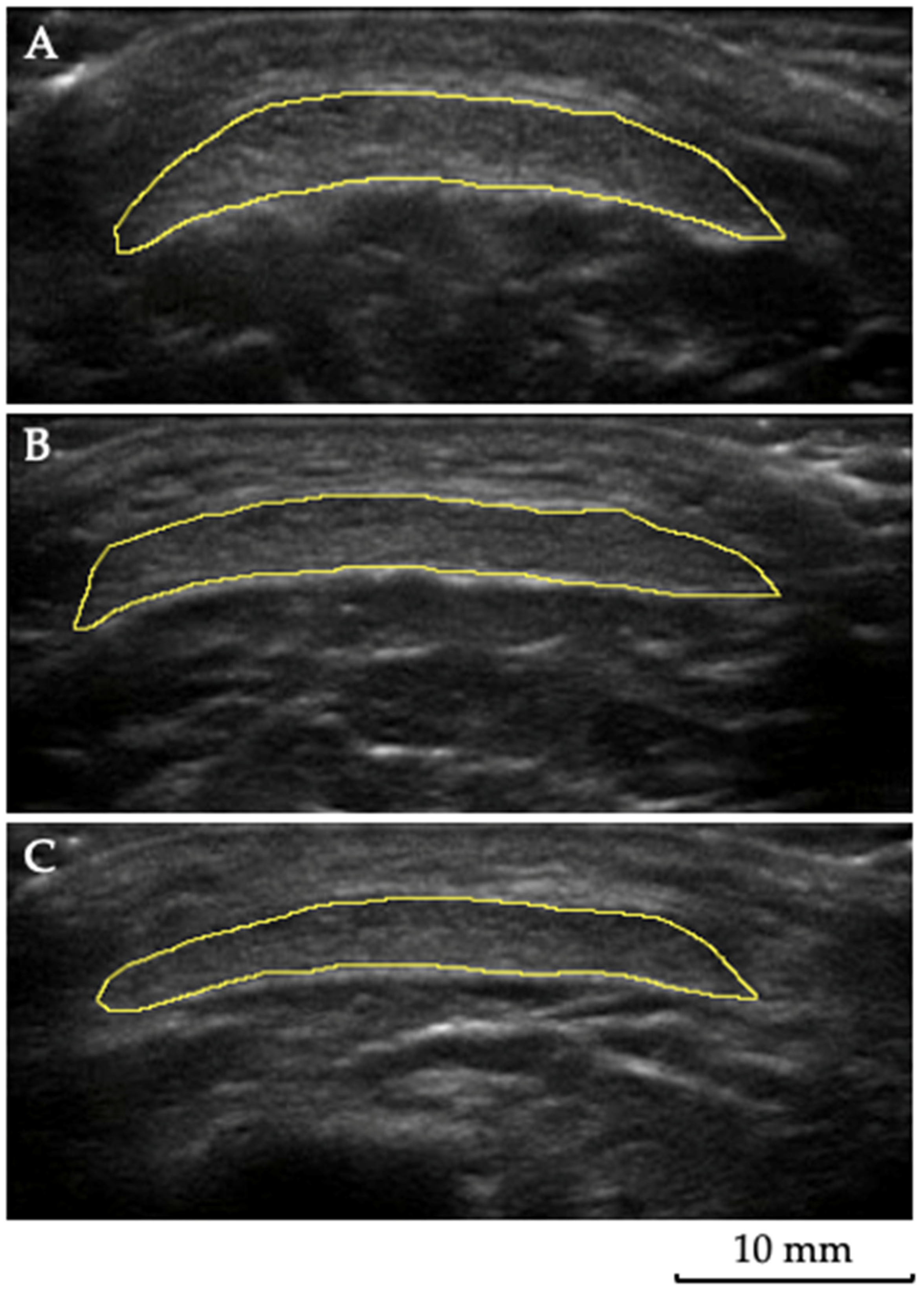
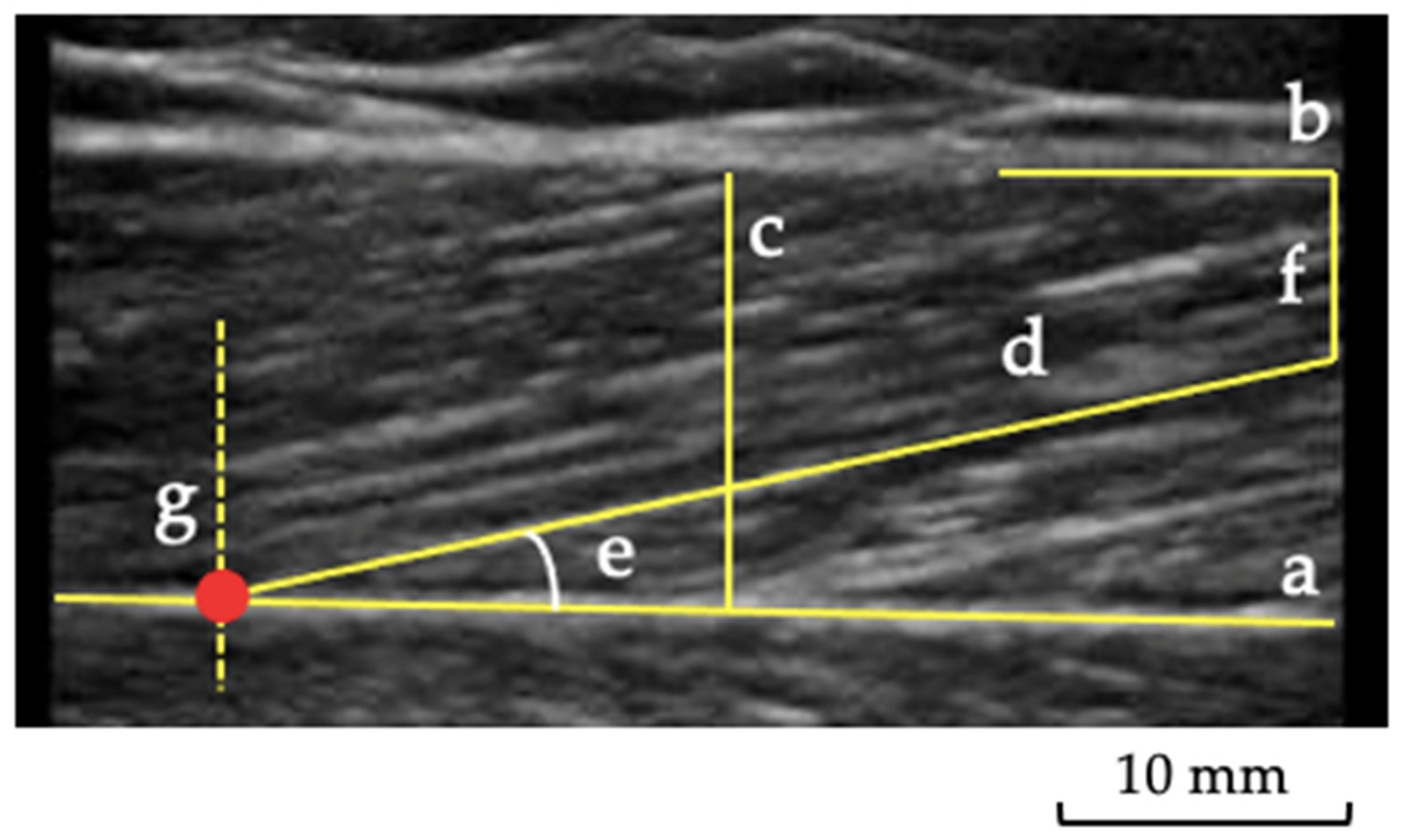
2.3. Muscle and Tendon Ultrasound Imaging
2.4. Statistical Analysis and Sample Size
3. Results
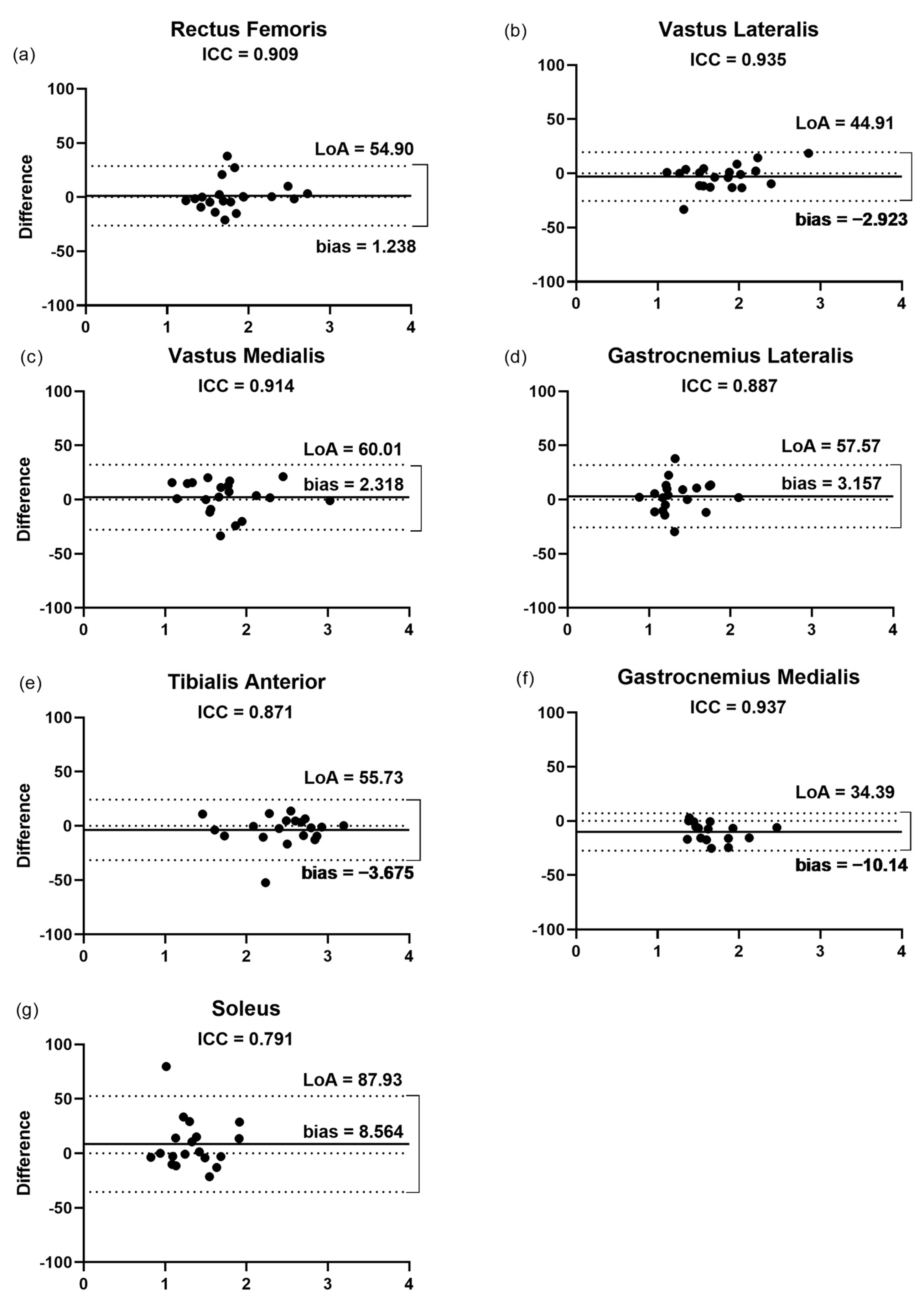
3.1. Intra-Rater Reliability
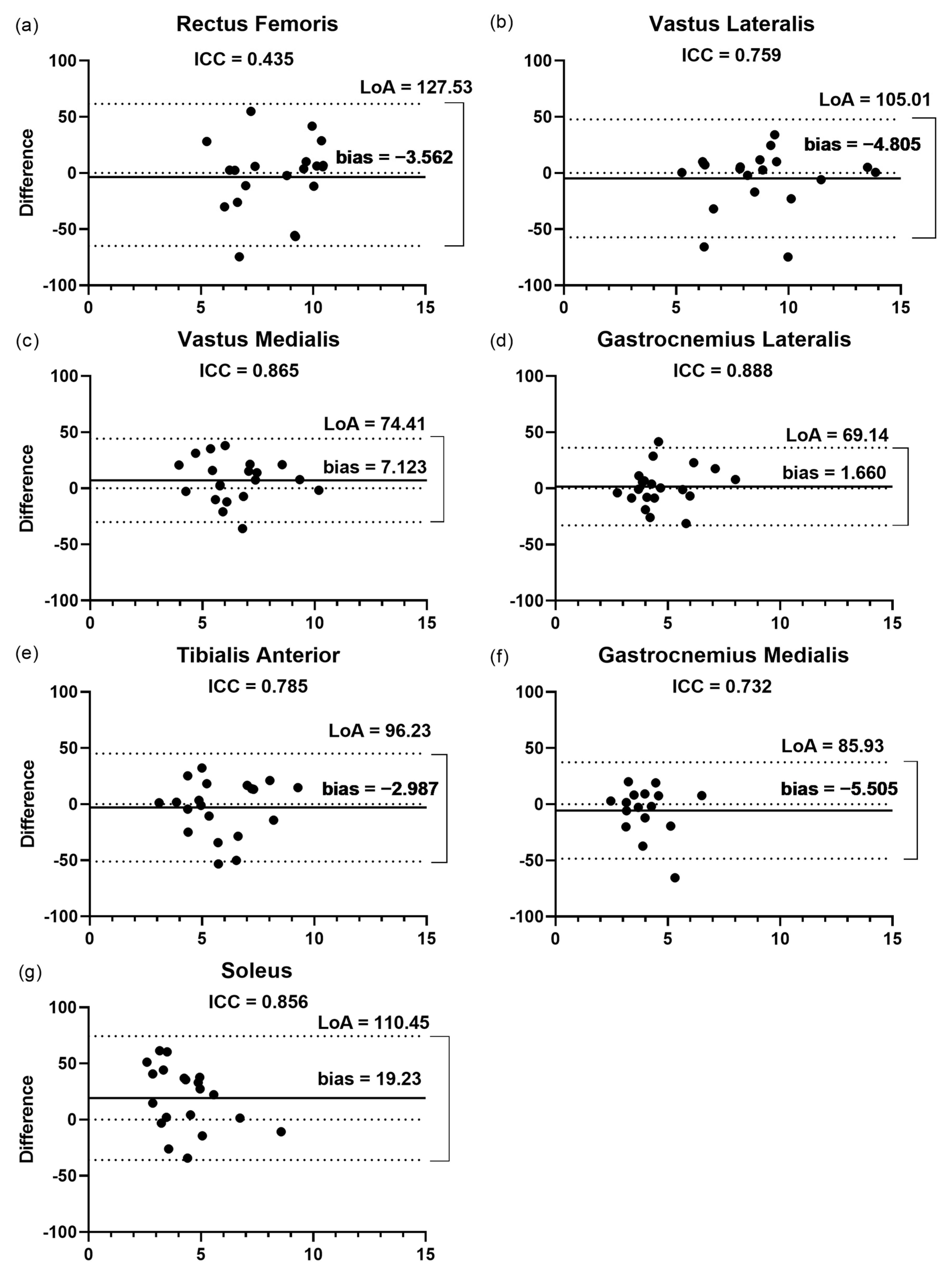
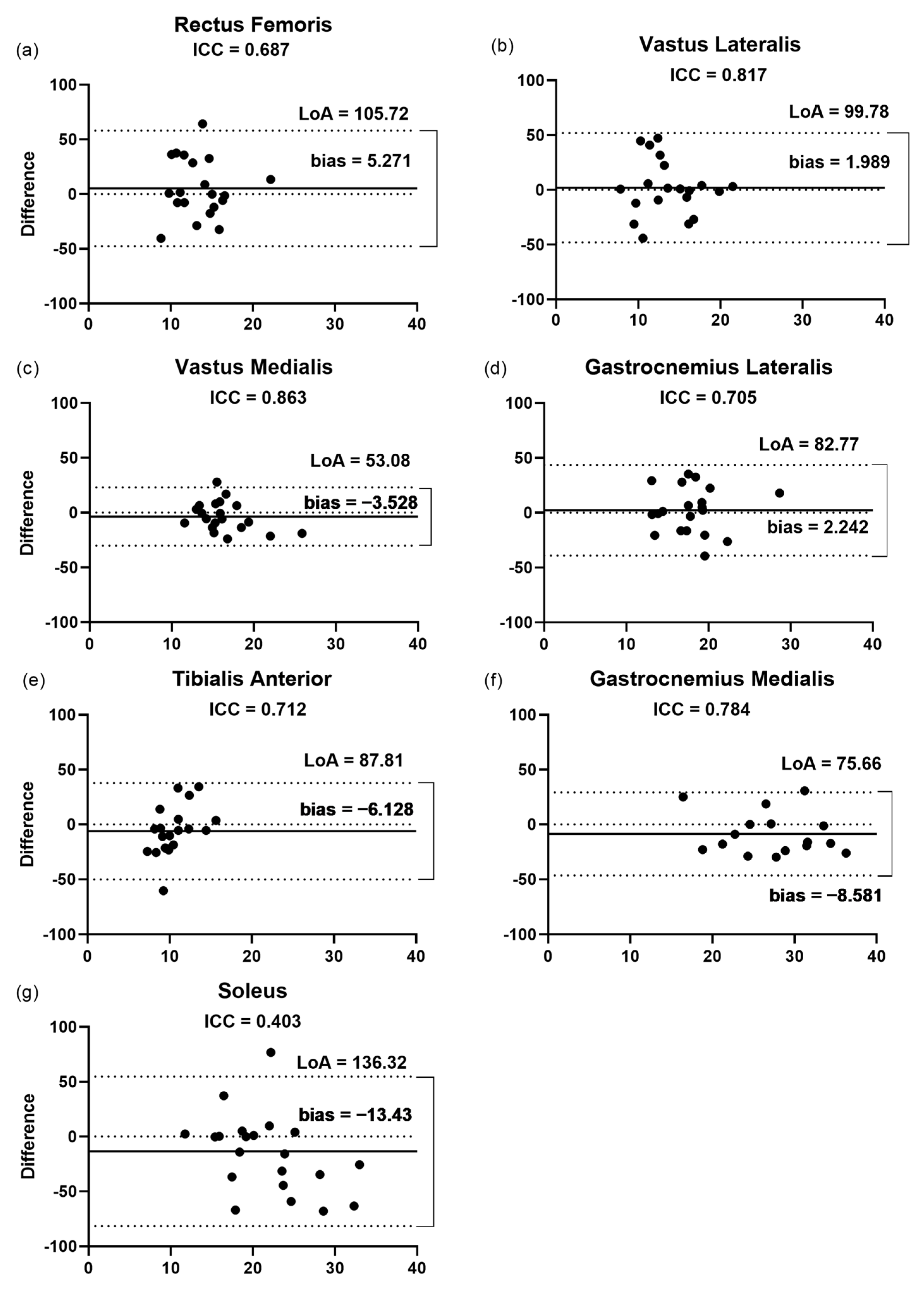
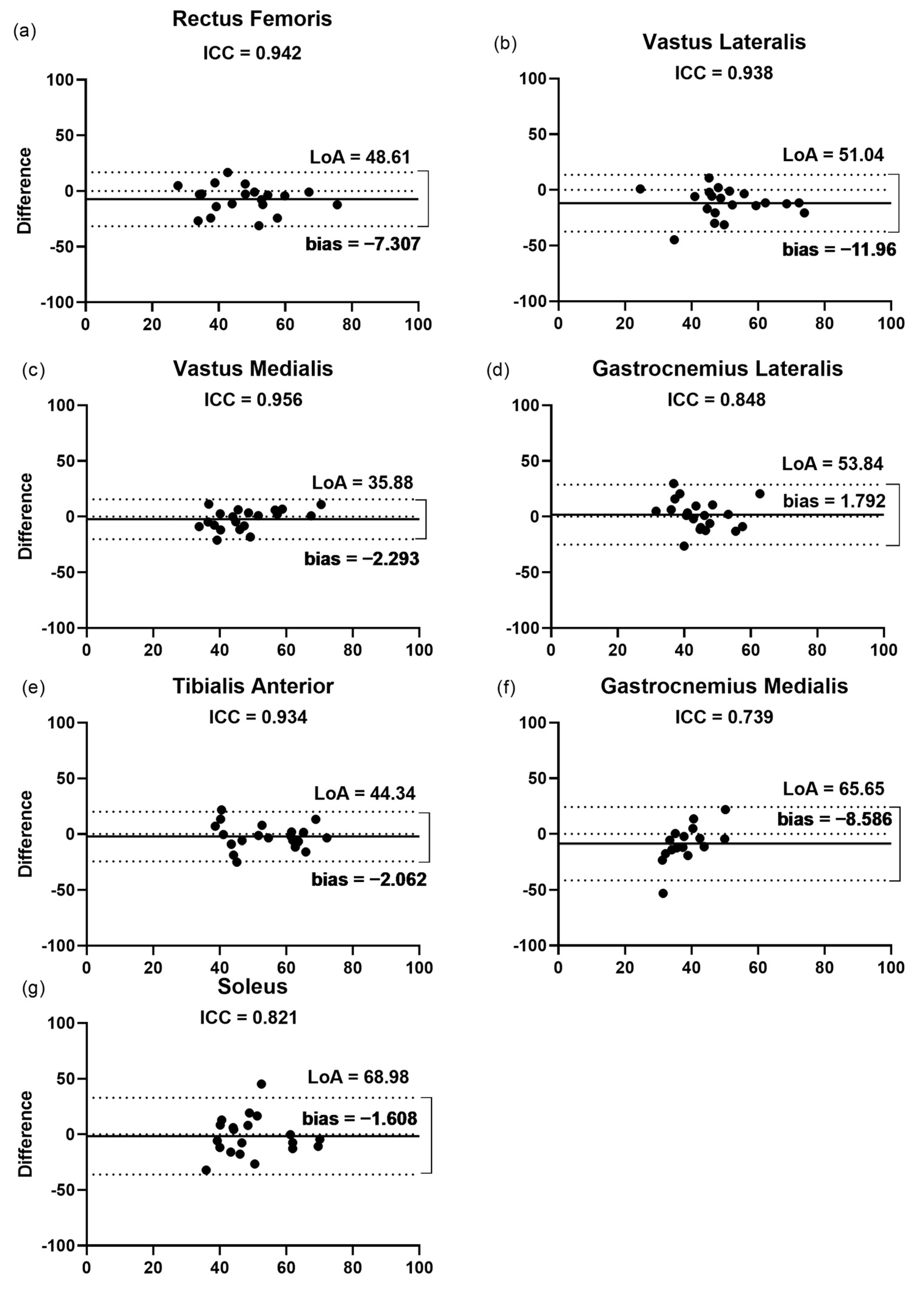
3.2. Inter-Rater Reliability
3.2.1. Fascicular Length
3.2.2. Pennation Angle
3.2.3. Thickness
3.2.4. Echogenicity
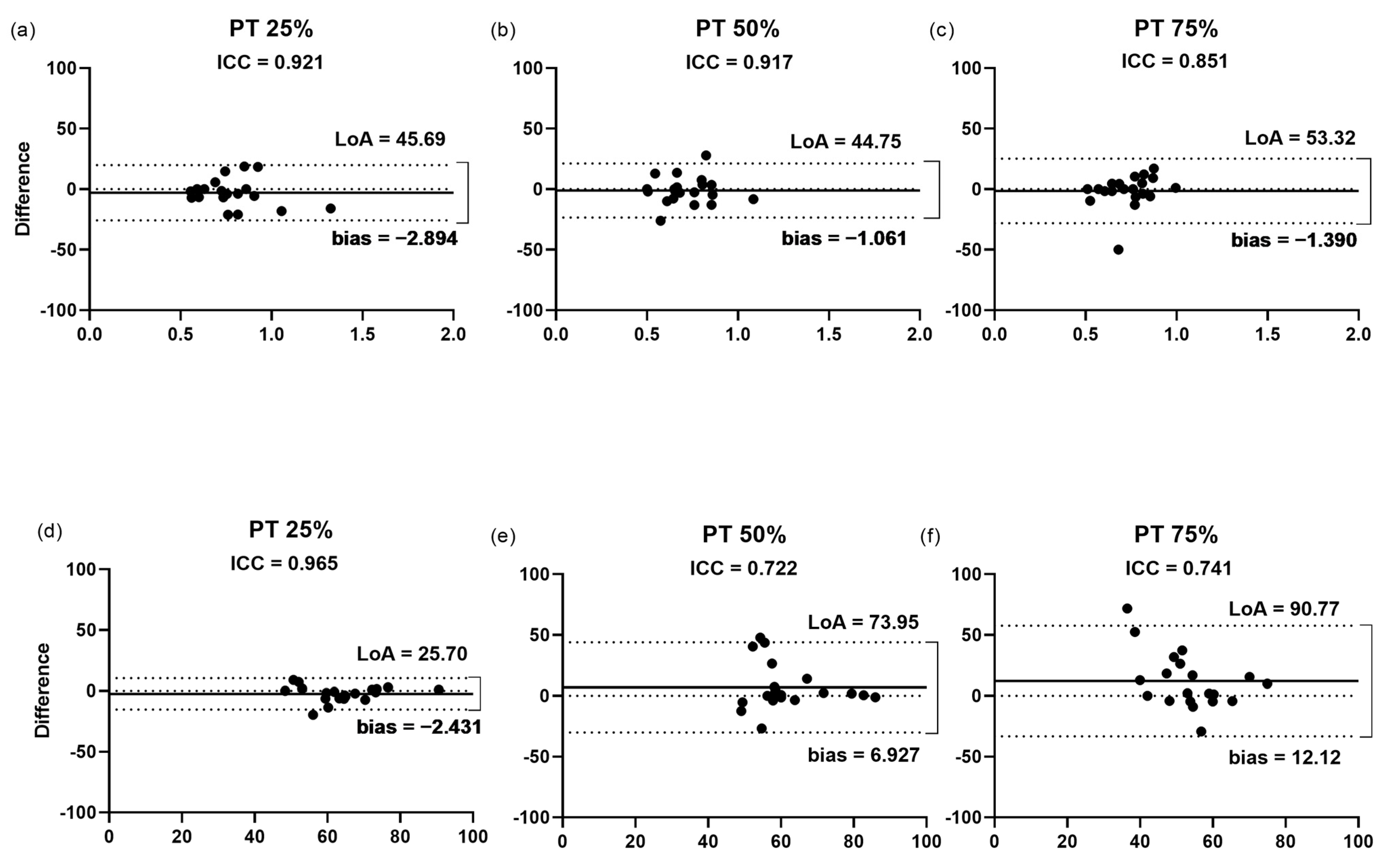
3.2.5. Cross-Sectional Area of the Patellar Tendon
3.2.6. Echogenicity of the Patellar Tendon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 17 May 2022).
- Siddiqi, H.K.; Mehra, M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical–Therapeutic Staging Proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of Post-COVID-19 Symptoms in Hospitalized and Non-Hospitalized COVID-19 Survivors: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A Major Cause of Cachexia and Sarcopenia? J. Cachexia Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Jacob Filho, W.; Shinjo, S.K.; Ferriolli, E.; Busse, A.L.; Avelino-Silva, T.J.; Longobardi, I.; de Oliveira Júnior, G.N.; Swinton, P.; Gualano, B.; et al. Muscle Strength and Muscle Mass as Predictors of Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Prospective Observational Study. J. Cachexia Sarcopenia Muscle 2021, 12, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.P.; Veronese, N. Post-COVID-19 Acute Sarcopenia: Physiopathology and Management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef]
- Van Ancum, J.M.; Scheerman, K.; Jonkman, N.H.; Smeenk, H.E.; Kruizinga, R.C.; Meskers, C.G.M.; Maier, A.B. Change in Muscle Strength and Muscle Mass in Older Hospitalized Patients: A Systematic Review and Meta-Analysis. Exp. Gerontol. 2017, 92, 34–41. [Google Scholar] [CrossRef]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular Presentations in Patients with COVID-19. Neurol. Sci. 2020, 41, 3039–3056. [Google Scholar] [CrossRef]
- Aschman, T.; Schneider, J.; Greuel, S.; Meinhardt, J.; Streit, S.; Goebel, H.H.; Büttnerova, I.; Elezkurtaj, S.; Scheibe, F.; Radke, J.; et al. Association between SARS-CoV-2 Infection and Immune-Mediated Myopathy in Patients Who Have Died. JAMA Neurol. 2021, 78, 948–960. [Google Scholar] [CrossRef]
- De Andrade, M.C., Jr.; de Salles, I.C.D.; de Brito, C.M.M.; Pastore, L., Jr.; Righetti, R.F.; Yamaguti, W.P. Skeletal Muscle Wasting and Function Impairment in Intensive Care Patients With Severe COVID-19. Front. Physiol. 2021, 12, 640973. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Kjaer, M. In Vivo Investigation of Tendon Responses to Mechanical Loading. J. Musculoskelet. Neuronal Interact. 2011, 11, 115–123. [Google Scholar]
- Magnusson, S.P.; Kjaer, M. The Impact of Loading, Unloading, Ageing and Injury on the Human Tendon. J. Physiol. 2019, 597, 1283–1298. [Google Scholar] [CrossRef]
- Castro, J.; Livino de Carvalho, K.; Silva, P.E.; Fachin-Martins, E.; Babault, N.; de Cássia Marqueti, R.; Quagliotti Durigan, J.L. Intra- and Inter-Rater Reproducibility of Ultrasound Imaging of Patellar and Quadriceps Tendons in Critically Ill Patients. PLoS ONE 2019, 14, e0219057. [Google Scholar] [CrossRef]
- Cavalcante, J.G.T.; Marqueti, R.d.C.; Corrigan, P.; Sousa, A.M.M.; Bottaro, M.; Babault, N.; Geremia, J.M.; Silbernagel, K.G.; Durigan, J.L.Q. The Effects of Knee and Hip Joint Angles on Patellar Tendon Loading during Quadriceps Neuromuscular Electrical Stimulation. Transl. Sports Med. 2021, 4, 587–596. [Google Scholar] [CrossRef]
- Lieber, R.L.; Fridén, J. Functional and Clinical Significance. Muscle Nerve 2000, 23, 1647–1666. [Google Scholar] [CrossRef]
- Lambell, K.J.; Tierney, A.C.; Wang, J.C.; Nanjayya, V.; Forsyth, A.; Goh, G.S.; Vicendese, D.; Ridley, E.J.; Parry, S.M.; Mourtzakis, M.; et al. Comparison of Ultrasound-Derived Muscle Thickness with Computed Tomography Muscle Cross-Sectional Area on Admission to the Intensive Care Unit: A Pilot Cross-Sectional Study. J. Parenter. Enter. Nutr. 2021, 45, 136–145. [Google Scholar] [CrossRef]
- Karapınar, M.; Atilla Ayyıldız, V.; Ünal, M.; Fırat, T. Ultrasound Imaging of Quadriceps Muscle in Patients with Knee Osteoarthritis: The Test-Retest and Inter-Rater Reliability and Concurrent Validity of Echo Intensity Measurement. Musculoskelet. Sci. Pract. 2021, 56, 102453. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Bonfiglio, M.; Castorina, E.G.; Antoci, S.A.M. The Primacy of Ultrasound in the Assessment of Muscle Architecture: Precision, Accuracy, Reliability of Ultrasonography. Physiatrist, Radiologist, General Internist, and Family Practitioner’s Experiences. Rev. Assoc. Med. Bras. 2019, 65, 165–170. [Google Scholar] [CrossRef]
- Betz, T.; Wehrstein, M.; Preisner, F.; Bendszus, M.; Friedmann-Bette, B. Reliability and Validity of a Standardised Ultrasound Examination Protocol to Quantify Vastus Lateralis Muscle. J. Rehabil. Med. 2021, 53, jrm00212. [Google Scholar] [CrossRef]
- Kottner, J.; Audigé, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) Were Proposed. J. Clin. Epidemiol. 2011, 64, 96–106. [Google Scholar] [CrossRef]
- Blazevich, A.J.; Gill, N.D.; Zhou, S. Intra- and Intermuscular Variation in Human Quadriceps Femoris Architecture Assessed In Vivo. J. Anat. 2006, 209, 289–310. [Google Scholar] [CrossRef]
- Arts, I.M.P.; Pillen, S.; Schelhaas, H.J.; Overeem, S.; Zwarts, M.J. Normal Values for Quantitative Muscle Ultrasonography in Adults. Muscle Nerve 2010, 41, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Geremia, J.M.; Baroni, B.M.; Bini, R.R.; Lanferdini, F.J.; de Lima, A.R.; Herzog, W.; Vaz, M.A. Triceps Surae Muscle Architecture Adaptations to Eccentric Training. Front. Physiol. 2019, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Muraoka, T.; Ito, S.; Kanehisa, H.; Fukunaga, T. In Vivo Muscle Fibre Behaviour during Counter-Movement Exercise in Humans Reveals a Significant Role for Tendon Elasticity. J. Physiol. 2002, 540, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.M.M.; de Oliveira, L.F. Confiabilidade Das Medidas de Arquitetura Do Músculo Vasto Lateral Pela Ultrassonografia. Motriz Rev. Educ. Fis. 2013, 19, 217–223. [Google Scholar] [CrossRef]
- Pillen, S.; van Keimpema, M.; Nievelstein, R.A.J.; Verrips, A.; van Kruijsbergen-Raijmann, W.; Zwarts, M.J. Skeletal Muscle Ultrasonography: Visual versus Quantitative Evaluation. Ultrasound Med. Biol. 2006, 32, 1315–1321. [Google Scholar] [CrossRef]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.Y.; Verrijp, K.N.; Arts, I.M.P.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.M.; Verrips, A. Skeletal Muscle Ultrasound: Correlation Between Fibrous Tissue and Echo Intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Phadke, R.; Rawal, J.; McPhail, M.J.W.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit. Care Med. 2015, 43, 1603–1611. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample Size and Optimal Designs for Reliability Studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Ramani, S.L.; Samet, J.; Franz, C.K.; Hsieh, C.; Nguyen, C.V.; Horbinski, C.; Deshmukh, S. Musculoskeletal Involvement of COVID-19: Review of Imaging. Skelet. Radiol. 2021, 50, 1763–1773. [Google Scholar] [CrossRef]
- Belluzzi, E.; Olivotto, E.; Toso, G.; Cigolotti, A.; Pozzuoli, A.; Biz, C.; Trisolino, G.; Ruggieri, P.; Grigolo, B.; Ramonda, R.; et al. Conditioned Media from Human Osteoarthritic Synovium Induces Inflammation in a Synoviocyte Cell Line. Connect Tissue Res. 2019, 60, 136–145. [Google Scholar] [CrossRef]
- Smith, J.; Finnoff, J.T. Diagnostic and Interventional Musculoskeletal Ultrasound: Part 1. Fundamentals. PM R 2009, 1, 64–75. [Google Scholar] [CrossRef]
- Damanti, S.; Cilla, M.; Tuscano, B.; de Lorenzo, R.; Manganaro, G.; Merolla, A.; Pacioni, G.; Pomaranzi, C.; Tiraferri, V.; Martinenghi, S.; et al. Evaluation of Muscle Mass and Stiffness with Limb Ultrasound in COVID-19 Survivors. Front. Endocrinol. 2022, 13, 801133. [Google Scholar] [CrossRef]
- Kwah, L.K.; Pinto, R.Z.; Diong, J.; Herbert, R.D. Reliability and Validity of Ultrasound Measurements of Muscle Fascicle Length and Pennation in Humans: A Systematic Review. J. Appl. Physiol. 2013, 114, 761–769. [Google Scholar] [CrossRef]
- Moreau, N.G.; Teefey, S.A.; Damiano, D.L. In Vivo Muscle Architecture and Size of the Rectus Femoris and Vastus Lateralis in Children and Adolescents with Cerebral Palsy. Dev. Med. Child Neurol. 2009, 51, 800–806. [Google Scholar] [CrossRef]
- Chleboun, G.S.; Busic, A.B.; Graham, K.K.; Stuckey, H.A. Fascicle Length Change of the Human Tibialis Anterior and Vastus Lateralis during Walking. J. Orthop. Sport. Phys. Ther. 2007, 37, 372–379. [Google Scholar] [CrossRef]
- Lanferdini, F.J.; Sonda, F.C.; Paz, I.A.; Oliveira, L.Z.; Wagner Neto, E.S.; Molinari, T.; Gomes, D.C.S.; Sbruzzi, G.; Vaz, M.A. Reliability of Knee Extensor Neuromuscular Structure and Function and Functional Tests’ Performance. J. Bodyw. Mov. Ther. 2021, 27, 584–590. [Google Scholar] [CrossRef]
- Aggeloussis, N.; Giannakou, E.; Albracht, K.; Arampatzis, A. Reproducibility of Fascicle Length and Pennation Angle of Gastrocnemius Medialis in Human Gait In Vivo. Gait Posture 2010, 31, 73–77. [Google Scholar] [CrossRef]
- König, N.; Cassel, M.; Intziegianni, K.; Mayer, F. Inter-Rater Reliability and Measurement Error of Sonographic Muscle Architecture Assessments. J. Ultrasound Med. 2014, 33, 769–777. [Google Scholar] [CrossRef]
- May, S.; Locke, S.; Kingsley, M. Reliability of Ultrasonographic Measurement of Muscle Architecture of the Gastrocnemius Medialis and Gastrocnemius Lateralis. PLoS ONE 2021, 16, e0258014. [Google Scholar] [CrossRef]
- Padhiar, N.; Al-Sayeagh, H.; Chan, O.; King, J.; Maffulli, N. Pennation Angle of the Soleus in Patients with Unilateral Achilles Tendinopathy. Disabil. Rehabil. 2008, 30, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Khoshkhoo, M.; Killingback, A.; Robertson, C.J.; Adds, P.J. The Effect of Exercise on Vastus Medialis Oblique Muscle Architecture: An Ultrasound Investigation. Clin. Anat. 2016, 29, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Baston, C.M.; Gellhorn, A.C.; Hough, C.L.; Bunnell, A.E. Interrater Reliability of Quantitative Ultrasound Measures of Muscle in Critically Ill Patients. PM R 2022, 14, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, M.G.; de Almeida, A.C.; Aily, J.B.; de Noronha, M.; Mattiello, S.M. Fatty Infiltration in the Thigh Muscles in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Rheumatol. Int. 2019, 39, 627–635. [Google Scholar] [CrossRef]
- Pardo, E.; el Behi, H.; Boizeau, P.; Verdonk, F.; Alberti, C.; Lescot, T. Reliability of Ultrasound Measurements of Quadriceps Muscle Thickness in Critically Ill Patients. BMC Anesthesiol. 2018, 18, 205. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Ferretti, G.; Narici, M.V. Influence of 90-Day Simulated Microgravity on Human Tendon Mechanical Properties and the Effect of Resistive Countermeasures. J. Appl. Physiol. 2005, 98, 2278–2286. [Google Scholar] [CrossRef]
- Gellhorn, A.C.; Carlson, M.J. Inter-Rater, Intra-Rater, and Inter-Machine Reliability of Quantitative Ultrasound Measurements of the Patellar Tendon. Ultrasound Med. Biol. 2013, 39, 791–796. [Google Scholar] [CrossRef]
- Dalmau-Pastor, M.; Fargues-Polo, B.; Casanova-Martínez, D.; Vega, J.; Golanó, P. Anatomy of the Triceps Surae: A Pictorial Essay. Foot Ankle Clin. 2014, 19, 603–635. [Google Scholar] [CrossRef]
- Flandry, F.; Hommel, G. Normal Anatomy and Biomechanics of the Knee. Sports Med. Arthrosc. Rev. 2011, 19, 82–92. [Google Scholar] [CrossRef]
- Klimstra, M.; Dowling, J.; Durkin, J.L.; MacDonald, M. The Effect of Ultrasound Probe Orientation on Muscle Architecture Measurement. J. Electromyogr. Kinesiol. 2007, 17, 504–514. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Mansur, H.; Estanislau, G.; de Noronha, M.; de Marqueti, R.C.; Fachin-Martins, E.; Durigan, J.L.Q. Intra- and Inter-Rater Reliability for the Measurement of the Cross-Sectional Area of Ankle Tendons Assessed by Magnetic Resonance Imaging. Acta Radiol. 2022, 63, 481–488. [Google Scholar] [CrossRef]
- Tamborrini, G.; Marx, C.; Micheroli, R. Inter-Rater Reliability in the Classification of Supraspinatus Tendon Tears Using 3D Ultrasound—A Question of Experience? J. Ultrason. 2016, 16, 252–259. [Google Scholar] [CrossRef]
- Biz, C.; Stecco, C.; Crimì, A.; Pirri, C.; Fosser, M.; Fede, C.; Fan, C.; Ruggieri, P.; de Caro, R. Are Patellofemoral Ligaments and Retinacula Distinct Structures of the Knee Joint? An Anatomic, Histological and Magnetic Resonance Imaging Study. Int. J. Environ. Res. Public Health 2022, 19, 1110. [Google Scholar] [CrossRef]
| Total (n = 20) | |
|---|---|
| Age (years) | 46.89 (11.64) |
| Sex | |
| Male | 10 (50%) |
| Female | 10 (50%) |
| Body mass (Kg) | 82.00 (19.41) |
| Height (m) | 1.67 (0.09) |
| BMI (kg/m2) | 28.92 (5.02) |
| Education | |
| College or Higher | 18 (90%) |
| Middle school or lower | 2 (10%) |
| Comorbidities | |
| Hypertension | 8 (40%) |
| Diabetes | 4 (20%) |
| Cardiovascular disease | 2 (10%) |
| Cerebrovascular disease | 1 (5%) |
| Hyperlipidemia | 6 (30%) |
| Depression | 1 (5%) |
| Panic Syndrome | 2 (10%) |
| Anxiety | 2 (10%) |
| Asthma | 3 (15%) |
| Tumors | 1 (5%) |
| Time from symptom onset to analysis (days) | 114.25 (89.91) |
| Hospitalization—yes/no | 8/12 |
| Length of hospital stay (days) | 20.87 (20.97) |
| Length of ICU stay (days) | 14.37 (18.76) |
| Vaccine | 10 (50%) |
| Smoking history | 3 (15%) |
| Rater 1 (T1) | Rater 2 (T2) | ||||
|---|---|---|---|---|---|
| Muscles | ICC | 95% CI | ICC | 95% CI | |
| RF | Lf | 0.89 | 0.77–0.95 | 0.88 | 0.75–0.95 |
| θp | 0.90 | 0.79–0.95 | 0.92 | 0.84–0.96 | |
| Thickness | 0.99 | 0.98–0.99 | 0.99 | 0.99–0.99 | |
| Echogenicity | 0.98 | 0.97–0.99 | 0.99 | 0.99–0.99 | |
| VL | Lf | 0.89 | 0.78–0.95 | 0.92 | 0.83–0.96 |
| θp | 0.89 | 0.78–0.95 | 0.91 | 0.81–0.96 | |
| Thickness | 0.99 | 0.98–0.99 | 0.99 | 0.99–0.99 | |
| Echogenicity | 0.98 | 0.96–0.99 | 0.99 | 0.99–0.99 | |
| VM | Lf | 0.80 | 0.58–0.91 | 0.95 | 0.90–0.98 |
| θp | 0.71 | 0.39–0.87 | 0.92 | 0.85–0.97 | |
| Thickness | 0.99 | 0.99–0.99 | 0.96 | 0.99–0.99 | |
| Echogenicity | 0.99 | 0.98–0.99 | 0.99 | 0.98–0.99 | |
| TA | Lf | 0.93 | 0.85–0.97 | 0.91 | 0.82–0.96 |
| θp | 0.87 | 0.73–0.94 | 0.69 | 0.37–0.87 | |
| Thickness | 0.99 | 0.97–0.99 | 0.99 | 0.98–0.99 | |
| Echogenicity | 0.98 | 0.97–0.99 | 0.98 | 0.96–0.99 | |
| GL | Lf | 0.96 | 0.91–0.98 | 0.94 | 0.87–0.97 |
| θp | 0.94 | 0.87–0.97 | 0.95 | 0.90–0.98 | |
| Thickness | 0.99 | 0.98–0.99 | 0.97 | 0.95–0.99 | |
| Echogenicity | 0.97 | 0.93–0.98 | 0.98 | 0.96–0.99 | |
| GM | Lf | 0.94 | 0.88–0.98 | 0.97 | 0.94–0.99 |
| θp | 0.94 | 0.87–0.97 | 0.96 | 0.92–0.98 | |
| Thickness | 0.98 | 0.96–0.99 | 0.99 | 0.99–0.99 | |
| Echogenicity | 0.99 | 0.97–0.99 | 0.95 | 0.89–0.98 | |
| SO | Lf | 0.92 | 0.84–0.96 | 0.97 | 0.95–0.99 |
| θp | 0.87 | 0.74–0.94 | 0.97 | 0.94–0.99 | |
| Thickness | 0.98 | 0.96–0.99 | 0.99 | 0.98–0.99 | |
| Echogenicity | 0.99 | 0.97–0.99 | 0.99 | 0.99–0.99 | |
| PT | |||||
| 25% | CSA | 0.97 | 0.95–0.99 | 0.97 | 0.95–0.99 |
| Echogenicity | 0.95 | 0.89–0.97 | 0.97 | 0.94–0.98 | |
| 50% | CSA | 0.96 | 0.91–0.98 | 0.97 | 0.95–0.99 |
| Echogenicity | 0.97 | 0.95–0.99 | 0.98 | 0.97–0.99 | |
| 75% | CSA | 0.94 | 0.88–0.97 | 0.96 | 0.93–0.98 |
| Echogenicity | 0.92 | 0.84–0.96 | 0.98 | 0.97–0.99 | |
| Lf (ICC–95% CI) | θp | Thickness (ICC–95% CI) | Echogenicity | |||||
|---|---|---|---|---|---|---|---|---|
| (ICC–95% CI) | (ICC–95% CI) | |||||||
| RF | 0.43 | 0.42–0.77 | 0.68 | 0.21–0.87 | 0.90 | 0.77–0.96 | 0.94 | 0.85–0.97 |
| VL | 0.75 | 0.39–0.90 | 0.81 | 0.53–0.92 | 0.93 | 0.83–0.97 | 0.93 | 0.84–0.97 |
| VM | 0.86 | 0.65–0.94 | 0.86 | 0.65–0.94 | 0.91 | 0.78–0.96 | 0.95 | 0.88–0.98 |
| TA | 0.78 | 0.45–0.91 | 0.71 | 0.27–0.88 | 0.87 | 0.67–0.94 | 0.93 | 0.83–0.97 |
| GL | 0.88 | 0.71–0.95 | 0.70 | 0.25–0.88 | 0.88 | 0.71–0.95 | 0.84 | 0.61–0.94 |
| GM | 0.73 | 0.23–0.90 | 0.78 | 0.32–0.92 | 0.93 | 0.81–0.97 | 0.73 | 0.25–0.90 |
| SO | 0.85 | 0.63–0.94 | 0.40 | 0.50–0.76 | 0.79 | 0.47–0.91 | 0.82 | 0.54–0.92 |
| CSA (ICC–95% CI) | Echogenicity (ICC–95% CI) | |||||||
| PT 25% | 0.92 | 0.79–0.96 | 0.96 | 0.91–0.98 | ||||
| PT 50% | 0.91 | 0.79–0.96 | 0.72 | 0.29–0.89 | ||||
| PT 75% | 0.85 | 0.62–0.94 | 0.74 | 0.34–0.89 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus Ferreira, L.G.; de Almeida Ventura, Á.; da Silva Almeida, I.; Mansur, H.; Babault, N.; Durigan, J.L.Q.; de Cássia Marqueti, R. Intra- and Inter-Rater Reliability and Agreement of Ultrasound Imaging of Muscle Architecture and Patellar Tendon in Post-COVID-19 Patients Who Had Experienced Moderate or Severe COVID-19 Infection. J. Clin. Med. 2022, 11, 6934. https://doi.org/10.3390/jcm11236934
de Jesus Ferreira LG, de Almeida Ventura Á, da Silva Almeida I, Mansur H, Babault N, Durigan JLQ, de Cássia Marqueti R. Intra- and Inter-Rater Reliability and Agreement of Ultrasound Imaging of Muscle Architecture and Patellar Tendon in Post-COVID-19 Patients Who Had Experienced Moderate or Severe COVID-19 Infection. Journal of Clinical Medicine. 2022; 11(23):6934. https://doi.org/10.3390/jcm11236934
Chicago/Turabian Stylede Jesus Ferreira, Leandro Gomes, Álvaro de Almeida Ventura, Isabella da Silva Almeida, Henrique Mansur, Nicolas Babault, João Luiz Quagliotti Durigan, and Rita de Cássia Marqueti. 2022. "Intra- and Inter-Rater Reliability and Agreement of Ultrasound Imaging of Muscle Architecture and Patellar Tendon in Post-COVID-19 Patients Who Had Experienced Moderate or Severe COVID-19 Infection" Journal of Clinical Medicine 11, no. 23: 6934. https://doi.org/10.3390/jcm11236934
APA Stylede Jesus Ferreira, L. G., de Almeida Ventura, Á., da Silva Almeida, I., Mansur, H., Babault, N., Durigan, J. L. Q., & de Cássia Marqueti, R. (2022). Intra- and Inter-Rater Reliability and Agreement of Ultrasound Imaging of Muscle Architecture and Patellar Tendon in Post-COVID-19 Patients Who Had Experienced Moderate or Severe COVID-19 Infection. Journal of Clinical Medicine, 11(23), 6934. https://doi.org/10.3390/jcm11236934







