The Role of Platelets in the Pathogenesis and Pathophysiology of Adenomyosis
Abstract
1. Introduction
2. A Primer on Tissue Repair, Platelets, and the Coagulation Pathways
3. Role of Platelets in the Pathogenesis of Adenomyosis
4. Adenomyotic Lesions as Wounds
5. Platelets Promote Progression of Adenomyotic Lesions
5.1. Platelet-Derived Growth Factors
5.2. Serotonin
5.3. Conflicting Findings
5.4. Platelet Activation by Thrombin/Thromboxane
5.5. Platelets-Mediated Suppression of Cytotoxicity in NK Cells in Ectopic Endometrium
5.6. Platelets and Coagulation in Adenomyosis-Induced HMB
6. Platelets and Coagulation in Adenomyosis-Induced Dysmenorrhea
7. Putting Pieces Together
8. Therapeutic Implications
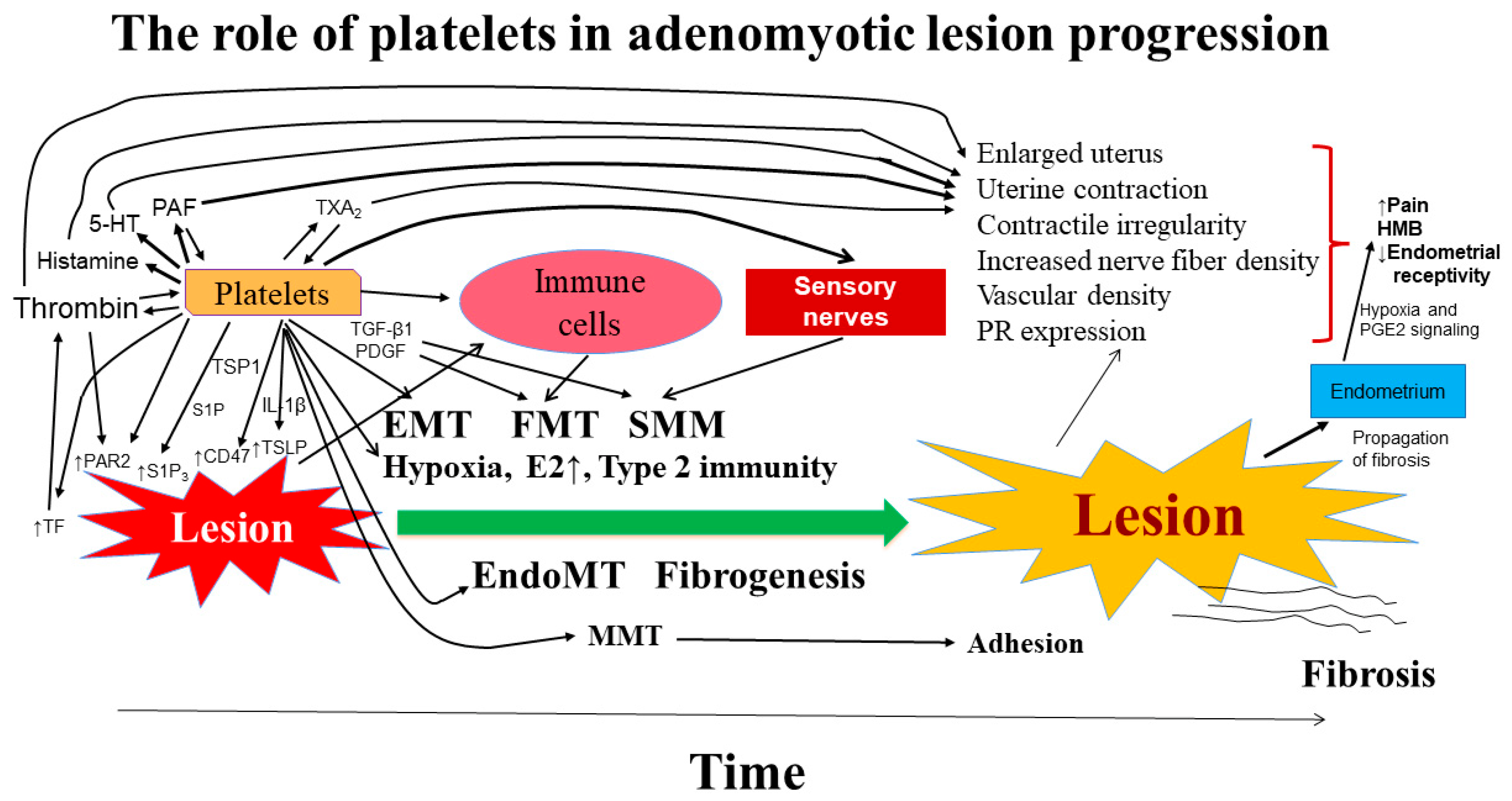
9. Summary and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Farquhar, C.; Brosens, I. Medical and surgical management of adenomyosis. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The impact of adenomyosis on women’s fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388.e1. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.C.; Sneeden, V.D. Adenomyosis: A reappraisal of symptomatology. Am. J. Obstet. Gynecol. 1958, 76, 1044–1057. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Guo, S.W. Clinical profiles of 710 premenopausal women with adenomyosis who underwent hysterectomy. J. Obstet. Gynaecol. Res. 2014, 40, 485–494. [Google Scholar] [CrossRef]
- Alcalde, A.M.; Martinez-Zamora, M.A.; Gracia, M.; Ros, C.; Rius, M.; Castelo-Branco, C.; Carmona, F. Assessment of Quality of Life, Sexual Quality of Life, and Pain Symptoms in Deep Infiltrating Endometriosis Patients With or Without Associated Adenomyosis and the Influence of a Flexible Extended Combined Oral Contraceptive Regimen: Results of a Prospective, Observational Study. J. Sex. Med. 2022, 19, 311–318. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Amano, H.; Kurozawa, Y.; Ideno, Y.; Hayashi, K.; Harada, T.; Japan, E.; Children’s Study, G. Adverse obstetrical outcomes for women with endometriosis and adenomyosis: A large cohort of the Japan Environment and Children’s Study. PLoS ONE 2019, 14, e0220256. [Google Scholar] [CrossRef]
- Kitawaki, J. Adenomyosis: The pathophysiology of an oestrogen-dependent disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 493–502. [Google Scholar] [CrossRef]
- Wu, M.Y.; Ho, H.N. The role of cytokines in endometriosis. Am. J. Reprod. Immunol. 2003, 49, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ulukus, M.; Ulukus, E.C.; Tavmergen Goker, E.N.; Tavmergen, E.; Zheng, W.; Arici, A. Expression of interleukin-8 and monocyte chemotactic protein 1 in women with endometriosis. Fertil. Steril. 2008, 91, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Igarashi, S.; Sasaki, M.; Tanaka, T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum. Reprod. 2001, 16, 561–566. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Hatazawa, J.; Tanaka, T. Immunohistochemical assessment of superoxide dismutase expression in the endometrium in endometriosis and adenomyosis. Fertil. Steril. 1999, 72, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Igarashi, S.; Hatazawa, J.; Tanaka, T. Endothelial nitric oxide synthase in the endometrium during the menstrual cycle in patients with endometriosis and adenomyosis. Fertil. Steril. 1998, 69, 303–308. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Kato, N.; Tanaka, T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2000, 74, 313–318. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2001, 75, 785–790. [Google Scholar] [CrossRef]
- Goteri, G.; Lucarini, G.; Montik, N.; Zizzi, A.; Stramazzotti, D.; Fabris, G.; Tranquilli, A.L.; Ciavattini, A. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2009, 28, 157–163. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.W. Aberrant immunoreactivity of deoxyribonucleic acid methyltransferases in adenomyosis. Gynecol. Obstet. Investig. 2012, 74, 100–108. [Google Scholar] [CrossRef]
- Liu, X.; Nie, J.; Guo, S.W. Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol. Obstet. Investig. 2012, 74, 50–55. [Google Scholar] [CrossRef]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T.; et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Luisi, S.; Tosti, C.; Sorbi, F.; Petraglia, F. Role of medical therapy in the management of uterine adenomyosis. Fertil. Steril. 2018, 109, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Groothuis, P.G. Is it time for a paradigm shift in drug research and development in endometriosis/adenomyosis? Hum. Reprod. Update 2018, 24, 577–598. [Google Scholar] [CrossRef]
- Brosens, I.A. Endometriosis—A disease because it is characterized by bleeding. Am. J. Obstet. Gynecol. 1997, 176, 263–267. [Google Scholar] [CrossRef]
- Guo, S.W. Fibrogenesis resulting from cyclic bleeding: The Holy Grail of the natural history of ectopic endometrium. Hum. Reprod. 2018, 33, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair: A showcase for cell plasticity and migration. Curr. Opin. Cell. Biol. 2016, 42, 29–37. [Google Scholar] [CrossRef]
- Cheong, Y.; Cameron, I.T.; Critchley, H.O.D. Abnormal uterine bleeding. Br. Med. Bull. 2017, 123, 103–114, Correction in Br. Med. Bull. 2019, 131, 119. [Google Scholar] [CrossRef]
- Hong, E.Y.; Lin, H.Z.; Fong, Y.F. Venous Thromboembolism and Adenomyosis: A Retrospective Review. Gynecol. Minim. Invasive. Ther. 2020, 9, 64–68. [Google Scholar] [CrossRef]
- Kim, B.; Kim, S.H.; Kim, T. Cerebral Infarcts by Nonbacterial Thrombotic Endocarditis Associated with Adenomyosis: A Case Report. J. Stroke Cerebrovasc. Dis. 2018, 27, e50–e53. [Google Scholar] [CrossRef]
- Yamashiro, K.; Tanaka, R.; Nishioka, K.; Ueno, Y.; Shimura, H.; Okuma, Y.; Hattori, N.; Urabe, T. Cerebral infarcts associated with adenomyosis among middle-aged women. J. Stroke Cerebrovasc. Dis. 2012, 21, e911–e915. [Google Scholar] [CrossRef]
- Yin, X.; Wu, J.; Song, S.; Zhang, B.; Chen, Y. Cerebral infarcts associated with adenomyosis: A rare risk factor for stroke in middle-aged women: A case series. BMC. Neurol. 2018, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, X.; Luo, F.; Shi, G.; He, Y.; Yao, Y.; Xu, L. Acute disseminated intravascular coagulation developed after dilation and curettage in an adenomyosis patient: A case report. Blood. Coagul. Fibrinolysis 2013, 24, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Aiura, R.; Nakayama, S.; Yamaga, H.; Kato, Y.; Fujishima, H. Systemic thromboembolism including multiple cerebral infarctions with middle cerebral artery occlusion caused by the progression of adenomyosis with benign gynecological tumor: A case report. BMC. Neurol. 2021, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell. Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. A J. Virtual Libr. 2008, 13, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Yun, S.H.; Sim, E.H.; Goh, R.Y.; Park, J.I.; Han, J.Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed. Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer. 2011, 11, 123–134. [Google Scholar] [CrossRef]
- Ntelis, K.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.; Daoussis, D. Platelets in Systemic Sclerosis: The Missing Link Connecting Vasculopathy, Autoimmunity, and Fibrosis? Curr. Rheumatol. Rep. 2019, 21, 15. [Google Scholar] [CrossRef]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, G.A.; Weyrich, A.S. Signal-dependent protein synthesis by activated platelets: New pathways to altered phenotype and function. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s17–s24. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Rampton, D.S. Review article: Platelets in inflammatory bowel disease—Pathogenetic role and therapeutic implications. Aliment. Pharmacol. Ther. 1997, 11, 237–247. [Google Scholar] [CrossRef]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M., 3rd; Morrell, C.N. Platelets present antigen in the context of MHC class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Solomou, E.E.; Katsanaki, K.; Kalyvioti, E.; Gizas, V.; Perperis, A.; Babali, D.; Verigou, E.; Gogos, H.; Hahalis, G.; Davlouros, P.; et al. Platelets from patients with myocardial infarction can activate T cells. Haematologica 2021, 106, 288–290. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Key, N.S.; Levy, J.H. Blood coagulation: Hemostasis and thrombin regulation. Anesth. Analg. 2009, 108, 1433–1446. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Ippolito, L.; Franchini, M.; Favaloro, E.J. Shortened activated partial thromboplastin time: Causes and management. Blood. Coagul. Fibrinolysis 2010, 21, 459–463. [Google Scholar] [CrossRef]
- Korte, W.; Clarke, S.; Lefkowitz, J.B. Short activated partial thromboplastin times are related to increased thrombin generation and an increased risk for thromboembolism. Am. J. Clin. Pathol. 2000, 113, 123–127. [Google Scholar] [CrossRef]
- Mina, A.; Favaloro, E.J.; Mohammed, S.; Koutts, J. A laboratory evaluation into the short activated partial thromboplastin time. Blood. Coagul. Fibrinolysis 2010, 21, 152–157. [Google Scholar] [CrossRef]
- Mina, A.; Favaloro, E.J.; Koutts, J. Relationship between short activated partial thromboplastin times, thrombin generation, procoagulant factors and procoagulant phospholipid activity. Blood. Coagul. Fibrinolysis 2012, 23, 203–207. [Google Scholar] [CrossRef]
- Tripodi, A.; Chantarangkul, V.; Martinelli, I.; Bucciarelli, P.; Mannucci, P.M. A shortened activated partial thromboplastin time is associated with the risk of venous thromboembolism. Blood 2004, 104, 3631–3634. [Google Scholar] [CrossRef] [PubMed]
- Madi, A.M.; Greci, L.S.; Nawaz, H.; Katz, D.L. The activated partial thromboplastin time in early diagnosis of myocardial infarction. Blood. Coagul. Fibrinolysis 2001, 12, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Guidi, G.C.; Favaloro, E.J. Epidemiological association between fasting plasma glucose and shortened APTT. Clin. Biochem. 2009, 42, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Wu, J. Diabetes mellitus is associated with shortened activated partial thromboplastin time and increased fibrinogen values. PLoS ONE 2011, 6, e16470. [Google Scholar] [CrossRef]
- Garcia-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Vannuccini, S.; Petraglia, F. Recent advances in understanding and managing adenomyosis. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021, 28, 2087–2097. [Google Scholar] [CrossRef]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod. Biomed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Update 2007, 13, 87–101. [Google Scholar] [CrossRef]
- Ferenczy, A. Pathophysiology of adenomyosis. Hum. Reprod. Update 1998, 4, 312–322. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Bottcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Laschke, M.W.; Mall, G. Archimetrosis: The evolution of a disease and its extant presentation: Pathogenesis and pathophysiology of archimetrosis (uterine adenomyosis and endometriosis). Arch. Gynecol. Obstet. 2022, 21, 1–20. [Google Scholar] [CrossRef]
- Guo, S.W. The Pathogenesis of Adenomyosis vis-a-vis Endometriosis. J. Clin. Med. 2020, 9, 485. [Google Scholar] [CrossRef]
- Wang, X.; Benagiano, G.; Liu, X.; Guo, S.W. Unveiling the Pathogenesis of Adenomyosis through Animal Models. J. Clin. Med. 2022, 11, 1744. [Google Scholar] [CrossRef]
- Curtis, K.M.; Hillis, S.D.; Marchbanks, P.A.; Peterson, H.B. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am. J. Obstet. Gynecol. 2002, 187, 543–544. [Google Scholar] [CrossRef]
- Levgur, M.; Abadi, M.A.; Tucker, A. Adenomyosis: Symptoms, histology, and pregnancy terminations. Obstet. Gynecol. 2000, 95, 688–691. [Google Scholar] [CrossRef]
- Panganamamula, U.R.; Harmanli, O.H.; Isik-Akbay, E.F.; Grotegut, C.A.; Dandolu, V.; Gaughan, J.P. Is prior uterine surgery a risk factor for adenomyosis? Obstet. Gynecol. 2004, 104, 1034–1038. [Google Scholar] [CrossRef]
- Parazzini, F.; Mais, V.; Cipriani, S.; Busacca, M.; Venturini, P.; On behalf of GISE. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: Results from a prospective multicentric study in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 143, 103–106. [Google Scholar] [CrossRef]
- Taran, F.A.; Weaver, A.L.; Coddington, C.C.; Stewart, E.A. Understanding adenomyosis: A case control study. Fertil. Steril. 2010, 94, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Liu, X.; Guo, S.W. Adenomyosis in mice resulting from mechanically or thermally induced endometrial-myometrial interface disruption and its possible prevention. Reprod. Biomed. Online 2020, 41, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, T.; Hirota, Y.; Aikawa, S.; Iida, R.; Ishizawa, C.; Kaku, T.; Hirata, T.; Fukui, Y.; Akaeda, S.; Matsuo, M.; et al. Constant Activation of STAT3 Contributes to the Development of Adenomyosis in Females. Endocrinology 2022, 163, bqac044. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, M.; Koga, K.; Hiraoka, T.; Kumasawa, K.; Maki, E.; Satake, E.; Taguchi, A.; Makabe, T.; Takeuchi, A.; Izumi, G.; et al. Establishment of a novel mouse model of adenomyosis suitable for longitudinal and quantitative analysis and perinatal outcome studies. Sci. Rep. 2022, 12, 17515. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; McElwain, J.C. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 2010, 25, 272–279. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Qi, Q.; Guo, S.-W.; Liu, X. Activated Platelets Induce Hypoxia-Inducible Factor-1α Expression Likely through Transforming Growth Factor-β1 in Human Endometrial Stromal Cells. Reprod. Dev. Med. 2019, 3, 69–76. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, X.; Zhang, Q.; Guo, S.W. Platelets induce increased estrogen production through NF-kappaB and TGF-beta1 signaling pathways in endometriotic stromal cells. Sci. Rep. 2020, 10, 1281. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, H.Y.; Huang, C.H.; Twu, N.F.; Yen, M.S.; Wang, P.H.; Chou, T.Y.; Liu, Y.N.; Chao, K.C.; Yang, M.H. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J. Pathol. 2010, 222, 261–270. [Google Scholar] [CrossRef]
- Horng, H.C.; Chang, W.H.; Yeh, C.C.; Huang, B.S.; Chang, C.P.; Chen, Y.J.; Tsui, K.H.; Wang, P.H. Estrogen Effects on Wound Healing. Int. J. Mol. Sci. 2017, 18, 2325. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. The role of estrogen in cutaneous ageing and repair. Maturitas 2017, 103, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Dodsworth, J.; van Boxtel, E.; Tarnuzzer, R.W.; Horan, M.A.; Schultz, G.S.; Ferguson, M.W. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997, 3, 1209–1215. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Mills, S.J.; Lei, K.; Gibbons, L.; Jeong, M.J.; Taniguchi, M.; Burow, M.; Horan, M.A.; Wahl, S.M.; Nakayama, T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig. 2003, 111, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Hardman, M.J.; Emmerson, E.; Campbell, L.; Ashcroft, G.S. Selective estrogen receptor modulators accelerate cutaneous wound healing in ovariectomized female mice. Endocrinology 2008, 149, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Braga, D.; Renzi, T.A.; Villa, A.; Bolego, C.; D’Avila, F.; Barlassina, C.; Maggi, A.; Locati, M.; Vegeto, E. Self-renewal and phenotypic conversion are the main physiological responses of macrophages to the endogenous estrogen surge. Sci. Rep. 2017, 7, 44270. [Google Scholar] [CrossRef]
- Mukai, K.; Nakajima, Y.; Urai, T.; Komatsu, E.; Nasruddin; Sugama, J.; Nakatani, T. 17beta-Estradiol administration promotes delayed cutaneous wound healing in 40-week ovariectomised female mice. Int. Wound J. 2016, 13, 636–644. [Google Scholar] [CrossRef]
- Holt, J.C.; Niewiarowski, S. Biochemistry of alpha granule proteins. Semin. Hematol. 1985, 22, 151–163. [Google Scholar]
- Gear, A.R.; Camerini, D. Platelet chemokines and chemokine receptors: Linking hemostasis, inflammation, and host defense. Microcirculation 2003, 10, 335–350. [Google Scholar] [CrossRef]
- Wagner, D.D.; Burger, P.C. Platelets in inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2131–2137. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Wang, W.; Wang, Y.; Zhu, F.; Wang, W. Interleukin-1beta Promotes Schwann Cells De-Differentiation in Wallerian Degeneration via the c-JUN/AP-1 Pathway. Front. Cell. Neurosci. 2019, 13, 304. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Guo, S.-W. Perioperative Suppression of Schwann Cell Dedifferentiation Reduces the Risk of Adenomyosis Resulting from Endometrial–Myometrial Interface Disruption in Mice. Biomedicines 2022, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Carrarelli, P.; Yen, C.F.; Funghi, L.; Arcuri, F.; Tosti, C.; Bifulco, G.; Luddi, A.; Lee, C.L.; Petraglia, F. Expression of Inflammatory and Neurogenic Mediators in Adenomyosis. Reprod. Sci. 2017, 24, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, M.; Liu, X.; Guo, S.W. Constitutive and tumor necrosis factor-alpha-induced activation of nuclear factor-kappaB in adenomyosis and its inhibition by andrographolide. Fertil. Steril. 2013, 100, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Lu, Y.; Liu, X.; Guo, S.W. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil. Steril. 2009, 92, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, S.H.; Cho, Y.M.; Ihm, H.J.; Oh, Y.S.; Hong, S.H.; Chae, H.D.; Kim, C.H.; Kang, B.M. Increased expression of nuclear factor kappa-B p65 subunit in adenomyosis. Obstet. Gynecol. Sci. 2016, 59, 123–129. [Google Scholar] [CrossRef]
- Propst, A.M.; Quade, B.J.; Nowak, R.A.; Stewart, E.A. Granulocyte macrophage colony-stimulating factor in adenomyosis and autologous endometrium. J. Soc. Gynecol. Investig. 2002, 9, 93–97. [Google Scholar] [CrossRef]
- Petaja, J. Inflammation and coagulation. An overview. Thromb. Res. 2011, 127, S34–S37. [Google Scholar] [CrossRef]
- Lipinski, S.; Bremer, L.; Lammers, T.; Thieme, F.; Schreiber, S.; Rosenstiel, P. Coagulation and inflammation. Molecular insights and diagnostic implications. Hamostaseologie 2011, 31, 94–102, 104. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L.; Strum, J.M. BRS. Cell Biology and Histology, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Vieira-de-Abreu, A.; Campbell, R.A.; Weyrich, A.S.; Zimmerman, G.A. Platelets: Versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012, 34, 5–30. [Google Scholar] [CrossRef]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nacher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Lai, M.D.; Lei, H.Y.; Wing, L.Y. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 2006, 147, 1278–1286. [Google Scholar] [CrossRef]
- Hastings, J.M.; Jackson, K.S.; Mavrogianis, P.A.; Fazleabas, A.T. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol. Reprod. 2006, 75, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Magnelli, V.; Reno, F. NETosis in Wound Healing: When Enough Is Enough. Cells 2021, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Munros, J.; Tassies, D.; Reverter, J.C.; Martin, L.; Perez, A.; Carmona, F.; Martinez-Zamora, M.A. Circulating Neutrophil Extracellular Traps Are Elevated in Patients With Deep Infiltrating Endometriosis. Reprod. Sci. 2019, 26, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune. Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, A.Q.; Zhu, S.; Yu, L.; Sun, J.F.; Xu, W.; Wang, X.L. Changes of coagulation function in patients with adenomyosis. Zhonghua Fu Chan Ke Za Zhi 2022, 57, 179–189. [Google Scholar] [CrossRef]
- Huang, P.; Lv, C.; Zhang, C.; Feng, H.; Li, C.; Zhang, H.; Zhao, X.; Li, M. Expression and significance of T-cell immunoglobulin mucin molecule 3 and its ligand galectin-9 in patients with adenomyosis. Gynecol. Endocrinol. 2020, 36, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, L.; Zheng, J. Expression of annexin A2 in adenomyosis and dysmenorrhea. Arch. Gynecol. Obstet. 2019, 300, 711–716. [Google Scholar] [CrossRef]
- Liu, L.X.; Wu, Y.G.; Zheng, J. Increased annexin A2 and decreased beta-catenin in adenomyosis contribute to adenomyosis-associated dysmenorrhea. Histol. Histopathol. 2017, 32, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yi, T.; Liu, R.; Bian, C.; Qi, X.; He, X.; Wang, K.; Li, J.; Zhao, X.; Huang, C.; et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol. Cell. Proteom. MCP 2012, 11, M112 017988. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Nakashima, M.; Masuzaki, H. Involvement of hepatocyte growth factor-induced epithelial-mesenchymal transition in human adenomyosis. Biol. Reprod. 2015, 92, 35. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Wernig, G.; Chen, S.Y.; Cui, L.; Van Neste, C.; Tsai, J.M.; Kambham, N.; Vogel, H.; Natkunam, Y.; Gilliland, D.G.; Nolan, G.; et al. Unifying mechanism for different fibrotic diseases. Proc. Natl. Acad. Sci. USA 2017, 114, 4757–4762. [Google Scholar] [CrossRef]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef]
- Stein, E.V.; Miller, T.W.; Ivins-O’Keefe, K.; Kaur, S.; Roberts, D.D. Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1beta Production and Activation through CD47. Sci. Rep. 2016, 6, 19684. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Suto, M.J. Thrombospondin-1 regulation of latent TGF-beta activation: A therapeutic target for fibrotic disease. Matrix. Biol. 2018, 68–69, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Wei, C.; Tang, L.; Sheng, Y.; Liu, Y.; Li, D.; Ding, D.; Qiu, J.; Zhu, X. TSP1-CD47-SIRPalpha signaling facilitates the development of endometriosis by mediating the survival of ectopic endometrium. Am. J. Reprod. Immunol. 2020, 83, e13236. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, S.; Li, Q.; Huang, Y.; Ji, M.; Jiao, X.; Yuan, M.; Wang, G. Macrophage-associated immune checkpoint CD47 blocking ameliorates endometriosis. Mol. Hum. Reprod. 2022, 28, gaac010. [Google Scholar] [CrossRef]
- Shazand, K.; Baban, S.; Prive, C.; Malette, B.; Croteau, P.; Lagace, M.; Racine, J.B.; Hugo, P. FOXO1 and c-jun transcription factors mRNA are modulated in endometriosis. Mol. Hum. Reprod. 2004, 10, 871–877. [Google Scholar] [CrossRef]
- Beste, M.T.; Pfaffle-Doyle, N.; Prentice, E.A.; Morris, S.N.; Lauffenburger, D.A.; Isaacson, K.B.; Griffith, L.G. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci. Transl. Med. 2014, 6, 222ra216. [Google Scholar] [CrossRef]
- Cinar, O.; Seval, Y.; Uz, Y.H.; Cakmak, H.; Ulukus, M.; Kayisli, U.A.; Arici, A. Differential regulation of Akt phosphorylation in endometriosis. Reprod. Biomed. Online 2009, 19, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Liu, S.; Li, J.; Wen, Z.; Li, M. 17betaE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFkappaB-dependent pathway. Mol. Cell Endocrinol. 2010, 317, 31–43. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhang, J.; Qi, Y.H.; Kong, M.; Liu, S.A.; Hu, J.J. Linc-ROR promotes endometrial cell proliferation by activating the PI3K-Akt pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2218–2225. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhao, X.; Liang, B.; Wen, Z.; Li, M. Loss of PP2A and PTEN immunoexpression coexists with survivin overexpression in adenomyosis. Reprod. Biol. 2014, 14, 200–205. [Google Scholar] [CrossRef]
- Yun, B.H.; Chon, S.J.; Choi, Y.S.; Cho, S.; Lee, B.S.; Seo, S.K. Pathophysiology of Endometriosis: Role of High Mobility Group Box-1 and Toll-Like Receptor 4 Developing Inflammation in Endometrium. PLoS ONE 2016, 11, e0148165. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Guo, S.W. Plasma High Mobility Group Box 1 (HMGB1), Osteopontin (OPN), and Hyaluronic Acid (HA) as Admissible Biomarkers for Endometriosis. Sci. Rep. 2019, 9, 9272. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, M.; Qi, Q.; Zhang, H.; Guo, S.W. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum. Reprod. 2016, 31, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, X.; Zhang, H.; Guo, S.W. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum. Reprod. 2016, 31, 355–369. [Google Scholar]
- Zhang, Q.; Duan, J.; Liu, X.; Guo, S.W. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol. Cell. Endocrinol. 2016, 428, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.W. Cellular Changes Consistent With Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, D.; Liu, X.; Guo, S.W. Activated Platelets Induce Estrogen Receptor beta Expression in Endometriotic Stromal Cells. Gynecol. Obstet. Investig. 2015, 80, 187–190. [Google Scholar] [CrossRef]
- Merlo, S.; Frasca, G.; Canonico, P.L.; Sortino, M.A. Differential involvement of estrogen receptor alpha and estrogen receptor beta in the healing promoting effect of estrogen in human keratinocytes. J. Endocrinol. 2009, 200, 189–197. [Google Scholar] [CrossRef]
- Campbell, L.; Emmerson, E.; Davies, F.; Gilliver, S.C.; Krust, A.; Chambon, P.; Ashcroft, G.S.; Hardman, M.J. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its antiinflammatory activities. J. Exp. Med. 2010, 207, 1825–1833. [Google Scholar] [CrossRef]
- Mehasseb, M.K.; Panchal, R.; Taylor, A.H.; Brown, L.; Bell, S.C.; Habiba, M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil. Steril. 2011, 95, 2228–2235.e2221. [Google Scholar] [CrossRef]
- Brandenberger, A.W.; Lebovic, D.I.; Tee, M.K.; Ryan, I.P.; Tseng, J.F.; Jaffe, R.B.; Taylor, R.N. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol. Hum. Reprod. 1999, 5, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Hirose, R.; Sakaguchi, H.; Tamaya, T. Expression of oestrogen receptor-alpha and -beta in ovarian endometriomata. Mol. Hum. Reprod. 1999, 5, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef] [PubMed]
- Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 2015, 112, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Mukai, K.; Urai, T.; Asano, K.; Nakajima, Y.; Nakatani, T. Evaluation of Effects of Topical Estradiol Benzoate Application on Cutaneous Wound Healing in Ovariectomized Female Mice. PLoS ONE 2016, 11, e0163560. [Google Scholar] [CrossRef]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef]
- Kitawaki, J.; Noguchi, T.; Amatsu, T.; Maeda, K.; Tsukamoto, K.; Yamamoto, T.; Fushiki, S.; Osawa, Y.; Honjo, H. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol. Reprod. 1997, 57, 514–519. [Google Scholar] [CrossRef]
- Ding, D.; Liu, X.; Duan, J.; Guo, S.W. Platelets are an unindicted culprit in the development of endometriosis: Clinical and experimental evidence. Hum. Reprod. 2015, 30, 812–832. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen Receptor beta Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Sztachelska, M.; Ponikwicka-Tyszko, D.; Martinez-Rodrigo, L.; Bernaczyk, P.; Palak, E.; Polchlopek, W.; Bielawski, T.; Wolczynski, S. Functional Implications of Estrogen and Progesterone Receptors Expression in Adenomyosis, Potential Targets for Endocrinological Therapy. J. Clin. Med. 2022, 11, 4407. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Prunotto, M.; Budd, D.C.; Gabbiani, G.; Meier, M.; Formentini, I.; Hartmann, G.; Pomposiello, S.; Moll, S. Epithelial-mesenchymal crosstalk alteration in kidney fibrosis. J. Pathol. 2012, 228, 131–147. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horne, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial-Mesenchymal Transition in Endometriosis-When Does It Happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef]
- Guo, S.W. Cracking the enigma of adenomyosis: An update on its pathogenesis and pathophysiology. Reproduction 2022, 164, R101–R121. [Google Scholar] [CrossRef]
- Darby, I.; Skalli, O.; Gabbiani, G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab. Investig. 1990, 63, 21–29. [Google Scholar]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell. Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, D.; Guo, S.W. Sensory nerve-derived neuropeptides accelerate the development and fibrogenesis of endometriosis. Hum. Reprod. 2019, 34, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, X.; Guo, S.W. Neuropeptides Substance P and Calcitonin Gene Related Peptide Accelerate the Development and Fibrogenesis of Endometriosis. Sci. Rep. 2019, 9, 2698. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Mi, J.W.; Zhang, H.C.; Gao, J.; Zhang, S.; Li, L.X.; Wu, M.Y.; Wang, J.M.; Huang, H. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during wound healing and scarring. J. Cosmet. Dermatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, X.; Xu, H.; Guo, S.W. Platelets induce endothelial-mesenchymal transition and subsequent fibrogenesis in endometriosis. Reprod. Biomed. Online 2020, 41, 500–517. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef]
- Duan, J.; Liu, X.; Wang, H.; Guo, S.W. The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice. Reprod. Biomed. Online 2018, 37, 254–268. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, X.; Guo, S.W. Platelets and Regulatory T Cells May Induce a Type 2 Immunity That Is Conducive to the Progression and Fibrogenesis of Endometriosis. Front. Immunol. 2020, 11, 610963. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, X.; Guo, S.W. Interleukin-33 Derived from Endometriotic Lesions Promotes Fibrogenesis through Inducing the Production of Profibrotic Cytokines by Regulatory T Cells. Biomedicines 2022, 10, 2893. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Involvement of the Wnt/beta-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS ONE 2013, 8, e76808. [Google Scholar] [CrossRef]
- Marcellin, L.; Santulli, P.; Chouzenoux, S.; Cerles, O.; Nicco, C.; Dousset, B.; Pallardy, M.; Kerdine-Romer, S.; Just, P.A.; Chapron, C.; et al. Alteration of Nrf2 and Glutamate Cysteine Ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free. Radic. Biol. Med. 2017, 110, 1–10. [Google Scholar] [CrossRef]
- Cai, X.; Shen, M.; Liu, X.; Guo, S.W. Reduced Expression of Eukaryotic Translation Initiation Factor 3 Subunit e and Its Possible Involvement in the Epithelial-Mesenchymal Transition in Endometriosis. Reprod. Sci. 2018, 25, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Shen, M.; Liu, X.; Nie, J. The Possible Role of Eukaryotic Translation Initiation Factor 3 Subunit e (eIF3e) in the Epithelial-Mesenchymal Transition in Adenomyosis. Reprod. Sci. 2019, 26, 377–385. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Duan, H.; Wang, Y.; Shen, X.; Dong, Q. Abnormal expression of connective tissue growth factor and its correlation with fibrogenesis in adenomyosis. Reprod. Biomed. Online 2021, 42, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Mehasseb, M.K.; Bell, S.C.; Pringle, J.H.; Habiba, M.A. Uterine adenomyosis is associated with ultrastructural features of altered contractility in the inner myometrium. Fertil. Steril. 2010, 93, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Streuli, I.; Santulli, P.; Chouzenoux, S.; Chapron, C.; Batteux, F. Activation of the MAPK/ERK Cell-Signaling Pathway in Uterine Smooth Muscle Cells of Women With Adenomyosis. Reprod. Sci. 2015, 22, 1549–1560. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Shen, X.; Duan, H.; Guo, Z.; Li, X.; Sun, F. The cannabinoid receptor CB1 affects the proliferation and apoptosis of adenomyotic human uterine smooth muscle cells of the junctional zone: A mechanism study. Reprod. Biol. Endocrinol. 2021, 19, 16. [Google Scholar] [CrossRef]
- Guo, S.W.; Mao, X.; Ma, Q.; Liu, X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil. Steril. 2013, 99, 231–240. [Google Scholar] [CrossRef]
- Mao, X.; Wang, Y.; Carter, A.V.; Zhen, X.; Guo, S.W. The retardation of myometrial infiltration, reduction of uterine contractility, and alleviation of generalized hyperalgesia in mice with induced adenomyosis by levo-tetrahydropalmatine (l-THP) and andrographolide. Reprod. Sci. 2011, 18, 1025–1037. [Google Scholar] [CrossRef]
- Liu, X.; Nie, J.; Guo, S.W. Elevated immunoreactivity to tissue factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Hum. Reprod. 2011, 26, 337–345. [Google Scholar] [CrossRef]
- Runic, R.; Schatz, F.; Wan, L.; Demopoulos, R.; Krikun, G.; Lockwood, C.J. Effects of norplant on endometrial tissue factor expression and blood vessel structure. J. Clin. Endocrinol. Metab. 2000, 85, 3853–3859. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G.; Lockwood, C.J.; Paidas, M.J. Tissue factor and the endometrium: From physiology to pathology. Thromb. Res. 2009, 124, 393–396. [Google Scholar] [CrossRef]
- Yang, B.; Gu, N.; Shi, S.; Zhang, C.; Chen, L.; Ouyang, J.; Lin, Y.; Sun, F.; Xu, H. Immunoreactivity of Plasminogen Activator Inhibitor 1 and Its Correlation with Dysmenorrhea and Lesional Fibrosis in Adenomyosis. Reprod. Sci. 2021, 28, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Osuga, Y.; Hirota, Y.; Yoshino, O.; Hirata, T.; Koga, K.; Taketani, Y. Proteinase-activated receptors in the endometrium and endometriosis. Front. Biosci. 2012, 4, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Weng, H.; Wang, X.; Zhou, B.; Yu, P.; Wang, Y. The role of tissue factor and protease-activated receptor 2 in endometriosis. Am. J. Reprod. Immunol. 2012, 68, 251–257. [Google Scholar] [CrossRef]
- Hirota, Y.; Osuga, Y.; Hirata, T.; Yoshino, O.; Koga, K.; Harada, M.; Morimoto, C.; Nose, E.; Yano, T.; Tsutsumi, O.; et al. Possible involvement of thrombin/protease-activated receptor 1 system in the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2005, 90, 3673–3679. [Google Scholar] [CrossRef]
- Hirota, Y.; Osuga, Y.; Hirata, T.; Harada, M.; Morimoto, C.; Yoshino, O.; Koga, K.; Yano, T.; Tsutsumi, O.; Taketani, Y. Activation of protease-activated receptor 2 stimulates proliferation and interleukin (IL)-6 and IL-8 secretion of endometriotic stromal cells. Hum. Reprod. 2005, 20, 3547–3553. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Canis, M.; Darcha, C.; Fukaya, T.; Yajima, A.; Bruhat, M.A. Increased mast cell density in peritoneal endometriosis compared with eutopic endometrium with endometriosis. Am. J. Reprod. Immunol. 1998, 40, 291–294. [Google Scholar] [CrossRef]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noel, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef]
- Saito, A.; Osuga, Y.; Yoshino, O.; Takamura, M.; Hirata, T.; Hirota, Y.; Koga, K.; Harada, M.; Takemura, Y.; Yano, T.; et al. TGF-beta1 induces proteinase-activated receptor 2 (PAR2) expression in endometriotic stromal cells and stimulates PAR2 activation-induced secretion of IL-6. Hum. Reprod. 2011, 26, 1892–1898. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Desmouliere, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound. Repair. Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth. Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, X.; Xu, H.; Guo, S.W. Mesothelial Cells Participate in Endometriosis Fibrogenesis Through Platelet-Induced Mesothelial-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2020, 105, e4124–e4127. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Osuga, Y.; Izumi, G.; Takamura, M.; Koga, K.; Nagai, M.; Harada, M.; Hirata, T.; Hirota, Y.; Yoshino, O.; et al. Interleukin-1beta stimulates the secretion of thymic stromal lymphopoietin (TSLP) from endometrioma stromal cells: Possible involvement of TSLP in endometriosis. Hum. Reprod. 2012, 27, 3028–3035. [Google Scholar] [CrossRef]
- Truchetet, M.E.; Demoures, B.; Eduardo Guimaraes, J.; Bertrand, A.; Laurent, P.; Jolivel, V.; Douchet, I.; Jacquemin, C.; Khoryati, L.; Duffau, P.; et al. Platelets Induce Thymic Stromal Lymphopoietin Production by Endothelial Cells: Contribution to Fibrosis in Human Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 2784–2794. [Google Scholar] [CrossRef]
- Eidukaite, A.; Siaurys, A.; Tamosiunas, V. Aberrant expression of CD95 and CD69 molecules among CD56 cells in women with endometriosis. Am. J. Reprod. Immunol. 2006, 55, 276–281. [Google Scholar] [CrossRef]
- Guo, M.; Bafligil, C.; Tapmeier, T.; Hubbard, C.; Manek, S.; Shang, C.; Martinez, F.O.; Schmidt, N.; Obendorf, M.; Hess-Stumpp, H.; et al. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: A characterisation study. BMC. Med. 2020, 18, 3. [Google Scholar] [CrossRef]
- Chegini, N.; Rossi, M.J.; Masterson, B.J. Platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and EGF and PDGF beta-receptors in human endometrial tissue: Localization and in vitro action. Endocrinology 1992, 130, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Overton, C.; Fernandez-Shaw, S.; Hicks, B.; Barlow, D.; Starkey, P. Peritoneal fluid cytokines and the relationship with endometriosis and pain. Hum. Reprod. 1996, 11, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kalu, E.; Sumar, N.; Giannopoulos, T.; Patel, P.; Croucher, C.; Sherriff, E.; Bansal, A. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J. Obstet. Gynaecol. Res. 2007, 33, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S.; Halme, J. Effect of platelet-derived growth factor on endometrial stromal cell proliferation in vitro: A model for endometriosis? Fertil. Steril. 1991, 56, 672–679. [Google Scholar] [CrossRef]
- Matsumoto, H.; Nasu, K.; Nishida, M.; Ito, H.; Bing, S.; Miyakawa, I. Regulation of proliferation, motility, and contractility of human endometrial stromal cells by platelet-derived growth factor. J. Clin. Endocrinol. Metab. 2005, 90, 3560–3567. [Google Scholar] [CrossRef]
- Gentilini, D.; Busacca, M.; Di Francesco, S.; Vignali, M.; Vigano, P.; Di Blasio, A.M. PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol. Hum. Reprod. 2007, 13, 317–322. [Google Scholar] [CrossRef]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine. Growth. Factor. Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef]
- Abdollahi, A.; Li, M.; Ping, G.; Plathow, C.; Domhan, S.; Kiessling, F.; Lee, L.B.; McMahon, G.; Grone, H.J.; Lipson, K.E.; et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J. Exp. Med. 2005, 201, 925–935. [Google Scholar] [CrossRef]
- Druce, M.; Rockall, A.; Grossman, A.B. Fibrosis and carcinoid syndrome: From causation to future therapy. Nat. Rev. Endocrinol. 2009, 5, 276–283. [Google Scholar] [CrossRef]
- Dees, C.; Akhmetshina, A.; Zerr, P.; Reich, N.; Palumbo, K.; Horn, A.; Jungel, A.; Beyer, C.; Kronke, G.; Zwerina, J.; et al. Platelet-derived serotonin links vascular disease and tissue fibrosis. J. Exp. Med. 2011, 208, 961–972. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, R.; Feng, Y.; Gao, W.; Bi, J.; Li, Z.; Liu, C. Serotonin Exhibits Accelerated Bleomycin-Induced Pulmonary Fibrosis through TPH1 Knockout Mouse Experiments. Mediators. Inflamm. 2018, 2018, 7967868. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Misra, D.P.; Prasad, N.; Rastogi, K.; Singh, H.; Rai, M.K.; Agarwal, V. 5-HT2 and 5-HT2B antagonists attenuate pro-fibrotic phenotype in human adult dermal fibroblasts by blocking TGF-beta1 induced non-canonical signaling pathways including STAT3: Implications for fibrotic diseases like scleroderma. Int. J. Rheum. Dis. 2018, 21, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Ryzhova, L.M.; Setola, V.; Merryman, W.D. 5-HT(2B) antagonism arrests non-canonical TGF-beta1-induced valvular myofibroblast differentiation. J. Mol. Cell. Cardiol. 2012, 53, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lofdahl, A.; Rydell-Tormanen, K.; Muller, C.; Martina Holst, C.; Thiman, L.; Ekstrom, G.; Wenglen, C.; Larsson-Callerfelt, A.K.; Westergren-Thorsson, G. 5-HT2B receptor antagonists attenuate myofibroblast differentiation and subsequent fibrotic responses in vitro and in vivo. Physiol. Rep. 2016, 4, e12873. [Google Scholar] [CrossRef]
- Mosele, S.; Stratopoulou, C.A.; Camboni, A.; Donnez, J.; Dolmans, M.M. Investigation of the role of platelets in the aetiopathogenesis of adenomyosis. Reprod. Biomed. Online 2021, 42, 826–834. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Guo, S.W. Histological and Immunohistochemical Characterization of the Similarity and Difference Between Ovarian Endometriomas and Deep Infiltrating Endometriosis. Reprod. Sci. 2018, 25, 329–340. [Google Scholar] [CrossRef]
- Lin, q.; Li, T.; Ding, S.; Yu, Q.; Zhang, X. Anemia-Associated Platelets and Plasma Prothrombin Time Increase in Patients with Adenomyosis. J. Clin. Med. 2022, 11, 4382. [Google Scholar] [CrossRef]
- Guo, S.W.; Du, Y.; Liu, X. Endometriosis-Derived Stromal Cells Secrete Thrombin and Thromboxane A2, Inducing Platelet Activation. Reprod. Sci. 2016, 23, 1044–1052. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer. 2002, 2, 850–861. [Google Scholar] [CrossRef]
- Lanier, L.L. Natural killer cell receptor signaling. Curr. Opin. Immunol. 2003, 15, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Cheent, K.; Khakoo, S.I. Natural killer cells: Integrating diversity with function. Immunology 2009, 126, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Santulli, P.; Jeljeli, M.; Vannuccini, S.; Marcellin, L.; Doridot, L.; Petraglia, F.; Batteux, F.; Chapron, C. Immunological changes associated with adenomyosis: A systematic review. Hum. Reprod. Update 2021, 27, 108–129. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Guo, S.W. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum. Reprod. 2017, 32, 794–810. [Google Scholar] [CrossRef]
- Guo, S.W.; Du, Y.; Liu, X. Platelet-derived TGF-beta1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis. Hum. Reprod. 2016, 31, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.; Kelly, R.W.; Baird, D.T.; Brenner, R.M. Regulation of human endometrial function: Mechanisms relevant to uterine bleeding. Reprod. Biol. Endocrinol. 2006, 4, S5. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S.; Committee, F.M.D. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynaecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, Y.; Shen, X.; Liu, X.; Guo, S.W. Anti-platelet therapy holds promises in treating adenomyosis: Experimental evidence. Reprod. Biol. Endocrinol. 2016, 14, 66. [Google Scholar] [CrossRef]
- Guo, S.W.; Ding, D.; Geng, J.G.; Wang, L.; Liu, X. P-selectin as a potential therapeutic target for endometriosis. Fertil. Steril. 2015, 103, 990–1000.e1008. [Google Scholar] [CrossRef]
- Guo, S.W.; Ding, D.; Liu, X. Anti-platelet therapy is efficacious in treating endometriosis induced in mouse. Reprod. Biomed. Online 2016, 33, 484–499. [Google Scholar] [CrossRef]
- Sheth, S.S.; Ray, S.S. Severe adenomyosis and CA125. J. Obstet. Gynaecol. 2014, 34, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Boosupalli, V.; Zivkovic, S.A.; Wechsler, L.R.; Gebel, J.M. High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology 2005, 64, 1944–1945. [Google Scholar] [CrossRef]
- Tas, F.; Kilic, L.; Bilgin, E.; Keskin, S.; Sen, F.; Ciftci, R.; Yildiz, I.; Yasasever, V. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2013, 23, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Winter, W.E.; Flax, S.D.; Harris, N.S. Coagulation Testing in the Core Laboratory. Lab. Med. 2017, 48, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Zwaginga, J.J.; Koomans, H.A.; Sixma, J.J.; Rabelink, T.J. Thrombus formation and platelet-vessel wall interaction in the nephrotic syndrome under flow conditions. J. Clin. Investig. 1994, 93, 204–211. [Google Scholar] [CrossRef]
- Tuktamyshov, R.; Zhdanov, R. The method of in vivo evaluation of hemostasis: Spatial thrombodynamics. Hematology 2015, 20, 584–586. [Google Scholar] [CrossRef]
- Bassus, S.; Herkert, O.; Kronemann, N.; Gorlach, A.; Bremerich, D.; Kirchmaier, C.M.; Busse, R.; Schini-Kerth, V.B. Thrombin causes vascular endothelial growth factor expression in vascular smooth muscle cells: Role of reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1550–1555. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Li, J.J.; Hu, L.; Lee, M.; Karpatkin, S. Thrombin induces increased expression and secretion of VEGF from human FS4 fibroblasts, DU145 prostate cells and CHRF megakaryocytes. Thromb. Haemost. 2001, 86, 1094–1098. [Google Scholar]
- Lockwood, C.J.; Krikun, G.; Koo, A.B.; Kadner, S.; Schatz, F. Differential effects of thrombin and hypoxia on endometrial stromal and glandular epithelial cell vascular endothelial growth factor expression. J. Clin. Endocrinol. Metab. 2002, 87, 4280–4286. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kawano, Y.; Fukuda, J.; Matsumoto, H.; Narahara, H. The production of vascular endothelial growth factor and metalloproteinase via protease-activated receptor in human endometrial stromal cells. Fertil. Steril. 2009, 91, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Babayev, S.N.; Kanchwala, M.; Xing, C.; Akgul, Y.; Carr, B.R.; Word, R.A. Thrombin Alters Human Endometrial Stromal Cell Differentiation During Decidualization. Reprod. Sci. 2019, 26, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, X.; Guo, S.W. Further Evidence for Hypercoagulability in Women With Ovarian Endometriomas. Reprod. Sci. 2018, 25, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, D.; Ren, Y.; Guo, S.W. Transvaginal Elastosonography as an Imaging Technique for Diagnosing Adenomyosis. Reprod. Sci. 2018, 25, 498–514. [Google Scholar] [CrossRef]
- Nie, J.; Zhao, C.; Lagana, A.S.; Liu, X.; Guo, S.W. Identification of lesional attributes of dysmenorrhea severity and the serum antimullerian hormone levels in women with ovarian endometriomas. Fertil. Steril. 2022, 118, 191–202. [Google Scholar] [CrossRef]
- Nishimura, F.; Mogami, H.; Moriuchi, K.; Chigusa, Y.; Mandai, M.; Kondoh, E. Mechanisms of thrombin-Induced myometrial contractions: Potential targets of progesterone. PLoS ONE 2020, 15, e0231944. [Google Scholar] [CrossRef]
- O’Sullivan, C.J.; Allen, N.M.; O’Loughlin, A.J.; Friel, A.M.; Morrison, J.J. Thrombin and PAR1-activating peptide: Effects on human uterine contractility in vitro. Am. J. Obstet. Gynecol. 2004, 190, 1098–1105. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Ascher-Landsberg, J.; Saunders, T.; Phillippe, M. The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am. J. Obstet. Gynecol. 2000, 183, 674–681. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Saunders, T.; Ascher-Landsberg, J.; Phillippe, M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am. J. Obstet. Gynecol. 2000, 183, 799–804. [Google Scholar] [CrossRef]
- Cruz, M.A.; Gonzalez, C.; Acevedo, C.G.; Sepulveda, W.H.; Rudolph, M.I. Effects of histamine and serotonin on the contractility of isolated pregnant and nonpregnant human myometrium. Gynecol. Obstet. Investig. 1989, 28, 1–4. [Google Scholar] [CrossRef]
- Martinez-Mir, M.I.; Estan, L.; Morales-Olivas, F.J.; Rubio, E. Effect of histamine and histamine analogues on human isolated myometrial strips. Br. J. Pharmacol. 1992, 107, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Cordeaux, Y.; Missfelder-Lobos, H.; Charnock-Jones, D.S.; Smith, G.C. Stimulation of contractions in human myometrium by serotonin is unmasked by smooth muscle relaxants. Reprod. Sci. 2008, 15, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Diao, M.; Ni, J.; Li, P.; Zhu, Q.; Wan, Y.L.; Ma, Y.S.; Lin, X.M. Stimulation of contractions in pregnant human myometrium is associated with 5-HT3 receptors. Int. J. Obstet. Anesth. 2016, 28, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Platelet-activating factor and pain. Biol. Pharm. Bull. 2011, 34, 1159–1162. [Google Scholar] [CrossRef]
- Simoni, J.; Simoni, G.; Lox, C.D.; McGunegle, D.E.; Feola, M. Cytokines and PAF release from human monocytes and macrophages: Effect of hemoglobin and contaminants. Artif. Cells. Blood. Substit. Immobil. Biotechnol. 1994, 22, 525–534. [Google Scholar] [CrossRef]
- Hemmings, R.; Miron, P.; Falcone, T.; Bourque, J.; Lepage, N.; Langlais, J. Platelet-activating factor acetylhydrolase activity in peritoneal fluids of women with endometriosis. Obstet. Gynecol. 1993, 81, 276–279. [Google Scholar]
- Hellman, K.M.; Yu, P.Y.; Oladosu, F.A.; Segel, C.; Han, A.; Prasad, P.V.; Jilling, T.; Tu, F.F. The Effects of Platelet-Activating Factor on Uterine Contractility, Perfusion, Hypoxia, and Pain in Mice. Reprod. Sci. 2018, 25, 384–394. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X.; Guo, S.W. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am. J. Obstet. Gynecol. 2010, 202, 346.e1–346.e8. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, B.; Zhang, H.; Ding, D.; Liu, X.; Guo, S.W. Possible Loss of GABAergic Inhibition in Mice With Induced Adenomyosis and Treatment With Epigallocatechin-3-Gallate Attenuates the Loss With Improved Hyperalgesia. Reprod. Sci. 2014, 21, 869–882. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, B.; Huang, X.; Xu, H.; Zhou, C.; Lin, J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil. Steril. 2010, 94, 730–737. [Google Scholar] [CrossRef]
- Lertvikool, S.; Sukprasert, M.; Pansrikaew, P.; Rattanasiri, S.; Weerakiet, S. Comparative study of nerve fiber density between adenomyosis patients with moderate to severe pain and mild pain. J. Med. Assoc. Thai. 2014, 97, 791–797. [Google Scholar] [PubMed]
- Zhang, X.; Lu, B.; Huang, X.; Xu, H.; Zhou, C.; Lin, J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil. Steril. 2009, 92, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cai, X.; Liu, X.; Guo, S.W. Possible involvement of neuropeptide and neurotransmitter receptors in Adenomyosis. Reprod. Biol. Endocrinol. 2021, 19, 25. [Google Scholar] [CrossRef]
- Guo, S.W.; Zhang, Q.; Liu, X. Social psychogenic stress promotes the development of endometriosis in mouse. Reprod. Biomed. Online 2017, 34, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Liu, X.; Qi, Q.; Guo, S.W. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor beta2. Hum. Reprod. 2016, 31, 2506–2519. [Google Scholar] [CrossRef]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef]
- Bourdon, M.; Oliveira, J.; Marcellin, L.; Santulli, P.; Bordonne, C.; Maitrot Mantelet, L.; Millischer, A.E.; Plu Bureau, G.; Chapron, C. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum. Reprod. 2021, 36, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.L. Platelet secretory mechanisms. Semin. Thromb. Hemost. 2004, 30, 441–450. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Wan, X.; Li, Y.; Yu, X.; Qin, Y.; Luo, Y.; Wang, F.; Huang, O. Elevated plasma levels of lysophosphatidic acid and aberrant expression of lysophosphatidic acid receptors in adenomyosis. BMC Womens Health 2017, 17, 118. [Google Scholar] [CrossRef]
- Dietze, R.; Starzinski-Powitz, A.; Scheiner-Bobis, G.; Tinneberg, H.R.; Meinhold-Heerlein, I.; Konrad, L. Lysophosphatidic acid triggers cathepsin B-mediated invasiveness of human endometriotic cells. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids 2018, 1863, 1369–1377. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, Y.; Li, Y.; Liang, Y.; Zhong, G.; Zhang, Q. Estrogen-increased SGK1 Promotes Endometrial Stromal Cell Invasion in Adenomyosis by Regulating with LPAR2. Reprod. Sci. 2022, 29, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk-Zieba, I.; Woclawek-Potocka, I.; Wasniewski, T.; Boruszewska, D.; Grycmacher, K.; Sinderewicz, E.; Staszkiewicz, J.; Wolczynski, S. LPAR2 and LPAR4 are the Main Receptors Responsible for LPA Actions in Ovarian Endometriotic Cysts. Reprod. Sci. 2019, 26, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Waldegger, S.; Klingel, K.; Barth, P.; Sauter, M.; Rfer, M.L.; Kandolf, R.; Lang, F. h-sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor beta in human intestine. Gastroenterology 1999, 116, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Schmid, E.; Russo, A.; Schmidt, E.M.; Burk, O.; Munzer, P.; Velic, A.; Macek, B.; Schaller, M.; Schwab, M.; et al. Impact of the serum- and glucocorticoid-inducible kinase 1 on platelet dense granule biogenesis and secretion. J. Thromb. Haemost. 2015, 13, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Boucharaba, A.; Serre, C.M.; Gres, S.; Saulnier-Blache, J.S.; Bordet, J.C.; Guglielmi, J.; Clezardin, P.; Peyruchaud, O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Investig. 2004, 114, 1714–1725. [Google Scholar] [CrossRef]
- Vannuzzi, V.; Bernacchioni, C.; Muccilli, A.; Castiglione, F.; Nozzoli, F.; Vannuccini, S.; Capezzuoli, T.; Ceccaroni, M.; Bruni, P.; Donati, C.; et al. Sphingosine 1-phosphate pathway is dysregulated in adenomyosis. Reprod. Biomed. Online 2022, 45, 15–18. [Google Scholar] [CrossRef]
- Yoshino, O.; Yamada-Nomoto, K.; Kano, K.; Ono, Y.; Kobayashi, M.; Ito, M.; Yoneda, S.; Nakashima, A.; Shima, T.; Onda, T.; et al. Sphingosine 1 Phosphate (S1P) Increased IL-6 Expression and Cell Growth in Endometriotic Cells. Reprod. Sci. 2019, 26, 1460–1467. [Google Scholar] [CrossRef]
- Ono, Y.; Kawakita, T.; Yoshino, O.; Sato, E.; Kano, K.; Ohba, M.; Okuno, T.; Ito, M.; Koga, K.; Honda, M.; et al. Sphingosine 1-Phosphate (S1P) in the Peritoneal Fluid Skews M2 Macrophage and Contributes to the Development of Endometriosis. Biomedicines 2021, 9, 1519. [Google Scholar] [CrossRef]
- Bernacchioni, C.; Capezzuoli, T.; Vannuzzi, V.; Malentacchi, F.; Castiglione, F.; Cencetti, F.; Ceccaroni, M.; Donati, C.; Bruni, P.; Petraglia, F. Sphingosine 1-phosphate receptors are dysregulated in endometriosis: Possible implication in transforming growth factor beta-induced fibrosis. Fertil. Steril. 2021, 115, 501–511. [Google Scholar] [CrossRef]
- Takeya, H.; Gabazza, E.C.; Aoki, S.; Ueno, H.; Suzuki, K. Synergistic effect of sphingosine 1-phosphate on thrombin-induced tissue factor expression in endothelial cells. Blood 2003, 102, 1693–1700. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Guo, S.W. Changing prostaglandin E2 (PGE2) signaling during lesional progression and exacerbation of endometriosis by inhibition of PGE2 receptor EP2 and EP4. Reprod. Med. Biol. 2022, 21, e12426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Guo, S.W. Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin E2 signaling. Reprod. Med. Biol. 2022, 21, e12423. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Cai, X.; Zheng, H.; Guo, S.W.; Liu, X. Scutellarin Suppresses Platelet Aggregation and Stalls Lesional Progression in Mouse With Induced Endometriosis. Reprod. Sci. 2019, 26, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X.; Guo, S.W. Therapeutic potential of andrographolide for treating endometriosis. Hum. Reprod. 2012, 27, 1300–1313. [Google Scholar] [CrossRef]
- Luo, M.; Cai, X.; Yan, D.; Liu, X.; Guo, S.W. Sodium tanshinone IIA sulfonate restrains fibrogenesis through induction of senescence in mice with induced deep endometriosis. Reprod. Biomed. Online 2020, 41, 373–384. [Google Scholar] [CrossRef]
- Nagira, K.; Taniguchi, F.; Nakamura, K.; Tokita, Y.; Tsuchiya, N.; Khine, Y.M.; Harada, T. Tokishakuyakusan, a Kampo medicine, attenuates endometriosis-like lesions and hyperalgesia in murine with endometriosis-like symptoms. Am. J. Reprod. Immunol. 2019, 82, e13182. [Google Scholar] [CrossRef]
- Lee, H.W.; Jun, J.H.; Kil, K.J.; Ko, B.S.; Lee, C.H.; Lee, M.S. Herbal medicine (Danggui Shaoyao San) for treating primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2016, 85, 19–26. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, B.; Zhang, H.; Liu, X.; Guo, S.W. Epigallocatechin-3-gallate reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice induced with adenomyosis. Reprod. Sci. 2013, 20, 1478–1491. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, Y.; Zhang, H.; Liu, X.; Guo, S.W. Resveratrol Reduces Myometrial Infiltration, Uterine Hyperactivity, and Stress Levels and Alleviates Generalized Hyperalgesia in Mice With Induced Adenomyosis. Reprod. Sci. 2015, 22, 1336–1349. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X. Quercetin alleviates generalized hyperalgesia in mice with induced adenomyosis. Mol. Med. Rep. 2017, 16, 5370–5376. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X. Leonurine Attenuates Hyperalgesia in Mice with Induced Adenomyosis. Med. Sci. Monit. 2017, 23, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, Y.; Guo, M.; Zhang, C.; Huang, L.; Pan, Q.; Lin, T.; Lu, Y.; Shen, X.; Zhang, H. Berberine attenuates hyperalgesia in mice with adenomyosis. Arch. Gynecol. Obstet. 2022, 3016, 115–125. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.W. Valproic acid alleviates generalized hyperalgesia in mice with induced adenomyosis. J. Obstet. Gynaecol. Res. 2011, 37, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zou, Y.; Wan, L.H.; Wang, L.Q.; Huang, M.Z.; Wu, J.; Zhu, Y.B.; Huang, O.P. Tanshinone IIA inhibits the proliferation, migration and invasion of ectopic endometrial stromal cells of adenomyosis via 14-3-3zeta downregulation. Arch. Gynecol. Obstet. 2015, 292, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, S.W. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 198–203. [Google Scholar] [CrossRef]
- Li, Y.D.; Ye, B.Q.; Zheng, S.X.; Wang, J.T.; Wang, J.G.; Chen, M.; Liu, J.G.; Pei, X.H.; Wang, L.J.; Lin, Z.X.; et al. NF-kappaB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J. Biol. Chem. 2009, 284, 4473–4483. [Google Scholar] [CrossRef]
- Paul, M.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free. Radic. Biol. Med. 2019, 130, 196–205. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Giovannini, L.; Giannessi, D.; Migliori, M.; Bernini, W.; Fregoni, M.; Bertelli, A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 1995, 17, 1–3. [Google Scholar]
- Beretz, A.; Cazenave, J.P.; Anton, R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: Structure-activity relationships. Agents. Actions. 1982, 12, 382–387. [Google Scholar] [CrossRef]
- Zhang, R.H.; Liu, Z.K.; Yang, D.S.; Zhang, X.J.; Sun, H.D.; Xiao, W.L. Phytochemistry and pharmacology of the genus Leonurus: The herb to benefit the mothers and more. Phytochemistry 2018, 147, 167–183. [Google Scholar] [CrossRef]
- Maione, F.; De Feo, V.; Caiazzo, E.; De Martino, L.; Cicala, C.; Mascolo, N. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J. Ethnopharmacol. 2014, 155, 1236–1242. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, L.; Mi, S.; Song, N.; Zhang, W.; Zhong, M. Tanshinone IIA prevents platelet activation and down-regulates CD36 and MKK4/JNK2 signaling pathway. BMC. Cardiovasc. Disord. 2020, 20, 81. [Google Scholar] [CrossRef]
- Davidson, D.C.; Hirschman, M.P.; Spinelli, S.L.; Morrell, C.N.; Schifitto, G.; Phipps, R.P.; Maggirwar, S.B. Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals. J. Immunol. 2011, 186, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.S.; Burridge, J. Danazol treatment and platelet function. Med. J. Aust. 1980, 1, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Zhang, Y.; Guo, S.W. Valproic acid and progestin inhibit lesion growth and reduce hyperalgesia in experimentally induced endometriosis in rats. Reprod. Sci. 2012, 19, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, S.W. A pilot study on the off-label use of valproic acid to treat adenomyosis. Fertil. Steril. 2008, 89, 246–250. [Google Scholar] [CrossRef]
- Liu, X.; Yu, S.; Guo, S.-W. A pilot study on the use of andrographolide to treat symptomatic adenomyosis. Gynecol. Minim. Invasive. Ther. 2014, 3, 119–126. [Google Scholar] [CrossRef]
- Igarashi, M.; Abe, Y.; Fukuda, M.; Ando, A.; Miyasaka, M.; Yoshida, M.; Shawki, O.A. Novel conservative medical therapy for uterine adenomyosis with a danazol-loaded intrauterine device. Fertil. Steril. 2000, 74, 412–413. [Google Scholar] [CrossRef]
- Guo, S.W. An overview of the current status of clinical trials on endometriosis: Issues and concerns. Fertil. Steril. 2014, 101, 183–190.e4. [Google Scholar] [CrossRef]
- Ludwig, N.; Hilger, A.; Zarbock, A.; Rossaint, J. Platelets at the Crossroads of Pro-Inflammatory and Resolution Pathways during Inflammation. Cells 2022, 11, 1957. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.-W. The Role of Platelets in the Pathogenesis and Pathophysiology of Adenomyosis. J. Clin. Med. 2023, 12, 842. https://doi.org/10.3390/jcm12030842
Guo S-W. The Role of Platelets in the Pathogenesis and Pathophysiology of Adenomyosis. Journal of Clinical Medicine. 2023; 12(3):842. https://doi.org/10.3390/jcm12030842
Chicago/Turabian StyleGuo, Sun-Wei. 2023. "The Role of Platelets in the Pathogenesis and Pathophysiology of Adenomyosis" Journal of Clinical Medicine 12, no. 3: 842. https://doi.org/10.3390/jcm12030842
APA StyleGuo, S.-W. (2023). The Role of Platelets in the Pathogenesis and Pathophysiology of Adenomyosis. Journal of Clinical Medicine, 12(3), 842. https://doi.org/10.3390/jcm12030842





