Diurnal Oscillations of Fibrinolytic Parameters in Patients with Acute Myocardial Infarction and Their Relation to Platelet Reactivity: Preliminary Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Design
2.2. Sample Preparation and Laboratory Tests
2.3. Platelet Aggregation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Maemura, K.; Takeda, N.; Nagai, R. Circadian rhythms in the CNS and peripheral clock disorders: Role of the biological clock in cardiovascular diseases. J. Pharmacol. Sci. 2007, 103, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Salvagno, G.L.; Lippi, G. Circadian variation within hemostasis: An underrecognized link between biology and disease? Semin. Thromb. Hemost. 2009, 35, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, M.; Bertolucci, C.; Portaluppi, F.; Colognesi, I.; Frigato, E.; Foà, A.; Bernardi, F. Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Boinska, J.; Koziński, M.; Kasprzak, M.; Ziołkowska, K.; Dziembowska, I.; Ziołkowski, M.; Kubica, J.; Rość, D. Diurnal variations in tissue factor and tissue factor pathway inhibitor concentrations in relation to on-treatment platelet reactivity: An analysis of patients with acute myocardial infarction. Platelets 2020, 31, 877–883. [Google Scholar] [CrossRef]
- Shimizu, T.; Uematsu, M.; Yoshizaki, T.; Obata, J.E.; Nakamura, T.; Fujioka, D.; Watanabe, K.; Watanabe, Y.; Kugiyama, K. Myocardial Production of Plasminogen Activator Inhibitor-1 is Associated with Coronary Endothelial and Ventricular Dysfunction after Acute Myocardial Infarction. J. Atheroscler. Thromb. 2016, 23, 557–566. [Google Scholar] [CrossRef]
- Neergaard-Petersen, S.; Larsen, S.B.; Grove, E.L.; Kristensen, S.D.; Ajjan, R.A.; Hvas, A.M. Imbalance between Fibrin Clot Formation and Fibrinolysis Predicts Cardiovascular Events in Patients with Stable Coronary Artery Disease. Thromb. Haemost. 2020, 120, 75–82. [Google Scholar] [CrossRef]
- Ząbczyk, M.; Natorska, J.; Undas, A. Fibrin Clot Properties in Atherosclerotic Vascular Disease: From Pathophysiology to Clinical Outcomes. J. Clin. Med. 2021, 10, 2999. [Google Scholar] [CrossRef]
- Bharadwaj, A.G.; Holloway, R.W.; Miller, V.A.; Waisman, D.M. Plasmin and Plasminogen System in the Tumor Microenvironment: Implications for Cancer Diagnosis, Prognosis, and Therapy. Cancers 2021, 13, 1838. [Google Scholar] [CrossRef]
- Yepes, M. The uPA/uPAR system in astrocytic wound healing. Neural Regen. Res. 2022, 17, 2404–2406. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.B.; Whyte, C.S.; Mutch, N.J. A Serpin With a Finger in Many PAIs: PAI-1’s Central Function in Thromboinflammation and Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 653655. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.L.; Houng, A.K.; Singh, S.; Wang, D. α2-Antiplasmin: New Insights and Opportunities for Ischemic Stroke. Semin. Thromb. Hemost. 2017, 43, 191–199. [Google Scholar] [PubMed]
- Miszta, A.; Huskens, D.; Donkervoort, D.; Roberts, M.J.M.; Wolberg, A.S.; de Laat, B. Assessing Plasmin Generation in Health and Disease. Int. J. Mol. Sci. 2021, 22, 2758. [Google Scholar] [CrossRef] [PubMed]
- Jovicić, A.; Ivanisević, V.; Nikolajević, R. Circadian variations of platelet aggregability and fibrinolytic activity in patients with ischemic stroke. Thromb. Res. 1991, 64, 487–491. [Google Scholar] [CrossRef]
- Andreotti, F.; Kluft, C. Circadian variation of fibrinolytic activity in blood. Chronobiol. Int. 1991, 8, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.B.; McLaren, M.; Scott, N.A.; Pringle, T.H.; McNeill, G.P.; Belch, J.J. Circadian variation of tissue plasminogen activator and its inhibitor, von willebrand factor antigen, and prostacyclin stimulating factor in men with ischaemic heart disease. Br. Heart J. 1993, 69, 121–124. [Google Scholar] [CrossRef][Green Version]
- Budkowska, M.; Lebiecka, A.; Marcinowska, Z.; Woźniak, J.; Jastrzębska, M.; Dołęgowska, B. The circadian rhythm of selected parameters of the hemostasis system in healthy people. Thromb. Res. 2019, 182, 79–88. [Google Scholar] [CrossRef]
- West, A.S.; Schønsted, I.; Iversen, K.H. Impact of the circadian clock on fibrinolysis and coagulation in healthy individuals and cardiovascular patients—A systematic review. Thromb. Res. 2021, 207, 75–84. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Myat, A.; Kubica, J.; Tantry, U.S. State of the art: Oral antiplatelet therapy. JRSM Cardiovasc. Dis. 2016, 5, 2048004016652514. [Google Scholar] [CrossRef]
- Koziński, M.; Bielis, L.; Wiśniewska-Szmyt, J.; Sukiennik, A.; Grabczewska, Z.; Swiatkiewicz, I.; Ziołkowski, M.; Rość, D.; Kubica, J. Increased morning ADP-dependent platelet aggregation persists despite dual antiplatelet therapy in patients with first ST-segment elevation myocardial infarction: Preliminary report. Cardiol. J. 2008, 15, 530–536. [Google Scholar] [PubMed]
- Kozinski, M.; Bielis, L.; Wisniewska-Szmyt, J.; Boinska, J.; Stolarek, W.; Marciniak, A.; Kubica, A.; Grabczewska, Z.; Navarese, E.P.; Andreotti, F.; et al. Diurnal variation in platelet inhibition by clopidogrel. Platelets 2011, 22, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Angleton, P.; Chandler, W.L.; Schmer, G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 1989, 79, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; Pott, A.; Yegnanarayan, R. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 levels in acute myocardial infarction. Pathophysiol. Haemost. Thromb. 2002, 32, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.B.; McLaren, M.; Saniabadi, A.; Fisher, T.C.; Belch, J.J. Circadian variation of endothelial cell function, red blood cell deformability and dehydro-thromboxane B2 in healthy volunteers. Blood Coagul. Fibrinolysis 1991, 2, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.R.; Rumley, A.; Lowe, G.D.O.; Strachan, D.P. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation 2007, 115, 996–1003. [Google Scholar] [CrossRef]
- Collet, J.P.; Montalescot, G.; Vicaut, E.; Ankri, A.; Walylo, F.; Lesty, C.; Choussat, R.; Beygui, F.; Borentain, M.; Vignolles, N.; et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation 2003, 108, 391–394. [Google Scholar] [CrossRef]
- Rapold, H.J.; Grimaudo, V.; Declerck, P.J.; Kruithof, E.K.; Bachmann, F. Plasma levels of plasminogen activator inhibitor type 1, beta-thromboglobulin, and fibrinopeptide A before, during, and after treatment of acute myocardial infarction with alteplase. Blood 1991, 78, 1490–1495. [Google Scholar] [CrossRef]
- Katsaros, K.M.; Speidl, W.S.; Kastl, S.P.; Zorn, G.; Huber, K.; Maurer, G.; Glogar, D.; Wojta, J.; Christ, G. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug-eluting stents. J. Thromb. Haemost. 2008, 6, 508–513. [Google Scholar] [CrossRef]
- Kapiotis, S.; Jilma, B.; Quehenberger, P.; Ruzicka, K.; Handler, S.; Speiser, W. Morning hypercoagulability and hypofibrinolysis. Diurnal variations in circulating activated factor VII, prothrombin fragment F1+2, and plasmin-plasmin inhibitor complex. Circulation 1997, 96, 19–30. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. Recent advances in circadian rhythms in cardiovascular system. Front. Pharmacol. 2015, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Zhang, S.; Zhao, L.; Jin, X.; Kim, W.; Cheng, X.W. Circadian and seasonal variation in onset of acute myocardial infarction. Medicine 2022, 101, e29839. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Kazama, M.; Tahara, C.; Shimazu, C.; Otake, J.; Kamei, K.; Nakatake, T.; Sakurai, N.; Yasumuro, Y.; Suzuki, S.; et al. Reference values of hemostasis related factors of healthy Japanese adults. I: Circadian fluctuation. Thromb. Res. 1990, 60, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.L.; Harpel, P.C.; Harpel, J.G.; Recht, P.A. Inhibition of tissue plasminogen activator activity by aspirin in vivo and its relationship to levels of tissue plasminogen activator inhibitor antigen, plasminogen activator and their complexes. Blood 1989, 74, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Geppert, A.; Graf, S.; Beckmann, R.; Hornykewycz, S.; Schuster, E.; Binder, B.R.; Huber, K. Concentration of endogenous tPA antigen in coronary artery disease: Relation to thrombotic events, aspirin treatment, hyperlipidemia, and multivessel disease. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1634–1642. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zorio, E.; Gilabert-Estellés, J.; España, F.; Ramón, L.A.; Cosín, R.; Estellés, A. Fibrinolysis: The key to new pathogenetic mechanisms. Curr. Med. Chem. 2008, 15, 923–929. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef]
- Zhao, L.; Gray, L.; Leonardi-Bee, J.; Weaver, C.S.; Heptinstall, S.; Bath, P.M.W. Effect of aspirin, clopidogrel and dipyridamole on soluble markers of vascular function in normal volunteers and patients with prior ischaemic stroke. Platelets 2006, 17, 100–104. [Google Scholar] [CrossRef]
- Sakata, T.; Kario, K. Antiplatelet therapy effectively reduces plasma plasminogen activator inhibitor-1 levels. Atherosclerosis 2011, 214, 490–491. [Google Scholar] [CrossRef]
- Fox, S.C.; May, J.A.; Dovlatova, N.; Glenn, J.R.; Johnson, A.; White, A.E.; Radhakrishnan, A.; Heptinstall, S. How does measurement of platelet P-selectin compare with other methods of measuring platelet function as a means of determining the effectiveness of antiplatelet therapy? Platelets 2019, 30, 290–295. [Google Scholar] [CrossRef]
- Ostrowska, M.; Kubica, J.; Adamski, P.; Kubica, A.; Eyileten, C.; Postula, M.; Toma, A.; Hengstenberg, A.; Siller-Matula, J.M. Stratified Approaches to Antiplatelet Therapies Based on Platelet Reactivity Testing. Front. Cardiovasc. Med. 2019, 6, 176. [Google Scholar] [CrossRef] [PubMed]
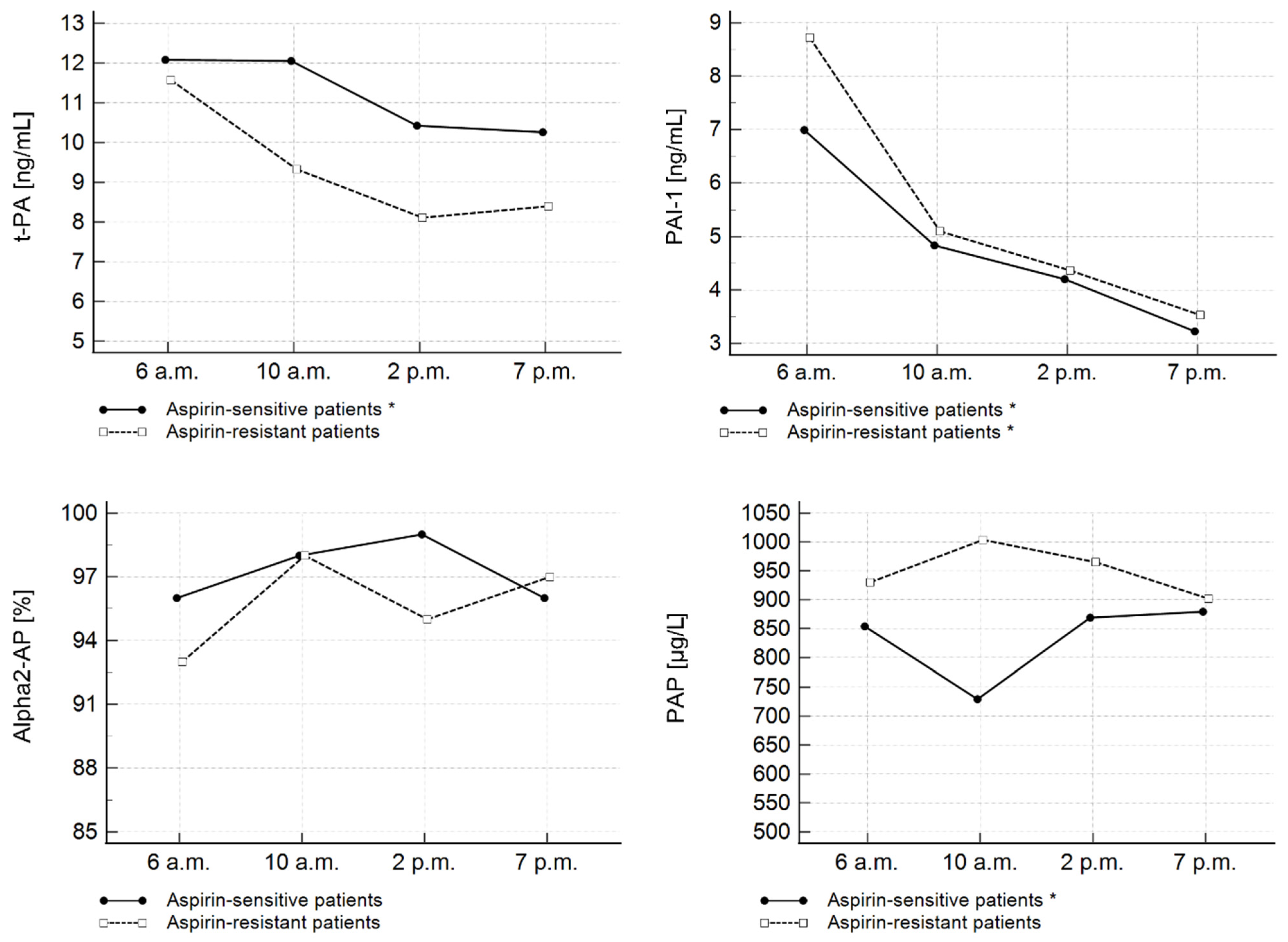
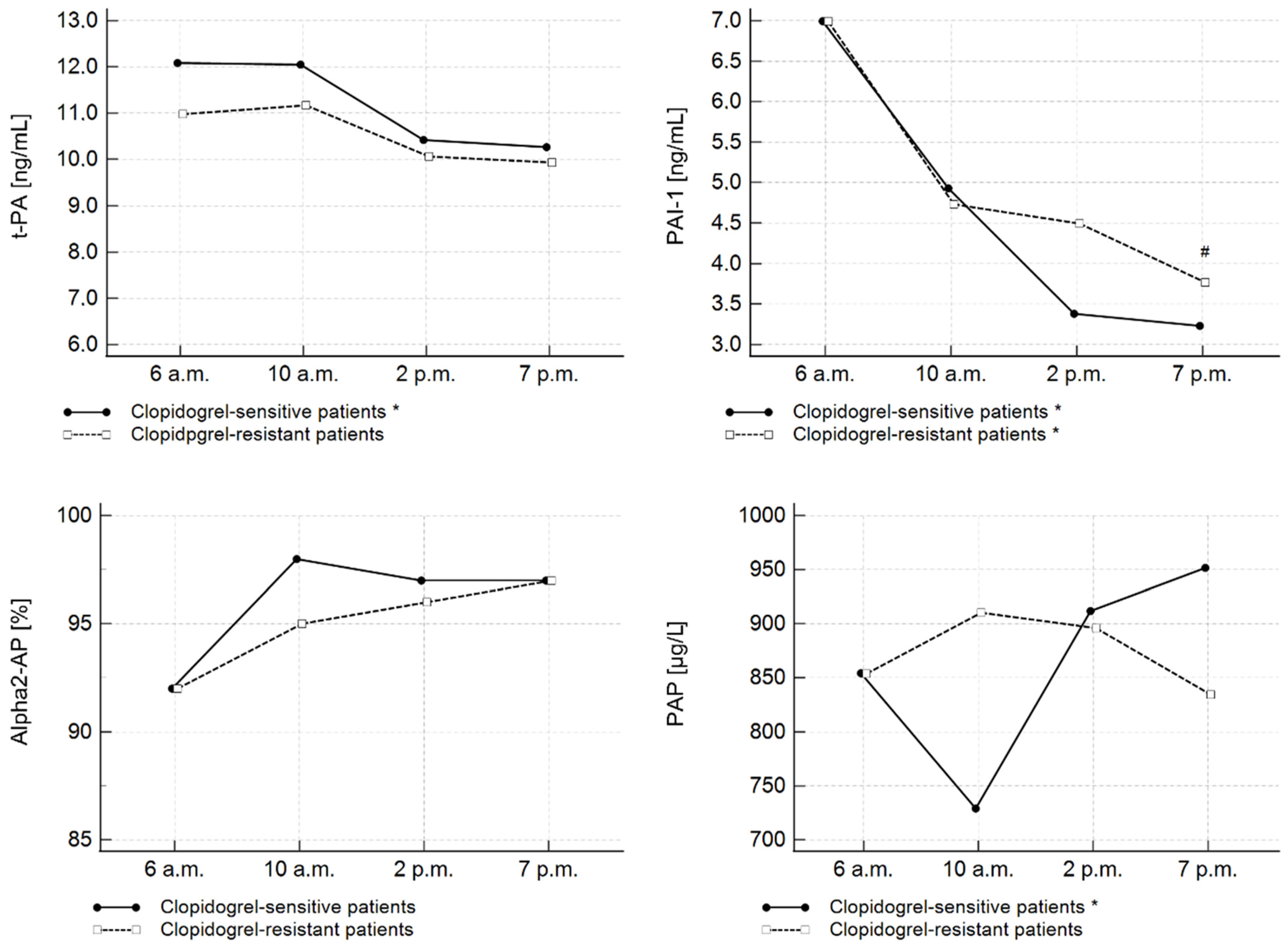
| Entire Study Cohort | Aspirin-Sensitive Patients | Aspirin-Resistant Patients | p | Clopidogrel-Sensitive Patients | Clopidogrel-Resistant Patients | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 30 | n = 22 | n = 8 | n = 18 | n = 12 | |||||||||
| n | % | n | % | n | % | n | % | n | % | ||||
| Sex | |||||||||||||
| female | 11 | 37% | 10 | 45% | 1 | 12.5% | 0.0976 | 7 | 39% | 4 | 33.3% | 0.7570 | |
| male | 19 | 63% | 12 | 55% | 7 | 87.5% | 11 | 61% | 8 | 66.7% | |||
| History of smoking | 14 | 47% | 10 | 45% | 5 | 62.5% | 0.4089 | 9 | 50% | 6 | 50% | 1.000 | |
| Hypertension | 22 | 73% | 15 | 68% | 6 | 75% | 0.7185 | 11 | 61% | 10 | 83% | 0.1931 | |
| Diabetes mellitus | 13 | 43% | 9 | 41% | 3 | 37.5% | 0.8661 | 7 | 39% | 5 | 42% | 0.8790 | |
| M | SD | M | SD | M | SD | M | SD | M | SD | ||||
| Age (years) | 57.5 | 9.5 | 57.1 | 7.6 | 58.7 | 14 | 0.9257 | 55.2 | 9.4 | 61 | 8.9 | 0.0687 | |
| BMI (kg/m2) | 28.1 | 4.2 | 27.7 | 3.73 | 29.1 | 5.4 | 0.6390 | 27.2 | 3.2 | 29.3 | 5.2 | 0.3302 | |
| Time from symptom onset (h) | 5.1 | 4.4 | 4.9 | 2.6 | 5.6 | 7.6 | 0.2515 | 5.8 | 5.4 | 4.1 | 2.2 | 0.4924 | |
| CK-MBmax (U/L) | 165 | 204 | 167 | 212 | 159 | 193 | 0.9625 | 184 | 219 | 136 | 184 | 0.8822 | |
| PLT (109/L) | 248 | 96 | 245 | 92 | 258 | 112 | 0.9252 | 225 | 84 | 283 | 106 | 0.0625 | |
| MPV (fL) | 11.1 | 1.1 | 10.9 | 1 | 11.5 | 1.2 | 0.1971 | 11.1 | 1 | 11 | 1.2 | 0.7189 | |
| TCH (mg/dL) | 210 | 46 | 214 | 47 | 201 | 4 | 0.4252 | 209 | 48 | 213 | 46 | 0.6566 | |
| HDL-CH (mg/dL) | 39 | 12 | 40 | 13 | 35 | 9 | 0.3021 | 41 | 13 | 35 | 9 | 0.1275 | |
| LDL-CH (mg/dL) | 149 | 40 | 150 | 40 | 145 | 4 | 0.7250 | 147 | 42 | 152 | 38 | 0.7508 | |
| TG (mg/dL) | 108 | 56 | 110 | 56 | 104 | 59 | 0.7964 | 92 | 46 | 133 | 61 | 0.0292 | |
| HsCRP (mg/L) | 12.9 | 10.9 | 12.0 | 10.6 | 16.8 | 14 | 0.6865 | 12.6 | 12.4 | 13.2 | 10 | 0.7928 | |
| 3rd Day After AMI (n = 30) | Friedman’s ANOVA p-Value | Dunn’s Test p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 a.m. | 10 a.m. | 2 p.m. | 7 p.m. | |||||||
| Me | IQR | Me | IQR | Me | IQR | Me | IQR | |||
| t-PA (ng/mL) | 12.09 | 10.43–14.66 | 12.05 | 9.28–15.51 | 10.42 | 7.27–13.74 | 10.26 | 7.58–11.21 | <0.001 | 1 vs. 4 |
| <0.05 | ||||||||||
| 2 vs. 4 | ||||||||||
| <0.05 | ||||||||||
| PAI-1 (ng/mL) | 8.95 | 4.43–12.13 | 5.38 | 3.64–9.79 | 4.78 | 2.46–6.36 | 3.22 | 1.75–5.08 | <0.001 | 1 vs. 3 |
| <0.05 | ||||||||||
| 1 vs. 4 | ||||||||||
| <0.05 | ||||||||||
| 2 vs. 4 | ||||||||||
| <0.05 | ||||||||||
| α2-AP (%) | 97 | 84–100 | 98 | 88–107 | 100 | 80–123 | 99 | 90–110 | 0.294 | N/A |
| PAP (µg/L) | 854.57 | 639.75–8938.63 | 817.47 | 639.75–1085.12 | 897.40 | 654.15–1055.82 | 855.06 | 693.04–1245.83 | 0.521 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boinska, J.; Koziński, M.; Kasprzak, M.; Ziołkowski, M.; Kubica, J.; Rość, D. Diurnal Oscillations of Fibrinolytic Parameters in Patients with Acute Myocardial Infarction and Their Relation to Platelet Reactivity: Preliminary Insights. J. Clin. Med. 2022, 11, 7105. https://doi.org/10.3390/jcm11237105
Boinska J, Koziński M, Kasprzak M, Ziołkowski M, Kubica J, Rość D. Diurnal Oscillations of Fibrinolytic Parameters in Patients with Acute Myocardial Infarction and Their Relation to Platelet Reactivity: Preliminary Insights. Journal of Clinical Medicine. 2022; 11(23):7105. https://doi.org/10.3390/jcm11237105
Chicago/Turabian StyleBoinska, Joanna, Marek Koziński, Michał Kasprzak, Michał Ziołkowski, Jacek Kubica, and Danuta Rość. 2022. "Diurnal Oscillations of Fibrinolytic Parameters in Patients with Acute Myocardial Infarction and Their Relation to Platelet Reactivity: Preliminary Insights" Journal of Clinical Medicine 11, no. 23: 7105. https://doi.org/10.3390/jcm11237105
APA StyleBoinska, J., Koziński, M., Kasprzak, M., Ziołkowski, M., Kubica, J., & Rość, D. (2022). Diurnal Oscillations of Fibrinolytic Parameters in Patients with Acute Myocardial Infarction and Their Relation to Platelet Reactivity: Preliminary Insights. Journal of Clinical Medicine, 11(23), 7105. https://doi.org/10.3390/jcm11237105






