Acute Ischemic Stroke and Transient Ischemic Attack in ST-Segment Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention
Abstract
1. Introduction
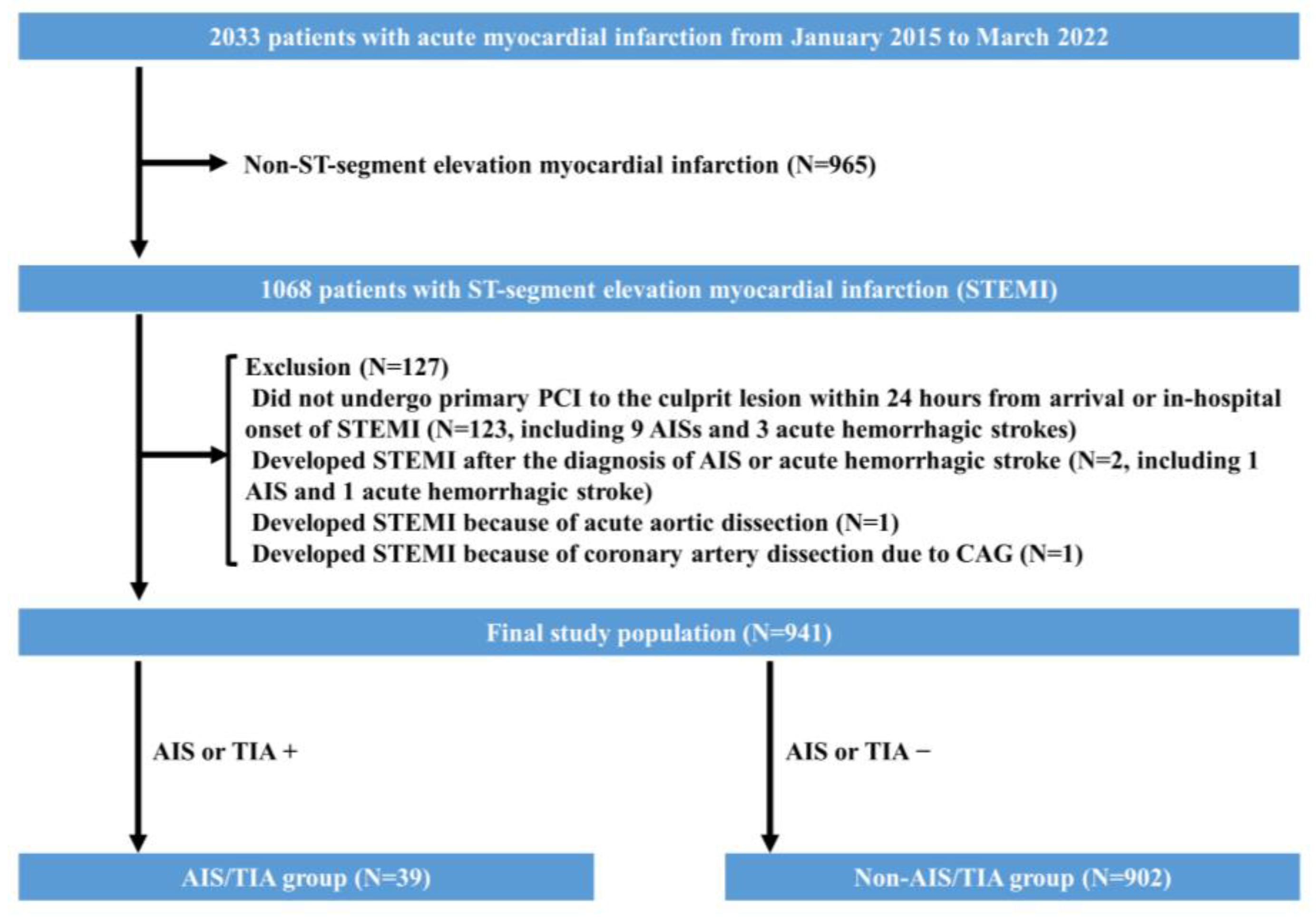
2. Materials and Methods
3. Statistical Analysis
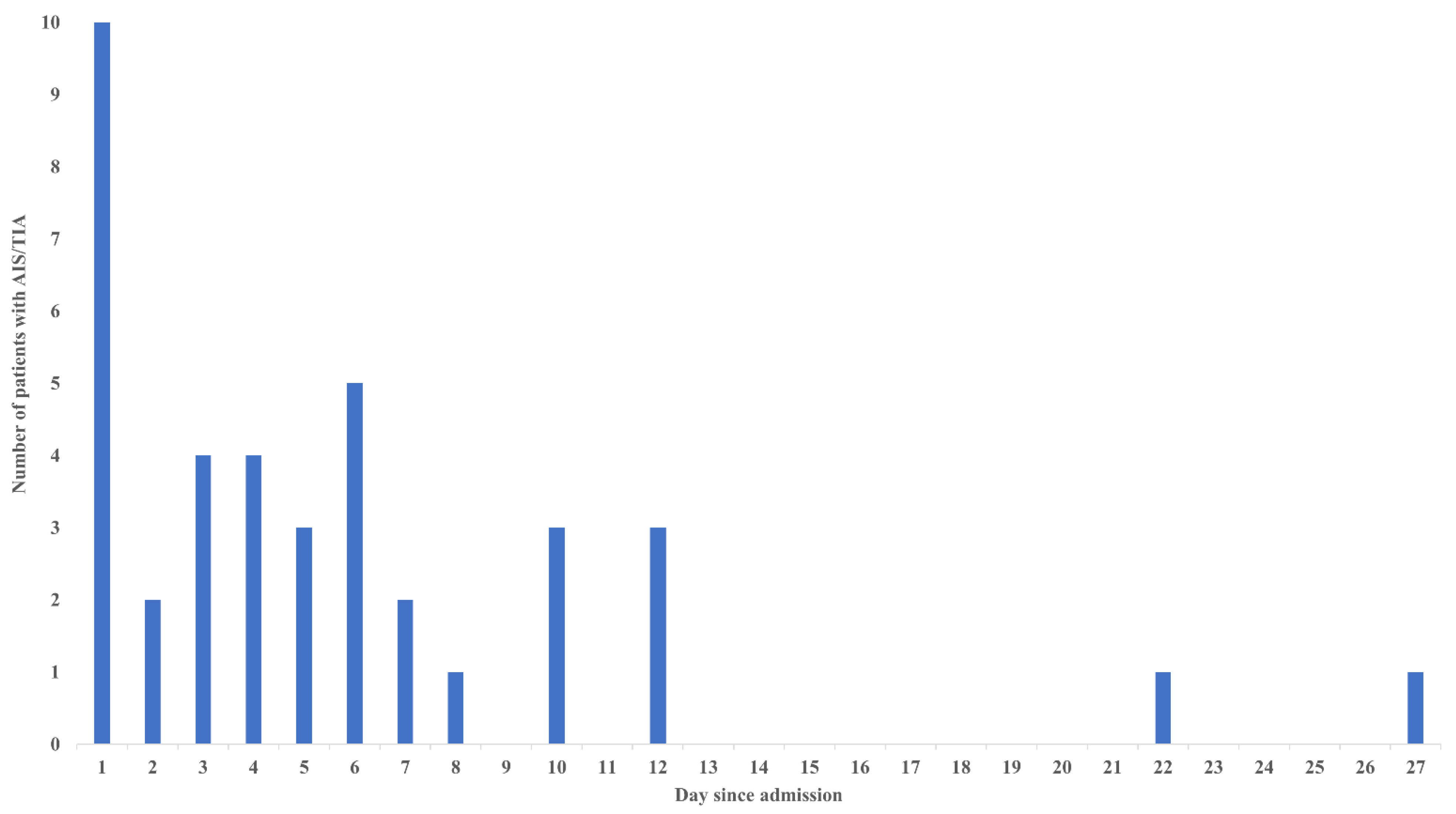
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hachet, O.; Guenancia, C.; Stamboul, K.; Daubail, B.; Richard, C.; Bejot, Y.; Yameogo, V.; Gudjoncik, A.; Cottin, Y.; Giroud, M.; et al. Frequency and predictors of stroke after acute myocardial infarction: Specific aspects of in-hospital and postdischarge events. Stroke 2014, 45, 3514–3520. [Google Scholar] [CrossRef]
- Aggarwal, G.; Patlolla, S.H.; Aggarwal, S.; Cheungpasitporn, W.; Doshi, R.; Sundaragiri, P.R.; Rabinstein, A.A.; Jaffe, A.S.; Barsness, G.W.; Cohen, M.; et al. Temporal Trends, Predictors, and Outcomes of Acute Ischemic Stroke in Acute Myocardial Infarction in the United States. J. Am. Heart Assoc. 2021, 10, e017693. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alqahtani, F.; Tarabishy, A.; Sandhu, G.; Rihal, C.S. Incidence, Predictors, and Outcomes of Acute Ischemic Stroke Following Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1497–1506. [Google Scholar] [CrossRef]
- Kajermo, U.; Ulvenstam, A.; Modica, A.; Jernberg, T.; Mooe, T. Incidence, trends, and predictors of ischemic stroke 30 days after an acute myocardial infarction. Stroke 2014, 45, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Al Suwaidi, J.; Al Habib, K.; Asaad, N.; Singh, R.; Hersi, A.; Al Falaeh, H.; Al Saif, S.; Al-Motarreb, A.; Almahmeed, W.; Sulaiman, K.; et al. Immediate and one-year outcome of patients presenting with acute coronary syndrome complicated by stroke: Findings from the 2nd Gulf Registry of Acute Coronary Events (Gulf RACE-2). BMC Cardiovasc. Disord. 2012, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Gonuguntla, K.; Rojulpote, C.; Kumar, M.; Nadadur, S.; Nardino, R.J.; Pickett, C. Prevalence and Determinants of Atrial Fibrillation-Associated In-Hospital Ischemic Stroke in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Am. J. Cardiol. 2021, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sotomi, Y.; Ueda, Y.; Hikoso, S.; Nakatani, D.; Suna, S.; Dohi, T.; Mizuno, H.; Okada, K.; Kida, H.; Oeun, B.; et al. Manual Thrombus Aspiration and its Procedural Stroke Risk in Myocardial Infarction. J. Am. Heart Assoc. 2021, 10, e022258. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Kohsaka, S.; Kumamaru, H.; Sawano, M.; Shiraishi, Y.; Ueda, I.; Noma, S.; Suzuki, M.; Numasawa, Y.; Hayashida, K.; et al. Stroke after Percutaneous Coronary Intervention in the Era of Transradial Intervention. Circ. Cardiovasc. Interv. 2018, 11, e006761. [Google Scholar] [CrossRef]
- Fuchs, S.; Stabile, E.; Kinnaird, T.D.; Mintz, G.S.; Gruberg, L.; Canos, D.A.; Pinnow, E.E.; Kornowski, R.; Suddath, W.O.; Satler, L.F.; et al. Stroke complicating percutaneous coronary interventions: Incidence, predictors, and prognostic implications. Circulation 2002, 106, 86–91. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Jr Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Taguchi, Y.; Kubo, S.; Ikuta, A.; Osakada, K.; Takamatsu, M.; Takahashi, K.; Ohya, M.; Shimada, T.; Miura, K.; Murai, R.; et al. Percutaneous coronary intervention for left main coronary artery malperfusion in acute type A aortic dissection. Cardiovasc. Interv. Ther. 2022, 37, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Sakakura, K.; Asada, S.; Taniguchi, Y.; Jinnouchi, H.; Tsukui, T.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Factors associated with difficulty in crossing the culprit lesion of acute myocardial infarction. Sci. Rep. 2021, 11, 21403. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Seguchi, M.; Jinnouchi, H.; Wada, H.; Fujita, H. Comparison of the cost in percutaneous coronary intervention between ST-segment elevation myocardial infarction vs. non-ST-segment elevation myocardial infarction. Cardiovasc. Interv. Ther. 2022, 37, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Comparison of door-to-balloon time and in-hospital outcomes in patients with ST-elevation myocardial infarction between before versus after COVID-19 pandemic. Cardiovasc. Interv. Ther. 2022, 37, 641–650. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Sakakura, K.; Adachi, Y.; Akashi, N.; Watanabe, Y.; Noguchi, M.; Yamamoto, K.; Ugata, Y.; Wada, H.; Momomura, S.I.; et al. In-hospital outcomes of acute myocardial infarction with cardiogenic shock caused by right coronary artery occlusion vs. left coronary artery occlusion. Cardiovasc. Interv. Ther. 2018, 33, 338–344. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Yasuda, S.; Honda, S.; Takegami, M.; Nishihira, K.; Kojima, S.; Asaumi, Y.; Suzuki, M.; Kosuge, M.; Takahashi, J.; Sakata, Y.; et al. Contemporary Antiplatelet Therapy and Clinical Outcomes of Japanese Patients with Acute Myocardial Infarction—Results from the Prospective Japan Acute Myocardial Infarction Registry (JAMIR). Circ. J. 2019, 83, 1633–1643. [Google Scholar] [CrossRef]
- Murakami, T.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Comparison of medical resource use and total admission cost in patients with acute myocardial infarction between on-hours visit versus off-hours visit. Cardiovasc. Interv. Ther. 2022, 37, 651–659. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Seguchi, M.; Tsukui, T.; Jinnouchi, H.; Wada, H.; Fujita, H. Long-term outcomes of the modest stent expansion strategy for the culprit lesion of acute myocardial infarction. Cardiovasc. Interv. Ther. 2022, 37, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Naito, R.; Sakakura, K.; Wada, H.; Funayama, H.; Sugawara, Y.; Kubo, N.; Ako, J.; Momomura, S. Comparison of long-term clinical outcomes between sirolimus-eluting stents and paclitaxel-eluting stents following rotational atherectomy. Int. Heart J. 2012, 53, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Okcun, B.; Peker, T.; Arslan, C.; Olcay, A.; Bulent Vatan, M. Prevalence of coronary artery anomalies in 12,457 adult patients who underwent coronary angiography. Clin. Cardiol. 2010, 33, E60–E64. [Google Scholar] [CrossRef]
- Guptill, J.T.; Mehta, R.H.; Armstrong, P.W.; Horton, J.; Laskowitz, D.; James, S.; Granger, C.B.; Lopes, R.D. Stroke after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: Timing, characteristics, and clinical outcomes. Circ. Cardiovasc. Interv. 2013, 6, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Takeji, Y.; Shiomi, H.; Morimoto, T.; Yoshikawa, Y.; Taniguchi, R.; Mutsumura-Nakano, Y.; Yamamoto, K.; Yamaji, K.; Tazaki, J.; Kato, E.T.; et al. Changes in demographics, clinical practices and long-term outcomes of patients with ST segment-elevation myocardial infarction who underwent coronary revascularisation in the past two decades: Cohort Study. BMJ Open 2021, 11, e043683. [Google Scholar] [CrossRef]
- Murai, M.; Hazui, H.; Sugie, A.; Hoshiga, M.; Negoro, N.; Muraoka, H.; Miyamoto, H.; Kobata, H.; Fukumoto, H.; Ishihara, T.; et al. Asymptomatic acute ischemic stroke after primary percutaneous coronary intervention in patients with acute coronary syndrome might be caused mainly by manipulating catheters or devices in the ascending aorta, regardless of the approach to the coronary artery. Circ. J. 2008, 72, 51–55. [Google Scholar]
- Korn-Lubetzki, I.; Farkash, R.; Pachino, R.M.; Almagor, Y.; Tzivoni, D.; Meerkin, D. Incidence and risk factors of cerebrovascular events following cardiac catheterization. J. Am. Heart Assoc. 2013, 2, e000413. [Google Scholar] [CrossRef]
- Varmdal, T.; Janszky, I.; Bakken, I.J.; Ellekjaer, H.; Fjaertoft, H.; Haberg, S.E.; Bonaa, K.H. Percutaneous Coronary Intervention as a Trigger for Stroke. Am. J. Cardiol. 2017, 119, 35–39. [Google Scholar] [CrossRef]
- Chandiramani, R.; Chen, H.; Aoi, S.; Giustino, G.; Claessen, B.; Sartori, S.; Aquino, M.; Sorrentino, S.; Cao, D.; Goel, R.; et al. Incidence, predictors and impact of stroke on mortality among patients with acute coronary syndromes following percutaneous coronary intervention-Results from the PROMETHEUS registry. Catheter. Cardiovasc. Interv. 2020, 95, 885–892. [Google Scholar] [CrossRef]
- Hoffman, S.J.; Routledge, H.C.; Lennon, R.J.; Mustafa, M.Z.; Rihal, C.S.; Gersh, B.J.; Holmes, D.R., Jr.; Gulati, R. Procedural factors associated with percutaneous coronary intervention-related ischemic stroke. JACC Cardiovasc. Interv. 2012, 5, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Tak, T. Aortic atheromas: Current knowledge and controversies: A brief review of the literature. Echocardiography 2011, 28, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Harloff, A.; Simon, J.; Brendecke, S.; Assefa, D.; Helbing, T.; Frydrychowicz, A.; Weber, J.; Olschewski, M.; Strecker, C.; Hennig, J.; et al. Complex plaques in the proximal descending aorta: An underestimated embolic source of stroke. Stroke 2010, 41, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.; Grines, C.L. Scraping of aortic debris by coronary guiding catheters. J. Am. Coll. Cardiol. 1998, 32, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Oldenburg, O.; Dirsch, O.; Haude, M.; Baumgart, D.; Welge, D.; Herrmann, J.; Arnold, G.; Schmid, K.W.; Erbel, R. Potential embolization by atherosclerotic debris dislodged from aortic wall during cardiac catheterization:: Histological and clinical findings in 7,621 patients. Catheter. Cardiovasc. Interv. 2000, 49, 389–394. [Google Scholar] [CrossRef]
- Joshi, N.V.; Toor, I.; Shah, A.S.; Carruthers, K.; Vesey, A.T.; Alam, S.R.; Sills, A.; Hoo, T.Y.; Melville, A.J.; Langlands, S.P.; et al. Systemic Atherosclerotic Inflammation Following Acute Myocardial Infarction: Myocardial Infarction Begets Myocardial Infarction. J. Am. Heart Assoc. 2015, 4, e001956. [Google Scholar] [CrossRef]
- Le May, M.; Wells, G.; So, D.; Chong, A.Y.; Dick, A.; Froeschl, M.; Glover, C.; Hibbert, B.; Marquis, J.F.; Blondeau, M.; et al. Safety and Efficacy of Femoral Access vs. Radial Access in ST-Segment Elevation Myocardial Infarction: The SAFARI-STEMI Randomized Clinical Trial. JAMA Cardiol. 2020, 5, 126–134. [Google Scholar] [CrossRef]
- Blackshear, J.L.; Pearce, L.A.; Hart, R.G.; Zabalgoitia, M.; Labovitz, A.; Asinger, R.W.; Halperin, J.L. Aortic plaque in atrial fibrillation: Prevalence, predictors, and thromboembolic implications. Stroke 1999, 30, 834–840. [Google Scholar] [CrossRef]
- Craiem, D.; Chironi, G.; Casciaro, M.E.; Graf, S.; Simon, A. Calcifications of the thoracic aorta on extended non-contrast-enhanced cardiac CT. PLoS ONE 2014, 9, e109584. [Google Scholar] [CrossRef]
- Kojima, K.; Kimura, S.; Hayasaka, K.; Mizusawa, M.; Misawa, T.; Yamakami, Y.; Sagawa, Y.; Ohtani, H.; Hishikari, K.; Sugiyama, T.; et al. Aortic Plaque Distribution, and Association between Aortic Plaque and Atherosclerotic Risk Factors: An Aortic Angioscopy Study. J. Atheroscler. Thromb. 2019, 26, 997–1006. [Google Scholar] [CrossRef]
- Kundu, A.; O’Day, K.; Shaikh, A.Y.; Lessard, D.M.; Saczynski, J.S.; Yarzebski, J.; Darling, C.E.; Thabet, R.; Akhter, M.W.; Floyd, K.C.; et al. Relation of Atrial Fibrillation in Acute Myocardial Infarction to In-Hospital Complications and Early Hospital Readmission. Am. J. Cardiol. 2016, 117, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Yamaji, K.; Watanabe, H.; Yoshikawa, Y.; Shiomi, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; et al. Prediction of Thrombotic and Bleeding Events after Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J. Am. Heart Assoc. 2018, 7, e008708. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Lee, K.H.; Torii, S.; Oguri, M.; Miyaji, T.; Kiyooka, T.; Ono, Y.; Asada, K.; Adachi, T.; Takahashi, A.; Ikari, Y. Reduction of door-to-balloon time in patients with ST-elevation myocardial infarction by single-catheter primary percutaneous coronary intervention method. Catheter. Cardiovasc. Interv. 2022, 99, 314–321. [Google Scholar] [CrossRef]
- Akashi, N.; Sakakura, K.; Yamamoto, K.; Taniguchi, Y.; Wada, H.; Momomura, S.I.; Fujita, H. Minimization of door-to-balloon time for ST-elevation acute myocardial infarction: A case report. Clin. Case Rep. 2017, 5, 787–791. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Ahmad, S.; Latif, A.; Lateef, N.; Ahsan, M.Z.; Abusnina, W.; Nathan, S.; Altin, S.E.; Kolte, D.S.; Messenger, J.C.; et al. Transradial versus transfemoral approach for percutaneous coronary intervention in patients with ST-elevation myocardial infarction complicated by cardiogenic shock: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 640–650. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Tanimoto, T.; Kitabata, H.; Arita, Y.; Yamamoto, Y.; Mori, K.; Terada, K.; Nishiguchi, T.; Taruya, A.; Kubo, T.; et al. Usefulness of rescue ultrasound guidance for transradial cardiac catheterization. Cardiovasc. Revasc. Med. 2019, 20, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wernly, B.; Seelmaier, C.; Leistner, D.; Stahli, B.E.; Pretsch, I.; Lichtenauer, M.; Jung, C.; Hoppe, U.C.; Landmesser, U.; Thiele, H.; et al. Mechanical circulatory support with Impella versus intra-aortic balloon pump or medical treatment in cardiogenic shock-a critical appraisal of current data. Clin. Res. Cardiol. 2019, 108, 1249–1257. [Google Scholar] [CrossRef]
| All (n = 941) | AIS/TIA Group (n = 39) | Non-AIS/TIA Group (n = 902) | p-Value | |
|---|---|---|---|---|
| Age, year | 71 (61–79) | 74 (68–77) | 71 (61–79) | 0.494 |
| Male, n (%) | 736 (78.2) | 33 (84.6) | 703 (77.9) | 0.428 |
| Body mass index >25 (kg/m2) | 319 (33.9) | 9 (23.1) | 310 (34.4) | 0.169 |
| Underlying disease | ||||
| Hypertension, n (%) | 712 (75.7) | 30 (76.9) | 682 (75.6) | 1.000 |
| Diabetes mellitus, n (%) | 391 (41.6) | 20 (51.3) | 371 (41.1) | 0.246 |
| Dyslipidemia, n (%) | 491 (52.2) | 18 (46.2) | 473 (52.4) | 0.513 |
| Hemodialysis, n (%) | 44 (4.7) | 1 (2.6) | 43 (4.8) | 1.000 |
| Previous atrial fibrillation, n (%) | 40 (4.3) | 1 (2.6) | 39 (4.3) | 1.000 |
| History of ischemic stroke or TIA, n (%) | 88 (9.4) | 7 (17.9) | 81 (9) | 0.083 |
| History of peripheral artery disease, n (%) | 31 (3.3) | 2 (5.1) | 29 (3.2) | 0.371 |
| History of previous PCI, n (%) | 120 (12.8) | 5 (12.8) | 115 (12.7) | 1.000 |
| History of previous CABG, n (%) | 13 (1.4) | 0 (0) | 13 (1.4) | 1.000 |
| History of previous MI, n (%) | 95 (10.1) | 5 (12.8) | 90 (10) | 0.583 |
| Medication before admission | ||||
| Aspirin, n (%) | 156/925 (16.9) | 5/34 (14.7) | 151/891 (16.9) | 1.000 |
| Thienopyridine, n (%) | 80/924 (8.7) | 3/34 (8.8) | 77/890 (8.7) | 1.000 |
| Beta-blocker, n (%) | 130/910 (14.3) | 4/32 (12.5) | 126/878 (14.4) | 1.000 |
| ACE-inhibitor, ARB, n (%) | 291/910 (32) | 9/32 (28.1) | 282/878 (32.1) | 0.704 |
| Statin, n (%) | 229/915 (25) | 6/33 (18.2) | 223/882 (25.3) | 0.419 |
| Hypoglycemic agents, n (%) | 221/915 (24.2) | 9/32 (28.1) | 212/883 (24) | 0.674 |
| Insulin, n (%) | 42/919 (4.6) | 3/34 (8.8) | 39/885 (4.4) | 0.200 |
| Warfarin, n (%) | 12/926 (1.3) | 1/34 (2.9) | 11/892 (1.2) | 0.363 |
| DOAC, n (%) | 20/926 (2.2) | 1/34 (2.9) | 19/892 (2.1) | 0.531 |
| Killip class | <0.001 | |||
| 1, n (%) | 656 (69.7) | 11 (28.2) | 645 (71.5) | |
| 2, n (%) | 65 (6.9) | 2 (5.1) | 63 (7) | |
| 3, n (%) | 70 (7.4) | 5 (12.8) | 65 (7.2) | |
| 4, n (%) | 150 (15.9) | 21 (53.8) | 129 (14.3) | |
| Cardiogenic shock, n (%) | 150 (15.9) | 21 (53.8) | 129 (14.3) | <0.001 |
| OHCA, n (%) | 62 (6.6) | 13 (33.3) | 49 (5.4) | <0.001 |
| Laboratory data at arrival | ||||
| Estimated GFR (mL/min/1.73 m2) | 65.1 (48.0–81.5) | 47.6 (36.6–63.4) | 65.9 (49.1–81.9) | <0.001 |
| Hemoglobin (g/dL) | 13.7 (12.2–15.0) | 13.9 (12.2–15.2) | 13.7 (12.2–15.0) | 0.726 |
| BNP (pg/mL) | 103.2 (30.4–352.4) (n = 902) | 177.9 (53.6–508.4) (n = 34) | 101.9 (30.0–346.1) (n = 868) | 0.095 |
| New-onset AF during admission, n (%) | 120 (12.8) | 11 (28.2) | 109 (12.1) | 0.011 |
| Left ventricular thrombus during admission, n (%) | 27 (2.9) | 2 (5.1) | 25 (2.8) | 0.309 |
| All (n = 941) | AIS/TIA Group (n = 39) | Non-AIS/TIA Group (n = 902) | p-Value | |
|---|---|---|---|---|
| Culprit lesion | 0.152 | |||
| LM-LAD, n (%) | 488 (51.9) | 20 (51.3) | 468 (51.9) | |
| RCA, n (%) | 361 (38.4) | 13 (33.3) | 348 (38.6) | |
| LCX, n (%) | 89 (9.5) | 5 (12.8) | 84 (9.3) | |
| Graft, n (%) | 3 (0.3) | 1 (2.6) | 2 (0.2) | |
| Triple vessels disease, n (%) | 198 (21) | 16 (41) | 182 (20.2) | 0.004 |
| Anomalous origin of coronary artery, n (%) | 24 (2.6) | 1 (2.6) | 23 (2.5) | 1.000 |
| Initial TIMI flow grade of culprit ≤2, n (%) | 785 (83.4) | 33 (84.6) | 752 (83.4) | 1.000 |
| Final TIMI flow grade of culprit ≤2, n (%) | 65 (6.9) | 6 (15.4) | 59 (6.5) | 0.046 |
| Lesion length (mm) | 13.6 (9.4–19.8) | 14.1 (7.1–21.8) | 13.6 (9.5–19.6) | 0.651 |
| Reference diameter (mm) | 2.4 (2.0–2.9) | 2.4 (1.9–2.7) | 2.4 (2.0–2.9) | 0.540 |
| Eccentricity, n (%) | 267 (28.4) | 5 (12.8) | 262 (29) | 0.029 |
| Moderately–extremely angulated lesion, n (%) | 92 (9.8) | 7 (17.9) | 85 (9.4) | 0.094 |
| Irregular contour, n (%) | 586 (62.3) | 23 (59) | 563 (62.4) | 0.736 |
| Ostial lesion, n (%) | 20 (2.1) | 1 (2.6) | 19 (2.1) | 0.575 |
| Bifurcation lesion, n (%) | 182 (19.3) | 4 (10.3) | 178 (19.7) | 0.211 |
| Excessive tortuosity, n (%) | 115 (12.2) | 7 (17.9) | 108 (12) | 0.312 |
| Moderate-severe calcification, n (%) | 160 (17) | 8 (20.5) | 152 (16.9) | 0.517 |
| Thrombus (TIMI Thrombus grade ≥3), n (%) | 414 (44) | 15 (38.5) | 399 (44.2) | 0.514 |
| ACC/AHA classification: type B2/C, n (%) | 794 (84.4) | 29 (74.4) | 765 (84.8) | 0.110 |
| Approach site | 0.001 | |||
| Trans-radial approach, n (%) | 637 (67.7) | 14 (35.9) | 623 (69.1) | |
| Trans-femoral approach, n (%) | 292 (31) | 25 (64.1) | 267 (29.6) | |
| Trans-brachial approach, n (%) | 12 (1.3) | 0 (0) | 12 (1.3) | |
| Number of used catheters | 3 (3–3) | 3 (3–4) | 3 (3–3) | <0.001 |
| Diagnostic catheters | 2 (2–2) | 2 (2–3) | 2 (2–2) | 0.001 |
| Guiding catheters | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.001 |
| Use of ≥4 catheters, n (%) | 174 (18.5) | 17 (43.6) | 157 (17.4) | <0.001 |
| Size of guiding catheter | 0.001 | |||
| 6 Fr, n (%) | 697 (74.1) | 19 (48.7) | 678 (75.2) | |
| 7 Fr, n (%) | 239 (25.4) | 19 (48.7) | 220 (24.4) | |
| 8 Fr, n (%) | 5 (0.5) | 1 (0.6) | 4 (0.4) | |
| Multivessel PCI at the time of primary PCI | 19 (2) | 5 (12.8) | 14 (1.6) | 0.001 |
| Final PCI procedure | 0.756 | |||
| POBA only, n (%) | 46 (4.9) | 1 (2.6) | 45 (5) | |
| Thrombus aspiration only, n (%) | 11 (1.2) | 1 (2.6) | 10 (1.1) | |
| Thrombus aspiration and POBA, n (%) | 11 (1.2) | 0 (0) | 11 (1.2) | |
| Bare metal stent, n (%) | 17 (1.8) | 0 (0) | 17 (1.9) | |
| Drug-eluting stent, n (%) | 831 (88.4) | 37 (94.9) | 794 (88.1) | |
| Drug-coated balloon, n (%) | 24 (2.6) | 0 (0) | 24 (2.7) | |
| Bougie with micro-catheter, n (%) | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Thrombus aspiration procedure, n (%) | 206 (21.9) | 9 (23.1) | 197 (21.8) | 0.844 |
| Use of guide extension catheters, n (%) | 92 (9.8) | 6 (15.4) | 86 (9.5) | 0.263 |
| IABP, n (%) | 96 (10.2) | 7 (17.9) | 89 (9.9) | 0.106 |
| V-A ECMO, n (%) | 57 (6.1) | 17 (43.6) | 40 (4.4) | <0.001 |
| Impella (Abiomed), n (%) | 3 (0.3) | 2 (5.1) | 1 (0.1) | 0.005 |
| Door-to-balloon time (minutes) | 72 (56–106) | 97 (68–137) | 71 (56–104) | 0.006 |
| Procedure time (minutes) | 52 (40–73) | 63 (49–96) | 51 (40–72) | 0.005 |
| BARC type 3 or 5 bleeding, n (%) | 69 (7.3) | 12 (30.8) | 57 (6.3) | <0.001 |
| Discontinuation of anti-thrombotic therapy, n (%) | 13 (1.4) | 3 (7.7) | 10 (1.1) | 0.014 |
| All (n = 941) | AIS/TIA Group (n = 39) | Non-AIS/TIA Group (n = 902) | p-Value | |
|---|---|---|---|---|
| In-hospital death, n (%) | 75 (8) | 18 (46.2) | 57 (6.3) | <0.001 |
| Among patients without cardiogenic shock (n = 791) | 20/791 (2.5) | 5/18 (27.8) | 15/773 (1.9) | <0.001 |
| Among patients with cardiogenic shock (n = 150) | 55/150 (36.7) | 13/21 (61.9) | 42/129 (32.6) | 0.014 |
| Favorable neurological function (CPC 1 or 2) at discharge, n (%) | 835 (88.7) | 14 (35.9) | 821 (91) | <0.001 |
| Tracheostomy, n (%) | 11 (1.2) | 3 (7.7) | 8 (0.9) | 0.009 |
| Mechanical ventilation (including NPPV), n (%) | 223 (23.7) | 30 (76.9) | 193 (21.4) | <0.001 |
| Ejection fraction at discharge (%) | 52.2 (41.8–60.6) (n = 877) | 42.5 (26.6–58.4) (n = 24) | 52.5 (42.0–60.7) (n = 853) | 0.021 |
| Number of cardiac catheterizations during admission | 1 (1–2) | 1 (1–1) | 1 (1–2) | 0.298 |
| Independent Variables | Dependent Variable: AIS/TIA | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| OHCA | 5.126 | 2.169–12.113 | <0.001 | |||
| Cardiogenic shock | 3.228 | 1.492–6.986 | 0.003 | |||
| New-onset AF | 2.280 | 1.033–5.031 | 0.041 | 2.914 | 1.296–6.551 | 0.010 |
| Lesion eccentricity | 0.446 | 0.167–1.196 | 0.109 | 0.406 | 0.148–1.113 | 0.080 |
| Final TIMI flow grade ≤2 | 2.490 | 0.911–6.811 | 0.075 | |||
| Triple vessels disease | 2.036 | 0.976–4.248 | 0.058 | |||
| Trans-femoral approach | 2.336 | 1.093–4.992 | 0.029 | 2.502 | 1.186–5.277 | 0.016 |
| Use of ≥4 catheters | 3.715 | 1.831–7.537 | <0.001 | 3.460 | 1.660–7.212 | 0.001 |
| BARC type 3 or 5 bleeding | 2.932 | 1.256–6.846 | 0.013 | 2.958 | 1.254–6.975 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Acute Ischemic Stroke and Transient Ischemic Attack in ST-Segment Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. J. Clin. Med. 2023, 12, 840. https://doi.org/10.3390/jcm12030840
Murakami T, Sakakura K, Jinnouchi H, Taniguchi Y, Tsukui T, Watanabe Y, Yamamoto K, Seguchi M, Wada H, Fujita H. Acute Ischemic Stroke and Transient Ischemic Attack in ST-Segment Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2023; 12(3):840. https://doi.org/10.3390/jcm12030840
Chicago/Turabian StyleMurakami, Tsukasa, Kenichi Sakakura, Hiroyuki Jinnouchi, Yousuke Taniguchi, Takunori Tsukui, Yusuke Watanabe, Kei Yamamoto, Masaru Seguchi, Hiroshi Wada, and Hideo Fujita. 2023. "Acute Ischemic Stroke and Transient Ischemic Attack in ST-Segment Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention" Journal of Clinical Medicine 12, no. 3: 840. https://doi.org/10.3390/jcm12030840
APA StyleMurakami, T., Sakakura, K., Jinnouchi, H., Taniguchi, Y., Tsukui, T., Watanabe, Y., Yamamoto, K., Seguchi, M., Wada, H., & Fujita, H. (2023). Acute Ischemic Stroke and Transient Ischemic Attack in ST-Segment Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine, 12(3), 840. https://doi.org/10.3390/jcm12030840







