Human Nanoplatelets as Living Vehicles for Tumor-Targeted Endocytosis In Vitro and Imaging In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Transferrin Conjugates
2.3. Nanoplatelets
2.4. High-Performance Liquid Chromatography (HPLC) and Cell Apoptosis Assay
2.5. Confocal Fluorescence Microscopy
2.6. Flow Cytometry
2.7. Electron Microscopy
2.8. Mice
2.9. NIR Fluorescence Imaging
3. Results
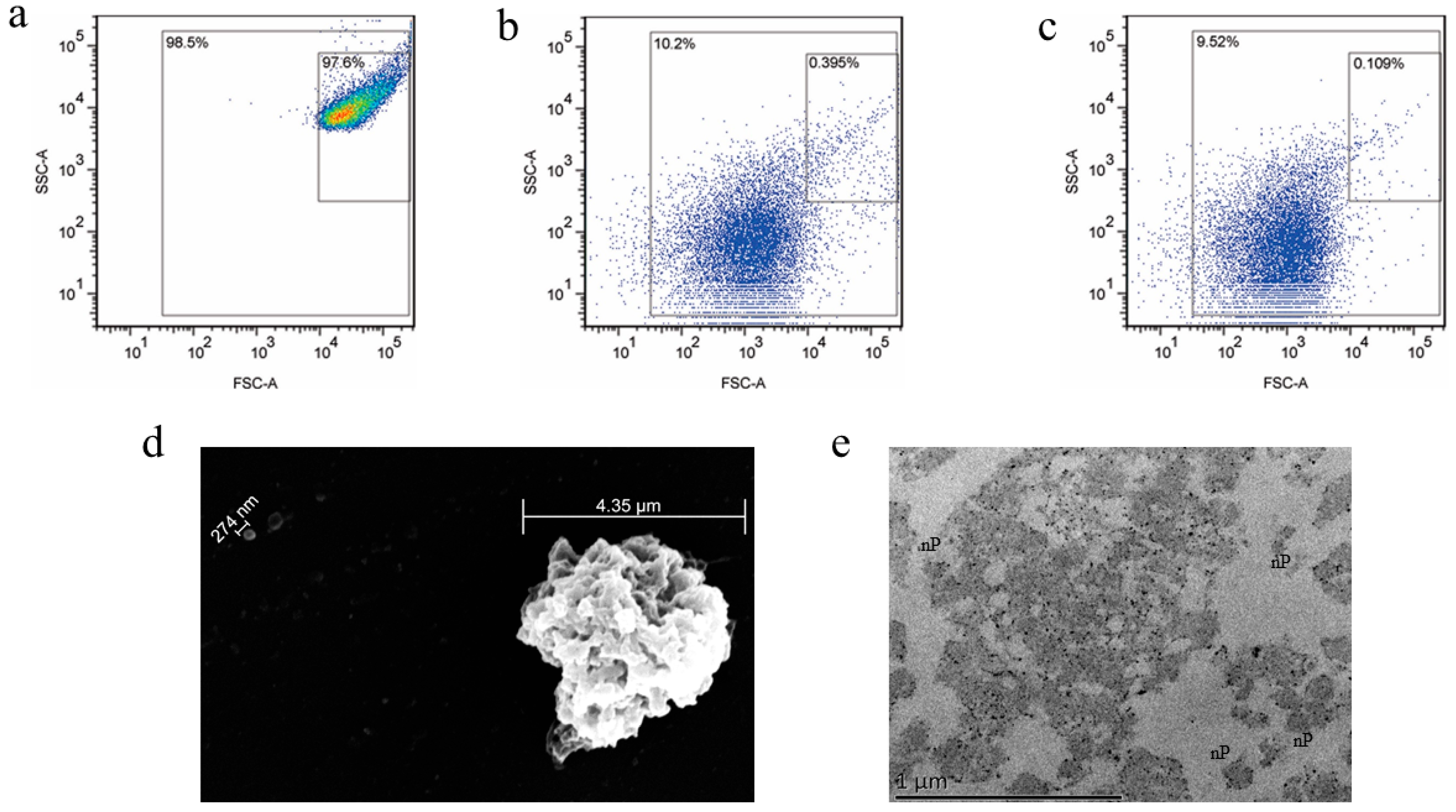
3.1. Preparation and Characterization of the Nanoplatelets
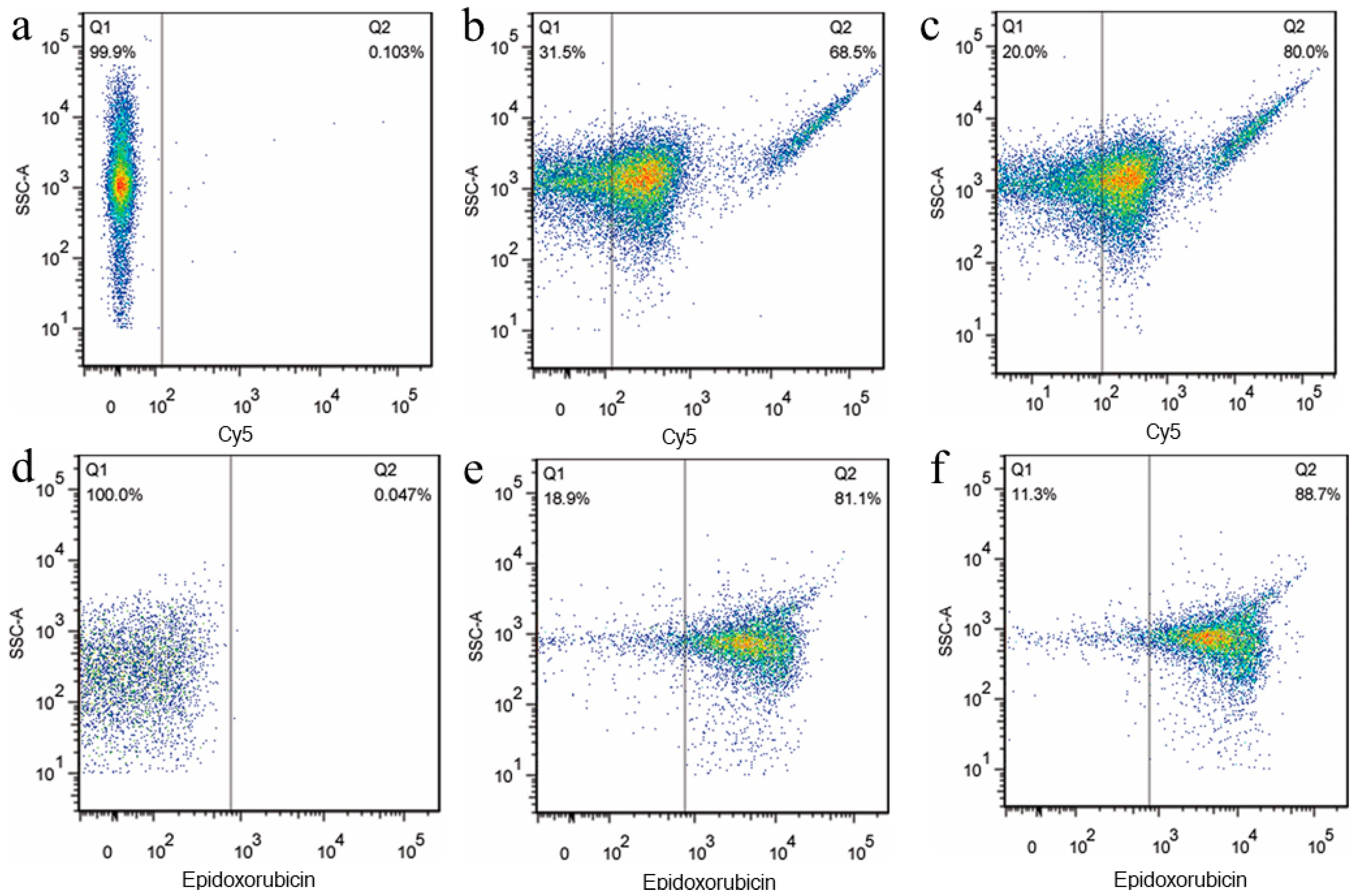
3.2. Preparation and Properties of the Functionalized Nanoplatelets
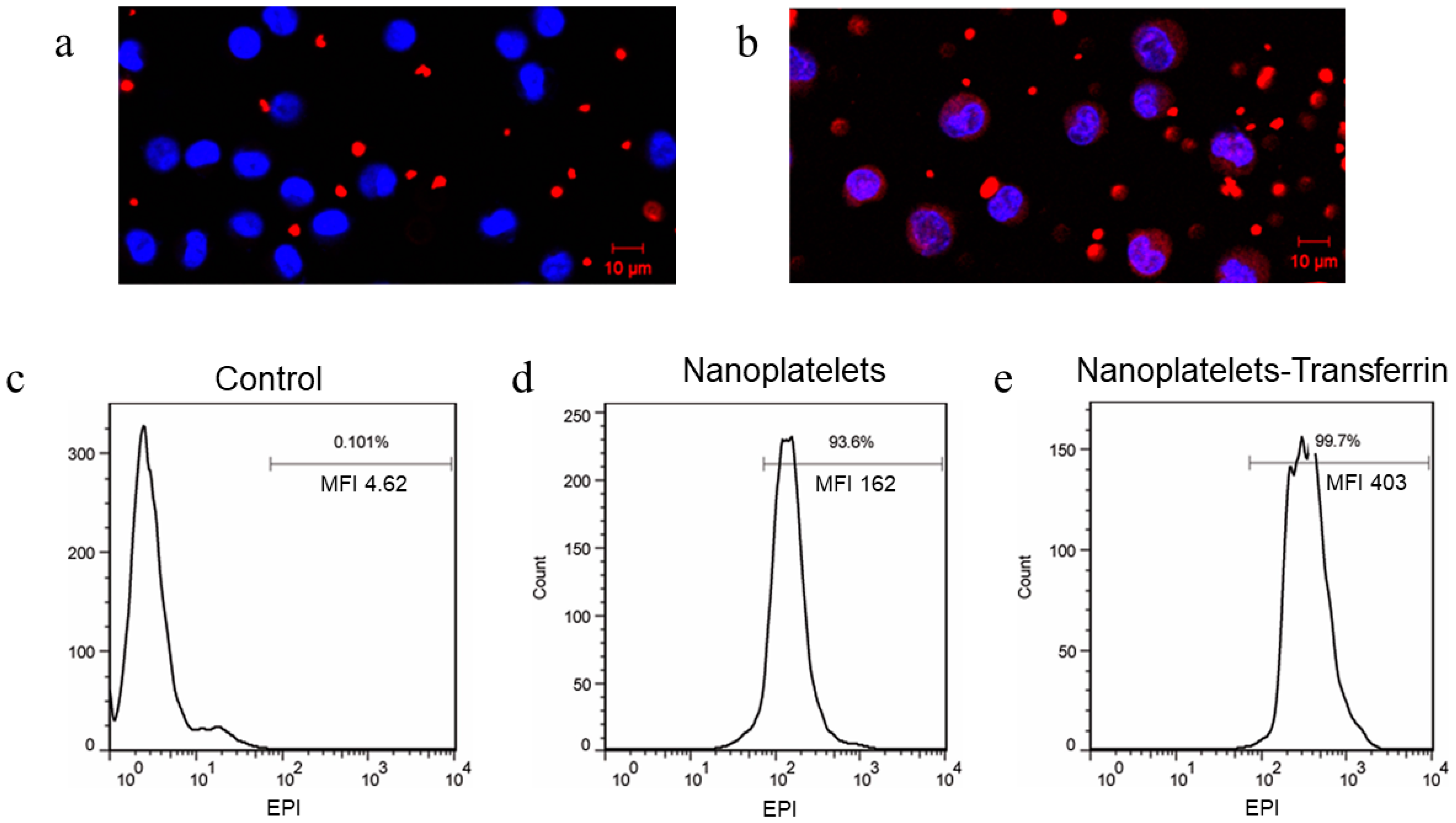
3.3. Imaging Interactions between Functionalized Nanoplatelets and RPMI8226 Cells
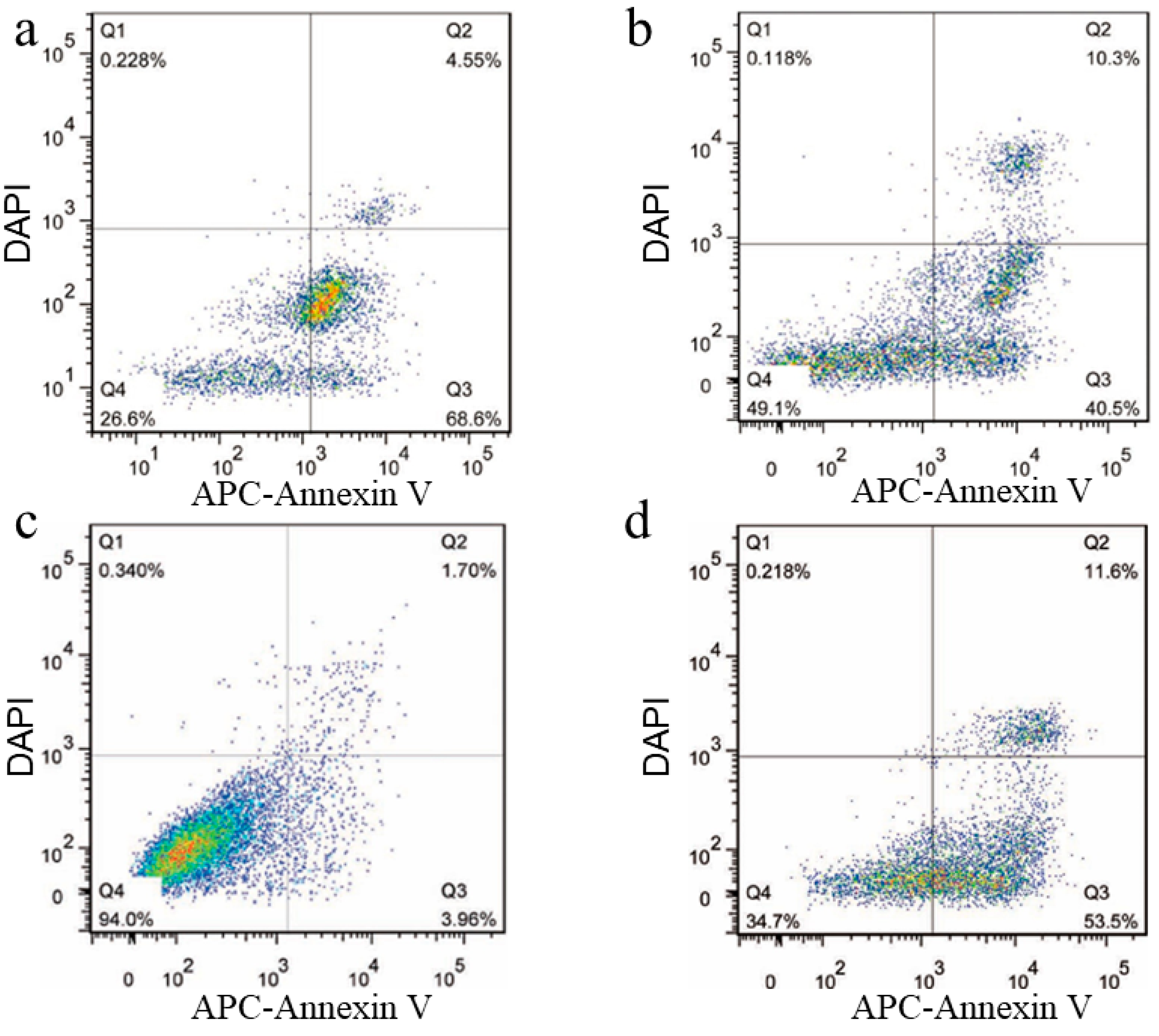
3.4. Apoptosis of RPMI8226 Cells Triggered by the Endocytosis of the Functionalized Nanoplatelets
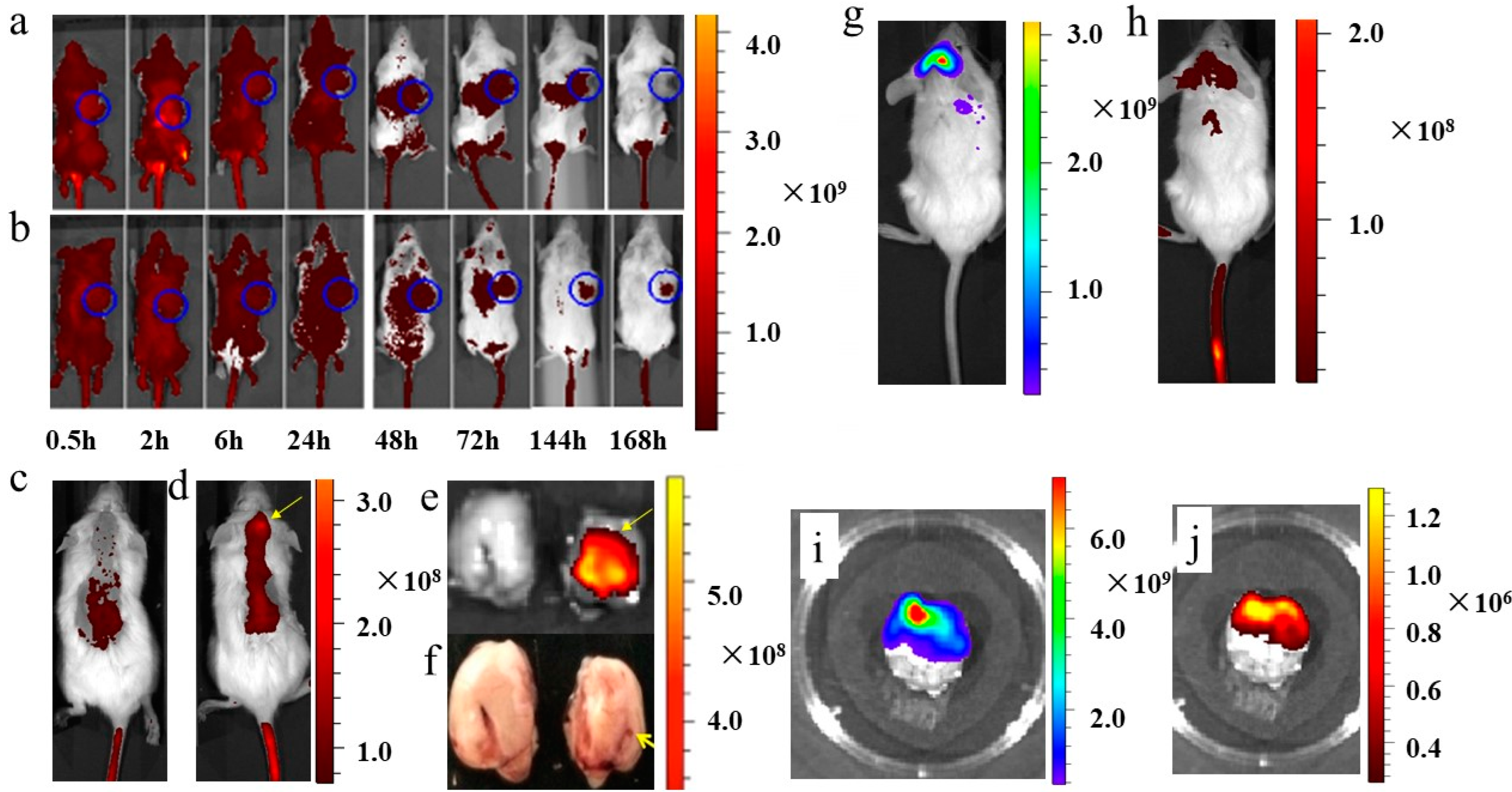
3.5. In Vivo Imaging of Functionalized Nanoplatelets in Myeloma Xenotransplants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bambace, N.M.; Holmes, C.E. The platelet contribution to cancer progression. J. Thromb. Haemost. 2011, 9, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, A.S.; Zimmerman, G.A. Platelets: Signaling cells in the immune continuum. Trends Immunol. 2004, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Jurasz, P.; Alonso-Escolano, D.; Radomski, M.W. Platelet-cancer interactions: Mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br. J. Pharmacol. 2004, 143, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Philippe, B.; Fouqueray, B.; Perez, J.; Lebret, M.; Baud, L. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am. J. Pathol. 1993, 143, 1713–1723. [Google Scholar] [PubMed]
- Dai, L.; Gu, N.; Chen, B.A.; Marriott, G. Human platelets repurposed as vehicles for in vivo imaging of myeloma xenotransplants. Oncotarget 2016, 7, 21076–21090. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Wang, K.-C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, Y.; Wang, L.; Bu, P.; Sharkey, C.C.; Wu, Q.; Wun, B.; Roy, S.; Shen, X.; King, M.R. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials 2016, 76, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Y.; He, W.; Ren, X.; Zhang, M.; Jiang, Y.; Zhou, Z.; Luan, Y. Platelet-armored nanoplatform to harmonize janus-faced IFN-γ against tumor recurrence and metastasis. J. Control. Release 2021, 338, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Yan, Y.; Choi, J.; Bai, J.; Klenchin, V.A.; Rayment, I.; Marriott, G. Biomolecular mimicry in the actin cytoskeleton: Mechanisms underlying the cytotoxicity of kabiramide C and related macrolides. Proc. Natl. Acad. Sci. USA 2003, 100, 13851–13856. [Google Scholar] [CrossRef] [PubMed]
- Petchprayoon, C.; Suwanborirux, K.; Tanaka, J.; Yan, Y.; Sakata, T.; Marriott, G. Fluorescent Kabiramides: New Probes to Quantify Actin in Vitro and in Vivo. Bioconjugate Chem. 2005, 16, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.; Petchprayoon, C.; Hoepker, A.C.; Moriarty, N.W.; Fink, S.J.; Cecere, G.; Paterson, I.; Adams, P.D.; Marriott, G. Structural and Biochemical Studies of Actin in Complex with Synthetic Macrolide Tail Analogues. Chemmedchem 2014, 9, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.E.; Read, M.S.; Reddick, R.L.; Garris, J.B.; Brinkhous, K.M. Thrombotic thrombocytopenia with von Willebrand factor deficiency induced by botrocetin. An animal model. Lab. Investig. 1988, 59, 443–452. [Google Scholar] [PubMed]
- Maksimenko, A.; Dosio, F.; Mougin, J.; Ferrero, A.; Wack, S.; Reddy, L.H.; Weyn, A.-A.; Lepeltier, E.; Bourgaux, C.; Stella, B.; et al. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc. Natl. Acad. Sci. USA 2014, 111, E217–E226. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 291–317. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Liu, Y.; Ding, S.; Wei, X.; Chen, B. Human Nanoplatelets as Living Vehicles for Tumor-Targeted Endocytosis In Vitro and Imaging In Vivo. J. Clin. Med. 2023, 12, 1592. https://doi.org/10.3390/jcm12041592
Dai L, Liu Y, Ding S, Wei X, Chen B. Human Nanoplatelets as Living Vehicles for Tumor-Targeted Endocytosis In Vitro and Imaging In Vivo. Journal of Clinical Medicine. 2023; 12(4):1592. https://doi.org/10.3390/jcm12041592
Chicago/Turabian StyleDai, Lu, Yehong Liu, Shuang Ding, Xiaowei Wei, and Baoan Chen. 2023. "Human Nanoplatelets as Living Vehicles for Tumor-Targeted Endocytosis In Vitro and Imaging In Vivo" Journal of Clinical Medicine 12, no. 4: 1592. https://doi.org/10.3390/jcm12041592
APA StyleDai, L., Liu, Y., Ding, S., Wei, X., & Chen, B. (2023). Human Nanoplatelets as Living Vehicles for Tumor-Targeted Endocytosis In Vitro and Imaging In Vivo. Journal of Clinical Medicine, 12(4), 1592. https://doi.org/10.3390/jcm12041592




