Women with Metabolic Syndrome and Unhealthy Lifestyle Factors Are at a Higher Risk for Hyperuricemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Source
2.2. Collection of Data
2.3. Definition of Variables
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Association between MetS, Cardiovascular Risk Factors, and Lifestyle Components and HUA
3.3. Subgroup Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borghi, C.; Cicero, A.F.G. Serum Uric Acid and Cardiometabolic Disease: Another Brick in the Wall? Hypertension 2017, 69, 1011–1013. [Google Scholar] [CrossRef]
- Borghi, C.; Rosei, E.A.; Bardin, T.; Dawson, J.; Dominiczak, A.; Kielstein, J.T.; Manolis, A.J.; Perez-Ruiz, F.; Mancia, G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015, 33, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.-H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759–766. [Google Scholar] [CrossRef]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 2006, 37, 1503–1507. [Google Scholar] [CrossRef]
- Kim, S.K.; Choe, J.Y. Association between smoking and serum uric acid in Korean population: Data from the seventh Korea na-tional health and nutrition examination survey 2016. Medicine 2019, 98, e14507. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, J.; Kim, G.-T. Prevalence of hyperuricemia and its associated factors in the general Korean population: An analysis of a population-based nationally representative sample. Clin. Rheumatol. 2018, 37, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Hense, H.-W.; Löwel, H.; Döring, A.; Tietze, M.; Keil, U. Association of Serum Uric Acid with All-Cause and Cardiovascular Disease Mortality and Incident Myocardial Infarction in the MONICA Augsburg Cohort. Epidemiology 1999, 10, 391–397. [Google Scholar] [CrossRef]
- Miao, Z.; Li, C.; Chen, Y.; Zhao, S.; Wang, Y.; Wang, Z.; Chen, X.; Xu, F.; Wang, F.; Sun, R.; et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J. Rheumatol. 2008, 35, 1859–1864. [Google Scholar]
- Weir, C.J.; Muir, S.W.; Walters, M.R.; Lees, K.R. Serum Urate as an Independent Predictor of Poor Outcome and Future Vascular Events After Acute Stroke. Stroke 2003, 34, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, X.; Jiang, L.; Mao, S.; Yin, X.; Guo, L. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2016, 16, 207. [Google Scholar] [CrossRef]
- Liu, L.; Lou, S.; Xu, K.; Meng, Z.; Zhang, Q.; Song, K. Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin. Rheumatol. 2013, 32, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Huang, J.; Xu, C.; Zhang, X.; Mi, F.; Xu, F.; Wang, Y.; Feng, Y.; Yin, J. Association of Sedentary Behavior and Physical Activity with Hyperuricemia and Sex Differences: Results from the China Multi-Ethnic Cohort Study. J. Rheumatol. 2022, 49, 513–522. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Zhang, L.; Liu, X.; Tu, R.; Wang, Y.; Li, R.; Li, L.; Hou, J.; Mao, Z.; et al. Independent and interactive effect of sitting time and physical activity on prevalence of hyperuricemia: The Henan Rural Cohort Study. Arthritis Res. Ther. 2021, 23, 7. [Google Scholar] [CrossRef]
- Halperin Kuhns, V.L.; Woodward, O.M. Sex Differences in Urate Handling. Int. J. Mol. Sci. 2020, 21, 4269. [Google Scholar] [CrossRef]
- Liu, M.; He, Y.; Jiang, B.; Wu, L.; Yang, S.; Wang, Y.; Li, X. Association between Serum Uric Acid Level and Metabolic Syndrome and Its Sex Difference in a Chinese Community Elderly Population. Int. J. Endocrinol. 2014, 2014, 754678. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, S.; Meng, Z.; Ren, X. Gender and age impacts on the correlations between hyperuricemia and metabolic syndrome in Chinese. Clin. Rheumatol. 2011, 30, 777–787. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Dong, L.; Gurka, M.J. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: An analysis of National Health and Nutrition Survey 1999–2006. Metabolism 2012, 61, 554–561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, S.; Liu, X.; Li, H.; Xu, W.; Jia, H. Sex difference in the association of serum uric acid with metabolic syndrome and its components: A cross-sectional study in a Chinese Yi population. Postgrad Med. 2017, 129, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Mao, Z.; Liu, X.; Zhang, X.; Yang, K.; Liu, R.; Qian, X.; Zhang, H.; Jiang, J.; et al. Sex-specific associations of serum uric acid with metabolic syndrome in Chinese rural population: The RuralDiab study. Clin. Chim. Acta 2018, 480, 119–125. [Google Scholar] [CrossRef]
- Sui, X.; Church, T.S.; Meriwether, R.A.; Lobelo, F.; Blair, S.N. Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008, 57, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chu, C.-H.; Bai, C.-H.; You, S.-L.; Chou, Y.-C.; Chou, W.-Y.; Chien, K.-L.; Hwang, L.-C.; Su, T.-C.; Tseng, C.-H.; et al. Uric acid level as a risk marker for metabolic syndrome: A Chinese cohort study. Atherosclerosis 2012, 220, 525–531. [Google Scholar] [CrossRef]
- Yu, S.; Yang, H.; Guo, X.; Zhang, X.; Zhou, Y.; Ou, Q.; Zheng, L.; Sun, Y. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: From lifestyle risk factors to metabolic comorbidities. Clin. Rheumatol. 2016, 35, 1207–1215. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Y.; Wei, J.; Zeng, C.; Li, L.-J.; Xie, X.; Wang, Y.-L.; Xie, D.-X.; Li, H.; Yang, C.; et al. Relationship between cigarette smoking and hyperuricemia in middle-aged and elderly population: A cross-sectional study. Rheumatol. Int. 2017, 37, 131–136. [Google Scholar] [CrossRef]
- Park, S.H. Smoking-Related Differential Influence of Alcohol Consumption on the Metabolic Syndrome. Subst. Use Misuse 2019, 54, 2351–2358. [Google Scholar] [CrossRef]
- Kim, I.Y.; Han, K.-D.; Kim, D.H.; Eun, Y.; Cha, H.-S.; Koh, E.-M.; Lee, J.; Kim, H. Women with Metabolic Syndrome and General Obesity Are at a Higher Risk for Significant Hyperuricemia Compared to Men. J. Clin. Med. 2019, 8, 837. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Dove, A.; Li, X.; Yang, H.; Li, S.; Wang, J.; Li, W.-D.; Zhao, H.; Xu, W.; et al. Healthy lifestyle counteracts the risk effect of genetic factors on incident gout: A large population-based longitudinal study. BMC Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Kurniasari, M.D.; Karwur, F.F.; Rayanti, R.E.; Dharmana, E.; Rias, Y.A.; Chou, K.R.; Tsai, H.T. Second-Hand Smoke and Its Synergistic Effect with a Body-Mass Index of >24.9 kg/m(2) Increase the Risk of Gout Arthritis in Indonesia. Int. J. Environ. Res. Public Health 2021, 18, 4324. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Ex-amination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- McCormick, N.; Yokose, C.; Lu, N.; Joshi, A.D.; Curhan, G.C.; Choi, H.K. Impact of adiposity on risk of female gout among those ge-netically predisposed: Sex-specific prospective cohort study findings over >32 years. Ann. Rheum. Dis. 2022, 81, 556–563. [Google Scholar] [CrossRef]
- Fanning, N.; Merriman, T.R.; Dalbeth, N.; Stamp, L.K. An association of smoking with serum urate and gout: A health paradox. Semin. Arthritis Rheum. 2018, 47, 825–842. [Google Scholar] [CrossRef]
- ter Horst, R.; Munckhof, I.C.v.D.; Schraa, K.; Aguirre-Gamboa, R.; Jaeger, M.; Smeekens, S.P.; Brand, T.; Lemmers, H.; Dijkstra, H.; Galesloot, T.E.; et al. Sex-Specific Regulation of Inflammation and Metabolic Syndrome in Obesity. Arter. Thromb. Vasc. Biol. 2020, 40, 1787–1800. [Google Scholar] [CrossRef]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef]
- Strasak, A.M.; Kelleher, C.C.; Brant, L.J.; Rapp, K.; Ruttmann, E.; Concin, H.; Diem, G.; Pfeiffer, K.P.; Ulmer, H. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: A prospective 21-year follow-up study. Int. J. Cardiol. 2008, 125, 232–239. [Google Scholar] [CrossRef]
- Vigna, L.; Vassalle, C.; Tirelli, A.S.; Gori, F.; Tomaino, L.; Sabatino, L.; Bamonti, F.; Boyraz, M.; Cekmez, F.; Karaoğlu, A.; et al. Gender-related association between uric acid, homocysteine, γ-glutamyltransferase, inflammatory biomarkers and metabolic syndrome in subjects affected by obesity. Biomarkers Med. 2017, 11, 857–865. [Google Scholar] [CrossRef]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res. 2010, 62, 170–180. [Google Scholar] [CrossRef]
- Ferrara, C.M.; Goldberg, A.P.; Nicklas, B.J.; Sorkin, J.D.; Ryan, A.S. Sex differences in insulin action and body fat distribution in over-weight and obese middle-aged and older men and women. Appl. Physiol. Nutr. Metab. 2008, 33, 784–790. [Google Scholar] [CrossRef]
- Bruns, C.M.; Kemnitz, J.W. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J. 2004, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, X.; Liu, Y.; Chang, Y.; Sun, Y.; Zhu, G.; Abraham, M.R. The Relation of Moderate Alcohol Consumption to Hyperuricemia in a Rural General Population. Int. J. Environ. Res. Public Health 2016, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.H.; Jung, K.J.; Park, Y.B.; Spiller, W.; Jee, S.H. Causal effect of alcohol consumption on hyperuricemia using a Mendelian ran-domization design. Int. J. Rheum. Dis. 2019, 22, 1912–1919. [Google Scholar] [CrossRef]
- Wu, Y.B.; Shin, D. Association between alcoholic beverage intake and hyperuricemia in Chinese adults: Findings from the China Health and Nutrition Survey. Medicine 2023, 102, e33861. [Google Scholar] [CrossRef]
- Bermudez, V.; Olivar, L.C.; Torres, W.; Navarro, C.; Gonzalez, R.; Espinoza, C.; Morocho, A.; Mindiola, A.; Chacin, M.; Arias, V.; et al. Cigarette smoking and metabolic syndrome components: A cross-sectional study from Maracaibo City, Venezuela. F1000Research 2018, 7, 565. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Ning, G. Active Smoking and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Han, K.; Lee, S.W.; Kim, K.; Kang, S.; Lee, S.; Cha, H.; Koh, E.; Kim, H.; Lee, J. Altered Risk of Incident Gout According to Changes in Metabolic Syndrome Status: A Nationwide, Population-Based Cohort Study of 1.29 million Young Men. Arthritis Rheumatol. 2023, 75, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Gaffo, A.L.; Jr, D.R.J.; Sijtsma, F.; E Lewis, C.; Mikuls, T.R.; Saag, K.G. Serum urate association with hypertension in young adults: Analysis from the Coronary Artery Risk Development in Young Adults cohort. Ann. Rheum. Dis. 2013, 72, 1321–1327. [Google Scholar] [CrossRef]
- Nishio, S.; Maruyama, Y.; Sugano, N.; Hosoya, T.; Yokoo, T.; Kuriyama, S. Gender interaction of uric acid in the development of hypertension. Clin. Exp. Hypertens. 2018, 40, 446–451. [Google Scholar] [CrossRef]
- Wang, S.F.; Shu, L.; Wang, S.; Wang, X.Q.; Mu, M.; Hu, C.Q.; Liu, K.Y.; Zhao, Q.H.; Hu, A.L.; Bo, Q.L.; et al. Gender difference in the association of hyperuricemia with hyper-tension in a middle-aged Chinese population. Blood Press 2014, 23, 339–344. [Google Scholar] [CrossRef]
- Wu, L.; He, Y.; Jiang, B.; Liu, M.; Wang, J.; Zhang, D.; Wang, Y.; Zeng, J. Association between serum uric acid level and hypertension in a Chinese elderly rural population. Clin. Exp. Hypertens. 2017, 39, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.G.; Shaper, A.G.; Thelle, D.S.; Whitehead, T.P. Serum uric acid, serum glucose and diabetes: Relationships in a population study. Postgrad. Med. J. 1986, 62, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Oda, E.; Kawai, R.; Sukumaran, V.; Watanabe, K. Uric acid is positively associated with metabolic syndrome but negatively asso-ciated with diabetes in Japanese men. Intern. Med. 2009, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-C.; Tsai, S.-T.; Lin, H.-Y.; Chou, P. Different progressions of hyperglycemia and diabetes among hyperuricemic men and women in the kinmen study. J. Rheumatol. 2004, 31, 1159–1165. [Google Scholar]
| Male (N = 7154) | Female (N = 9134) | p Value | |
|---|---|---|---|
| Uric acid (mg/dL) | 5.95 ± 0.02 | 4.41 ± 0.01 | <0.0001 |
| Hyperuricemia, % | 21.06 (0.63) | 6.61 (0.31) | <0.0001 |
| Age (years) | 45.76 ± 0.27 | 47.75 ± 0.28 | <0.0001 |
| Level of education, % | <0.0001 | ||
| Elementary school graduate or less | 8.77 (0.4) | 18.67 (0.63) | |
| Middle school graduate | 8.21 (0.41) | 9.08 (0.37) | |
| High school graduate | 37.17 (0.78) | 33.88 (0.69) | |
| University graduate or more | 45.85 (0.96) | 38.36 (0.84) | |
| Level of income, % | <0.0001 | ||
| Q1 | 13.03 (0.6) | 16.78 (0.65) | |
| Q2 | 22.64 (0.69) | 23.98 (0.65) | |
| Q3 | 30.97 (0.74) | 28.97 (0.67) | |
| Q4 | 33.36 (0.96) | 30.28 (0.89) | |
| Occupation, yes, % | 76.53 (0.66) | 54.64 (0.71) | <0.0001 |
| Spouse, yes, % | 93.09 (0.43) | 80.56 (0.62) | <0.0001 |
| Smoking, yes, % | 37.06 (0.77) | 5.66 (0.33) | <0.0001 |
| Amount of alcohol intake | <0.0001 | ||
| None | 13.8 (0.51) | 30.24 (0.61) | |
| Mild | 70.19 (0.66) | 66.92 (0.62) | |
| Heavy | 16 (0.52) | 2.84 (0.23) | |
| Physically active, % | 50.23 (0.79) | 43.56 (0.7) | <0.0001 |
| Diabetes, % | 11.64 (0.44) | 8.99 (0.38) | <0.0001 |
| Hypertension, % | 30.66 (0.66) | 23.65 (0.63) | <0.0001 |
| Hypercholesterolemia, % | 18.66 (0.55) | 21.58 (0.49) | <0.0001 |
| Chronic kidney disease, % | 3.08 (0.21) | 2.95 (0.19) | 0.6212 |
| BMI (Kg/m2) | 24.58 ± 0.05 | 23.32 ± 0.05 | <0.0001 |
| WC (cm) | 86.21 ± 0.14 | 78.18 ± 0.16 | <0.0001 |
| Total cholesterol (mg/dL) | 192.08 ± 0.55 | 193.8 ± 0.46 | 0.0084 |
| HDL-C (mg/dL) | 47.45 ± 0.16 | 55.04 ± 0.18 | <0.0001 |
| Fasting plasma glucose (mg/dL) | 102.15 ± 0.37 | 97.14 ± 0.27 | <0.0001 |
| Systolic BP (mmHg) | 119.89 ± 0.23 | 115 ± 0.26 | <0.0001 |
| Diastolic BP (mmHg) | 78.47 ± 0.16 | 73.48 ± 0.14 | <0.0001 |
| Metabolic syndrome, yes, % | 34.45 (0.69) | 26.13 (0.62) | <0.0001 |
| BMI | <0.0001 | ||
| <23 | 32.81 (0.66) | 52.27 (0.69) | |
| ≥23, <25 | 25.3 (0.61) | 19.75(0.47) | |
| ≥25 | 41.89 (0.71) | 27.98 (0.61) | |
| Eating alone (times/day) | <0.0001 | ||
| 0 times | 51.96 (0.88) | 40.64 (0.7) | |
| 1 time/day | 29.42 (0.71) | 33.47 (0.63) | |
| 2 times /day | 13.01 (0.53) | 17.81 (0.5) | |
| 3 times/day | 5.61 (0.35) | 8.08 (0.35) | |
| Menopause, yes | 40.1 (0.78) |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | Univariate | Model 1 | Model 2 | |
| Smoking | ||||||
| Non smoker | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Ex-smoker | 0.82 (0.69, 0.98) | 1.16 (0.91, 1.47) | 1.13 (0.88, 1.44) | 0.96 (0.62, 1.49) | 1.06 (0.62, 1.80) | 0.97 (0.55, 1.73) |
| Current smoker | 0.95 (0.81, 1.12) | 1.02 (0.80, 1.31) | 1.03 (0.80, 1.32) | 1.61 (1.14, 2.28) | 1.60 (1.02, 2.53) | 1.60 (1.01, 2.53) |
| Alcohol | ||||||
| None | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Mild | 1.41 (1.14, 1.75) | 1.13 (0.88, 1.44) | 1.21 (0.93, 1.56) | 0.78 (0.65, 0.94) | 1.16 (0.92, 1.46) | 1.34 (1.04, 1.72) |
| Heavy | 1.79 (1.39, 2.32) | 1.5 (1.10, 2.05) | 1.50 (1.08, 2.08) | 1.41 (0.88, 2.26) | 1.79 (0.92, 3.48) | 1.68 (0.85, 3.29) |
| Physically active exercise | ||||||
| No | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 1.166 (1.01, 1.34) | 1.038 (0.86, 1.26) | 1.072 (0.88, 1.31) | 0.928 (0.77, 1.11) | 0.945 (0.75, 1.18) | 1.064 (0.84, 1.35) |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | Univariate | Model 1 | Model 2 | |
| Metabolic Syndrome | ||||||
| No | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Yes | 1.87 (1.63, 2.14) | 2.26 (1.88, 2.72) | 1.80 (1.47, 2.21) | 4.04 (3.31, 4.93) | 3.66 (2.82, 4.75) | 2.40 (1.78, 3.23) |
| Metabolic Components, Yes vs. No (Reference) | ||||||
| Raised WC | 2.06 (1.81, 2.34) | 1.79 (1.50, 2.13) | 1.23 (0.99, 1.54) | 4.26 (3.51, 5.18) | 3.33 (2.63, 4.22) | 1.78 (1.31, 2.42) |
| Elevated TG | 1.81 (1.59, 2.06) | 2.13 (1.78, 2.55) | 1.84 (1.52, 2.21) | 2.88 (2.36, 3.50) | 2.27 (1.78, 2.89) | 1.81 (1.41, 2.34) |
| Hyperglycemia | 1.02 (0.89, 1.17) | 1.16 (0.97, 1.39) | 0.97 (0.81, 1.17) | 2.66 (2.20, 3.22) | 2.11 (1.569, 2.63) | 1.61 (1.26, 2.05) |
| Elevated BP | 1.54 (1.35, 1.75) | 2.10 (1.71, 2.58) | 1.73 (1.39, 2.15) | 2.86 (2.33, 3.50) | 2.70 (2.01, 3.62) | 2.11 (1.56, 2.86) |
| Low HDL | 2.86 (2.33, 3.50) | 2.70 (2.01, 3.62) | 2.11 (1.56, 2.86) | 2.30 (1.90, 2.79) | 1.94 (1.52, 2.47) | 1.53 (1.19, 1.97) |
| Number of Metabolic components | ||||||
| 0 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 1 | 1.42 (1.13, 1.79) | 1.40 (0.99, 1.99) | 1.23 (0.86, 1.77) | 1.37 (0.92, 2.02) | 1.38 (0.80, 2.36) | 1.12 (0.64, 1.95) |
| 2 | 1.70 (1.36, 2.12) | 2.09 (1.51, 2.88) | 1.74 (1.24, 2.43) | 3.80 (2.64, 5.46) | 4.44 (2.65, 7.43) | 3.15 (1.82, 5.44) |
| 3 | 2.36 (1.89, 2.95) | 2.94 (2.09, 4.14) | 2.28 (1.57, 3.30) | 5.64 (3.97, 8.01) | 6.87 (4.22, 11.18) | 4.35 (2.52, 7.50) |
| ≥4 | 2.71 (2.19, 3.35) | 4.1 (2.96, 5.68) | 2.90 (2.01, 4.18) | 7.76 (5.64, 10.68) | 9.02 (5.63, 14.45) | 4.80 (2.77, 8.30) |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | Univariate | Model 1 | Model 2 | |
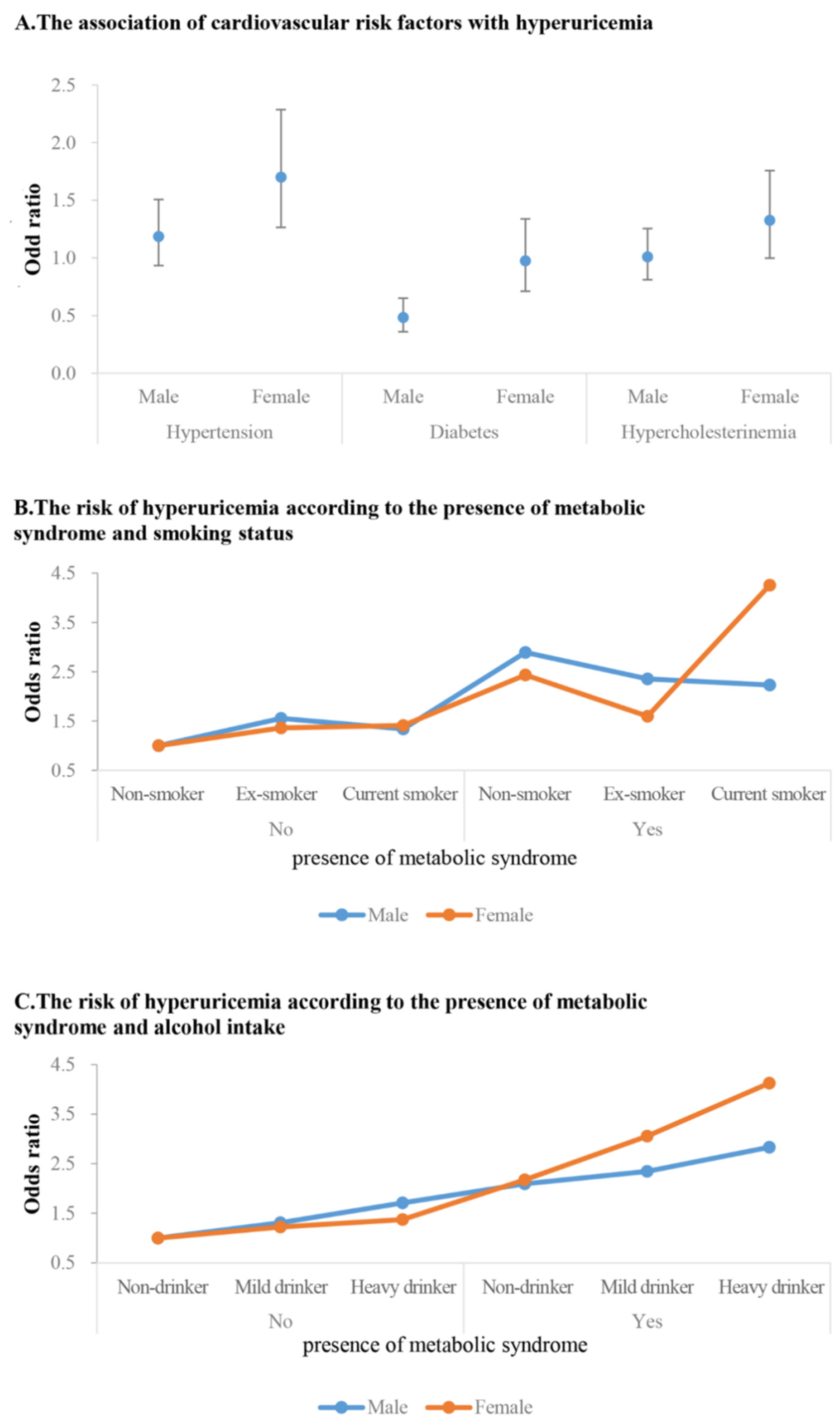
| Hypertension | 1.28 (1.11, 1.49) | 1.79 (1.45, 2.20) | 1.19 (0.93, 1.51) | 3.10 (2.55, 3.77) | 2.81 (2.13, 3.71) | 1.7 (1.27, 2.29) |
| Diabetes | 0.62 (0.49, 0.77) | 0.76 (0.59, 0.99) | 0.48 (0.36, 0.65) | 2.62 (2.05, 3.36) | 1.91 (1.45, 2.52) | 0.98 (0.71, 1.34) |
| Hypercholesterolema | 1.12 (0.95, 1.33) | 1.36 (1.11, 1.66) | 1.01 (0.81, 1.26) | 2.04 (1.67, 2.48) | 1.68 (1.31, 2.14) | 1.33 (0.99, 1.76) |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | Univariate | Model 1 | Model 2 | |
| Metabolic Syndrome, No | ||||||
| Non-smoker | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Ex-smoker | 0.83 (0.65, 1.05) | 1.57 (1.10, 2.23) | 1.56 (1.10, 2.21) | 1.3 (0.71, 2.37) | 1.48 (0.71, 3.10) | 1.36 (0.64, 2.91) |
| Current smoker | 0.99 (0.80,1.22) | 1.30 (0.91, 1.87) | 1.34 (0.94, 1.92) | 1.86 (1.11, 3.14) | 1.36 (0.60, 3.05) | 1.41 (0.61, 3.27) |
| Metabolic Syndrome, Yes | ||||||
| Non-smoker | 2.39 (1.87, 3.06) | 3.68 (2.52, 5.39) | 2.89 (1.93, 4.33) | 4.20 (3.40, 5.19) | 3.72 (2.83, 4.89) | 2.44 (1.77, 3.35) |
| Ex-smoker | 1.47 (1.17, 1.84) | 2.99 (2.11, 4.25) | 2.35 (1.63, 3.40) | 3.49 (1.79, 6.80) | 2.59 (1.20, 5.62) | 1.60 (0.69, 3.68) |
| Current smoker | 1.74 (1.38, 2.20) | 2.68 (1.84, 3.90) | 2.23 (1.51, 3.30) | 7.01 (4.49, 10.95) | 6.27 (3.69, 10.64) | 4.26 (2.47, 7.35) |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Univariate | Model 1 | Model 2 | Univariate | Model 1 | Model 2 | |
| Metabolic Syndrome, No | ||||||
| Non-drinker | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Mild drinker | 1.66 (1.21, 2.28) | 1.29 (0.89, 1.86) | 1.31 (0.91, 1.89) | 1.07 (0.76, 1.49) | 1.15 (0.75, 1.74) | 1.22 (0.80, 1.88) |
| Heavy drinker | 2.27 (1.55, 3.33) | 1.71 (1.04, 2.81) | 1.71 (1.03, 2.84) | 2.19 (1.09, 4.39) | 1.50 (0.51, 4.39) | 1.37 (0.46, 4.10) |
| Metabolic Syndrome, Yes | ||||||
| Non-drinker | 2.42 (1.64, 3.57) | 2.99 (1.9, 4.69) | 2.10 (1.30, 3.38) | 4.19 (3.01, 5.84) | 3.51 (2.38, 5.17) | 2.18 (1.42, 3.35) |
| Mild drinker | 3.19 (2.30, 4.44) | 2.90 (1.96, 4.25) | 2.35 (1.57, 3.50) | 4.43 (3.20, 6.13) | 4.29 (2.93, 6.28) | 3.06 (2.05, 4.57) |
| Heavy drinker | 3.18 (2.18, 4.65) | 3.27 (2.08, 5.13) | 2.83 (1.78, 4.5) | 6.79 (3.18, 14.5) | 5.71 (2.34, 13.9) | 4.13 (1.68, 10.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Han, K.; Jung, J.; Eun, Y.; Kim, I.Y.; Koh, E.-M.; Lee, S.; Cha, H.-S.; Kim, H.; Lee, J. Women with Metabolic Syndrome and Unhealthy Lifestyle Factors Are at a Higher Risk for Hyperuricemia. J. Clin. Med. 2023, 12, 7159. https://doi.org/10.3390/jcm12227159
Kang S, Han K, Jung J, Eun Y, Kim IY, Koh E-M, Lee S, Cha H-S, Kim H, Lee J. Women with Metabolic Syndrome and Unhealthy Lifestyle Factors Are at a Higher Risk for Hyperuricemia. Journal of Clinical Medicine. 2023; 12(22):7159. https://doi.org/10.3390/jcm12227159
Chicago/Turabian StyleKang, Seonyoung, Kyungdo Han, Jinhyoung Jung, Yeonghee Eun, In Young Kim, Eun-Mi Koh, Seulkee Lee, Hoon-Suk Cha, Hyungjin Kim, and Jaejoon Lee. 2023. "Women with Metabolic Syndrome and Unhealthy Lifestyle Factors Are at a Higher Risk for Hyperuricemia" Journal of Clinical Medicine 12, no. 22: 7159. https://doi.org/10.3390/jcm12227159
APA StyleKang, S., Han, K., Jung, J., Eun, Y., Kim, I. Y., Koh, E.-M., Lee, S., Cha, H.-S., Kim, H., & Lee, J. (2023). Women with Metabolic Syndrome and Unhealthy Lifestyle Factors Are at a Higher Risk for Hyperuricemia. Journal of Clinical Medicine, 12(22), 7159. https://doi.org/10.3390/jcm12227159





