The Effects of a Cardiac Rehabilitation Program on Endothelial Progenitor Cells and Inflammatory Profile in Patients with Chronic Heart Failure of Different Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Exercise Training Protocol
2.4. Flow Cytometry Analyses for EPCs and CECs
2.5. Inflammatory Indices
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Khan, M.A.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker Profiles in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e003989. [Google Scholar] [CrossRef]
- Djohan, A.H.; Sia, C.H.; Lee, P.S.; Poh, K.K. Endothelial Progenitor Cells in Heart Failure: An Authentic Expectation for Potential Future Use and a Lack of Universal Definition. J. Cardiovasc. Transl. Res. 2018, 11, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J.; Lip, G.Y.; Blann, A.D. Circulating endothelial cells in cardiovascular disease. J. Am. Coll. Cardiol. 2006, 48, 1538–1547. [Google Scholar] [CrossRef]
- Lopes, J.; Teixeira, M.; Cavalcante, S.; Gouveia, M.; Duarte, A.; Ferreira, M.; Simões, M.I.; Conceição, M.; Ribeiro, I.P.; Gonçalves, A.C.; et al. Reduced Levels of Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Heart Failure with Reduced Ejection Fraction. Arch. Med. Res. 2022, 53, 289–295. [Google Scholar] [CrossRef]
- Kourek, C.; Briasoulis, A.; Zouganeli, V.; Karatzanos, E.; Nanas, S.; Dimopoulos, S. Exercise Training Effects on Circulating Endothelial and Progenitor Cells in Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Kourek, C.; Karatzanos, E.; Psarra, K.; Ntalianis, A.; Mitsiou, G.; Delis, D.; Linardatou, V.; Pittaras, T.; Vasileiadis, I.; Dimopoulos, S.; et al. Endothelial progenitor cells mobilization after maximal exercise in patients with chronic heart failure. Hell. J. Cardiol. 2021, 62, 70–72. [Google Scholar] [CrossRef]
- Kourek, C.; Alshamari, M.; Mitsiou, G.; Psarra, K.; Delis, D.; Linardatou, V.; Pittaras, T.; Ntalianis, A.; Papadopoulos, C.; Panagopoulou, N.; et al. The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: Comparing two different exercise training protocols. Int. J. Cardiol. Heart Vasc. 2020, 32, 100702. [Google Scholar] [CrossRef]
- Kourek, C.; Karatzanos, E.; Psarra, K.; Georgiopoulos, G.; Delis, D.; Linardatou, V.; Gavrielatos, G.; Papadopoulos, C.; Nanas, S.; Dimopoulos, S. Endothelial progenitor cells mobilization after maximal exercise according to heart failure severity. World J. Cardiol. 2020, 12, 526–539. [Google Scholar] [CrossRef]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef]
- Nanas, S.; Anastasiou-Nana, M.; Dimopoulos, S.; Sakellariou, D.; Alexopoulos, G.; Kapsimalakou, S.; Papazoglou, P.; Tsolakis, E.; Papazachou, O.; Roussos, C.; et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int. J. Cardiol. 2006, 110, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Duda, D.G.; Cohen, K.S.; Scadden, D.T.; Jain, R.K. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat. Protoc. 2007, 2, 805–810. [Google Scholar] [CrossRef]
- Biosciences, B. BD Cytometric Bead Array (CBA) Human Soluble Protein Master Buffer Kit Instruction Manual; Becton, Dickinson and Company BD Biosciences: Franklin Lakes, NJ, USA, 2011. [Google Scholar]
- Flamme, I.; Breier, G.; Risau, W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev. Biol. 1995, 169, 699–712. [Google Scholar] [CrossRef]
- Erbs, S.; Höllriegel, R.; Linke, A.; Beck, E.B.; Adams, V.; Gielen, S.; Möbius-Winkler, S.; Sandri, M.; Kränkel, N.; Hambrecht, R.; et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ. Heart Fail. 2010, 3, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Viehmann, M.; Adams, V.; Rabald, K.; Mangner, N.; Höllriegel, R.; Lurz, P.; Erbs, S.; Linke, A.; Kirsch, K.; et al. Chronic heart failure and aging—Effects of exercise training on endothelial function and mechanisms of endothelial regeneration: Results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. Eur. J. Prev. Cardiol. 2016, 23, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Dopheide, J.F.; Geissler, P.; Rubrech, J.; Trumpp, A.; Zeller, G.C.; Daiber, A.; Münzel, T.; Radsak, M.P.; Espinola-Klein, C. Influence of exercise training on proangiogenic TIE-2 monocytes and circulating angiogenic cells in patients with peripheral arterial disease. Clin. Res. Cardiol. 2016, 105, 666–676. [Google Scholar] [CrossRef]
- Eleuteri, E.; Mezzani, A.; Di Stefano, A.; Vallese, D.; Gnemmi, I.; Delle Donne, L.; Taddeo, A.; Della Bella, S.; Giannuzzi, P. Aerobic training and angiogenesis activation in patients with stable chronic heart failure: A preliminary report. Biomarkers 2013, 18, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, J.; Zhao, B.; Wang, Y.; Zhang, L.; Wang, Y.; Zheng, M.; Liu, G. The Effects of High-Intensity Interval Training on Exercise Capacity and Prognosis in Heart Failure and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2022, 2022, 4273809. [Google Scholar] [CrossRef]
- Okamura, M.; Shimizu, M.; Yamamoto, S.; Nishie, K.; Konishi, M. High-intensity interval training versus moderate-intensity continuous training in patients with heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Lin, C.P.; Lin, Y.H.; Hsu, C.C.; Wang, J.S. High-intensity Interval training enhances mobilization/functionality of endothelial progenitor cells and depressed shedding of vascular endothelial cells undergoing hypoxia. Eur. J. Appl. Physiol. 2016, 116, 2375–2388. [Google Scholar] [CrossRef]
- Fuertes-Kenneally, L.; Manresa-Rocamora, A.; Blasco-Peris, C.; Ribeiro, F.; Sempere-Ruiz, N.; Sarabia, J.M.; Climent-Paya, V. Effects and Optimal Dose of Exercise on Endothelial Function in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Sports Med. Open 2023, 9, 8. [Google Scholar] [CrossRef]
- Anand, I.S.; Latini, R.; Florea, V.G.; Kuskowski, M.A.; Rector, T.; Masson, S.; Signorini, S.; Mocarelli, P.; Hester, A.; Glazer, R.; et al. C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef]
- Chia, Y.C.; Kieneker, L.M.; van Hassel, G.; Binnenmars, S.H.; Nolte, I.M.; van Zanden, J.J.; van der Meer, P.; Navis, G.; Voors, A.A.; Bakker, S.J.L.; et al. Interleukin 6 and Development of Heart Failure with Preserved Ejection Fraction in the General Population. J. Am. Heart Assoc. 2021, 10, e018549. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.J.; Smart, N.A. Aerobic Training Intensity for Improved Endothelial Function in Heart Failure Patients: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2017, 2017, 2450202. [Google Scholar] [CrossRef]
- Gielen, S.; Adams, V.; Möbius-Winkler, S.; Linke, A.; Erbs, S.; Yu, J.; Kempf, W.; Schubert, A.; Schuler, G.; Hambrecht, R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 861–868. [Google Scholar] [CrossRef]
- Cesari, F.; Marcucci, R.; Gori, A.M.; Burgisser, C.; Francini, S.; Sofi, F.; Gensini, G.F.; Abbate, R.; Fattirolli, F. Impact of a cardiac rehabilitation program and inflammatory state on endothelial progenitor cells in acute coronary syndrome patients. Int. J. Cardiol. 2013, 167, 1854–1859. [Google Scholar] [CrossRef]
- Smart, N.A.; Larsen, A.I.; Le Maitre, J.P.; Ferraz, A.S. Effect of exercise training on interleukin-6, tumour necrosis factor alpha and functional capacity in heart failure. Cardiol. Res. Pract. 2011, 2011, 532620. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Armani, A.; Iellamo, F.; Consoli, C.; Molinari, F.; Caminiti, G.; Volterrani, M.; Rosano, G.M. Effects of a short-term exercise training on serum factors involved in ventricular remodelling in chronic heart failure patients. Int. J. Cardiol. 2012, 155, 409–413. [Google Scholar] [CrossRef]
- Malandish, A.; Karimi, A.; Naderi, M.; Ghadamyari, N.; Gulati, M. Impacts of Exercise Interventions on Inflammatory Markers and Vascular Adhesion Molecules in Patients with Heart Failure: A Meta-analysis of RCTs. CJC Open 2023, 5, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Stafford, N.; Assrafally, F.; Prehar, S.; Zi, M.; De Morais, A.M.; Maqsood, A.; Cartwright, E.J.; Mueller, W.; Oceandy, D. Signaling via the Interleukin-10 Receptor Attenuates Cardiac Hypertrophy in Mice During Pressure Overload, but not Isoproterenol Infusion. Front. Pharmacol. 2020, 11, 559220. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, A.K.; Malik, A.; Akolkar, G.; Belló-Klein, A.; Khaper, N.; Singal, P.K. IL-10: A Key Molecule in the Mitigation of Heart Failure. In Biomedical Translational Research; Sobti, R., Ganju, A.K., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-Del-Olmo, M.Á.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Hoymans, V.Y.; Beckers, P.J.; Possemiers, N.M.; Wuyts, K.; Paelinck, B.P.; Vrints, C.J.; Conraads, V.M. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res. Cardiol. 2010, 105, 665–676. [Google Scholar] [CrossRef]
- Mezzani, A.; Grassi, B.; Jones, A.M.; Giordano, A.; Corrà, U.; Porcelli, S.; Della Bella, S.; Taddeo, A.; Giannuzzi, P. Speeding of pulmonary VO2 on-kinetics by light-to-moderate-intensity aerobic exercise training in chronic heart failure: Clinical and pathophysiological correlates. Int. J. Cardiol. 2013, 167, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, S.; Song, Y.; Ji, X.; Zeng, W.; Wang, X.; Wang, Y.; Feng, Q. The impact of cardiomotor rehabilitation on endothelial function in elderly patients with chronic heart failure. BMC Cardiovasc. Disord. 2021, 21, 524. [Google Scholar] [CrossRef]
- McPhate, L.; Simek, E.M.; Haines, T.P. Program-related factors are associated with adherence to group exercise interventions for the prevention of falls: A systematic review. J. Physiother. 2013, 59, 81–92. [Google Scholar] [CrossRef]
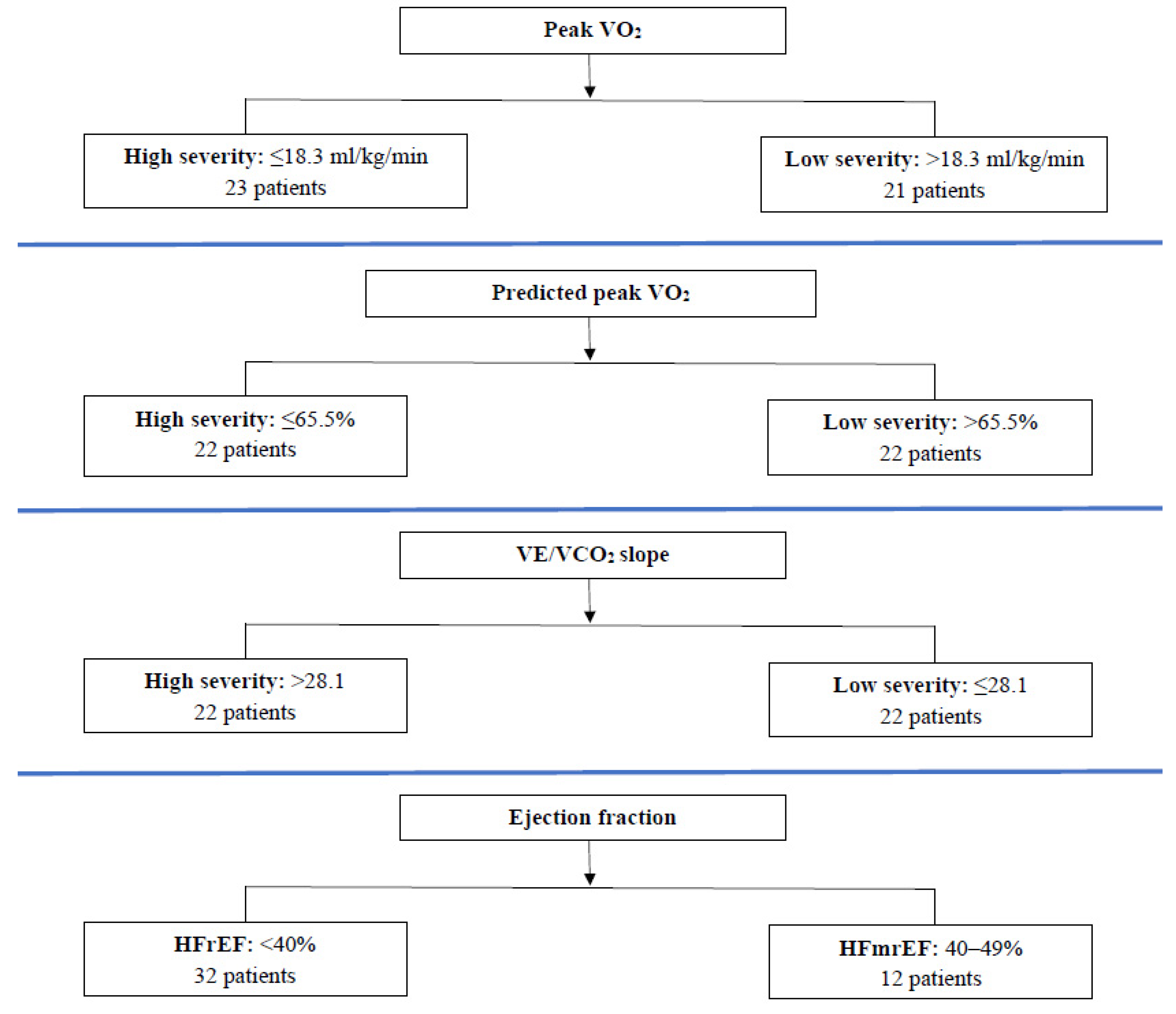
| Demographics | Peak VO2 (mL/kg/min) | Predicted Peak VO2 (%) | VE/VCO2 | Ejection Fraction (%) | ||||
|---|---|---|---|---|---|---|---|---|
| ≤18.3 | >18.3 | ≤65.5 | >65.5 | >28.1 | ≤28.1 | <40 | 40–49 | |
| Number of patients (N) | 23 | 21 | 22 | 22 | 22 | 22 | 32 | 12 |
| Gender (males/females) | 17/6 | 18/3 | 16/6 | 19/3 | 17/5 | 18/4 | 26/6 | 9/3 |
| Age (years) a | 57 ± 11 | 54 ± 9 | 51 ± 10 | 61 ± 7 * | 58 ± 9 | 54 ± 11 | 56 ± 10 | 56 ± 10 |
| Height (cm) a | 174 ± 11 | 176 ± 8 | 176 ± 12 | 174 ± 8 | 173 ± 11 | 177 ± 9 | 175 ± 10 | 175 ± 10 |
| Weight (kg) a | 90 ± 25 | 88 ± 22 | 96 ± 29 | 82 ± 14 * | 85 ± 24 | 94 ± 23 | 86 ± 21 | 97 ± 29 |
| NYHA stage (class II/III) | 17/6 | 17/4 | 17/5 | 17/5 | 14/8 | 20/2 | 23/9 | 11/1 |
| EF (%) b | 30 (25–40) | 35 (28–38) | 30 (25–41) | 33 (30–35) | 30 (25–39) | 33 (29–40) | 30 (25–35) | 44 (40–45) * |
| Baseline Cardiopulmonary Exercise Testing Indices | ||||||||
| Peak VO2 (mL/kg/min) a | 15.1 ± 2.8 | 22.1 ± 2.3 * | 16.2 ± 4.4 | 20.6 ± 3.2 * | 17.3 ± 4.2 | 19.5 ± 4.4 | 18.6 ± 4.3 | 17.9 ± 4.6 |
| Predicted peak VO2 (%) a | 55 ± 14 | 74 ± 11 * | 52 ± 9 | 77 ± 8 * | 63 ± 16 | 66 ± 15 | 65 ± 15 | 62 ± 18 |
| VE/VCO2 slope a | 29 ± 6 | 29 ± 4 | 28 ± 5 | 30 ± 5 | 33 ± 4 | 25 ± 3 * | 29 ± 5 | 28 ± 5 |
| Peak WR (watts) a | 82 ± 33 | 122 ± 33 * | 94 ± 41 | 108 ± 36 | 90 ± 38 | 112 ± 36 | 100 ± 39 | 104 ± 39 |
| Peak VO2 ≤ 18.3 mL/kg/min 23 Patients | Peak VO2 > 18.3 mL/kg/min 21 Patients | p-Value between Groups | |||
|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | ||
| Endothelial Cellular Populations b | |||||
| CD34+/CD45−/CD133+ | 54 (24–74) | 98 (76–131) * | 42 (20–71) | 85 (50–112) ** | 0.213 |
| CD34+/CD45−/CD133+/VEGFR2 | 2 (1–4) | 7 (4–9) * | 2 (1–3) | 5 (3–7) ** | 0.055 |
| CD34+/CD133+/VEGFR2 | 13 (9–16) | 22 (17–36) ** | 10 (7–19) | 23 (14–54) *** | 0.125 |
| CD34+/CD45−/CD133− | 186 (131–287) | 431 (301–618) ** | 234 (164–259) | 520 (297–866) * | 0.315 |
| CD34+/CD45−/CD133−/VEGFR2 | 1 (1–3) | 4 (3–8) ** | 1 (1–2) | 5 (3–8) * | 0.163 |
| Cardiopulmonary Exercise Testing Indices a | |||||
| Peak VO2 (mL/kg/min) | 15.1 ± 2.8 | 18.4 ± 5.1 ** | 22.1 ± 2.3 | 23.3 ± 5.4 | 0.147 |
| Predicted peak VO2 (%) | 55 ± 14 | 67 ± 21 ** | 74 ± 11 | 79 ± 21 | 0.216 |
| VE/VCO2 slope | 29 ± 6 | 28 ± 6 | 29 ± 4 | 27 ± 5 ** | 0.566 |
| Peak WR (watts) | 82 ± 33 | 102 ± 38 * | 122 ± 33 | 141 ± 43 * | 0.731 |
| Blood Sample Indices b | |||||
| CRP (mg/dL) | 0.2 (0.1–0.4) | 0.1 (0.1–0.2) ** | 0.2 (0.1–0.6) | 0.1 (0–0.4) ** | 0.798 |
| IL-6 (pg/mL) | 18.5 (15.9–23.5) | 15.1 (13–22.2) | 14.4 (12–18.6) | 14.6 (11–18.3) | 0.379 |
| IL-10 (pg/mL) | 24.5 (23.6–26.4) | 24.7 (23.3–29.5) | 24 (23.4–25.1) | 25.9 (22.9–29.2) | 0.642 |
| VEGF (pg/mL) | 14 (12–21) | 20 (15–45) * | 15 (13–19) | 24 (20–35) * | 0.235 |
| EF (%) b | 30 (25–40) | 35 (30–45) ** | 35 (28–38) | 39 (30–43) ** | 0.802 |
| Predicted Peak VO2 ≤ 65.5% 22 Patients | Predicted Peak VO2 > 65.5% 22 Patients | p-Value between Groups | |||
|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | ||
| Endothelial Cellular Populations b | |||||
| CD34+/CD45−/CD133+ | 50 (24–73) | 97 (71–107) * | 43 (22–85) | 83 (48–120) * | 0.624 |
| CD34+/CD45−/CD133+/VEGFR2 | 2 (1–4) | 6 (4–8) * | 2 (1–3) | 5 (3–8) * | 0.368 |
| CD34+/CD133+/VEGFR2 | 13 (8–16) | 22 (15–41) *** | 11 (7–18) | 24 (14–38) ** | 0.120 |
| CD34+/CD45−/CD133− | 218 (128–259) | 423 (297–575) ** | 201 (151–366) | 542 (306–738) * | 0.360 |
| CD34+/CD45−/CD133−/VEGFR2 | 1 (1–3) | 4 (3–9) ** | 1 (1–2) | 5 (3–7) * | 0.375 |
| Cardiopulmonary Exercise Testing Indices a | |||||
| Peak VO2 (mL/kg/min) | 16.2 ± 4.4 | 19.2 ± 6.4 *** | 20.6 ± 3.2 | 22.3 ± 4.6 | 0.368 |
| Predicted peak VO2 (%) | 52 ± 9 | 60 ± 16 ** | 77 ± 8 | 85 ± 20 *** | 0.360 |
| VE/VCO2 slope | 28 ± 5 | 28 ± 6 | 30 ± 5 | 27 ± 5 ** | 0.977 |
| Peak WR (watts) | 94 ± 41 | 116 ± 49 * | 108 ± 36 | 124 ± 41 * | 0.087 |
| Blood Sample Indices b | |||||
| CRP (mg/dL) | 0.4 (0.1–0.5) | 0.1 (0.1–0.3) * | 0.2 (0.1–0.4) | 0 (0–0.2) ** | 0.678 |
| IL-6 (pg/mL) | 17.5 (13.7–23.5) | 15.4 (12.9–21.4) | 16.4 (12–21) | 14.8 (11.8–18.3) | 0.228 |
| IL-10 (pg/mL) | 24.3 (23.5–26.1) | 24.4 (23.3–29.7) | 24.2 (23.5–25.6) | 26.8 (22.8–28.8) *** | 0.757 |
| VEGF (pg/mL) | 13 (12–19) | 20 (15–27) * | 15 (13–20) | 27 (20–63) * | 0.116 |
| EF (%) b | 30 (25–41) | 35 (30–44) ** | 33 (30–35) | 35 (30–45) ** | 0.717 |
| VE/VCO2 > 28.1 22 Patients | VE/VCO2 ≤ 28.1 22 Patients | p-Value between Groups | |||
|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | ||
| Endothelial Cellular Populations b | |||||
| CD34+/CD45−/CD133+ | 41 (20–66) | 88 (53–98) * | 51 (30–85) | 104 (54–127) * | 0.354 |
| CD34+/CD45−/CD133+/VEGFR2 | 2 (1–4) | 7 (4–9) * | 2 (1–2) | 5 (3–7) * | 0.114 |
| CD34+/CD133+/VEGFR2 | 12 (8–18) | 23 (17–37) ** | 12 (7–17) | 22 (14–45) ** | 0.760 |
| CD34+/CD45−/CD133− | 198 (144–380) | 425 (284–768) ** | 218 (147–246) | 452 (303–622) * | 0.903 |
| CD34+/CD45−/CD133−/VEGFR2 | 1 (1–2) | 6 (4–9) ** | 1 (1–2) | 4 (2–8) * | 0.740 |
| Cardiopulmonary Exercise Testing Indices a | |||||
| Peak VO2 (mL/kg/min) | 17.3 ± 4.2 | 19.5 ± 5.1 | 19.5 ± 4.4 | 22.0 ± 6.2 *** | 0.880 |
| Predicted peak VO2 (%) | 63 ± 16 | 72 ± 20 *** | 66 ± 15 | 74 ± 24 *** | 0.853 |
| VE/VCO2 slope | 33 ± 4 | 31 ± 5 | 25 ± 3 | 24 ± 3 | 0.498 |
| Peak WR (watts) | 90 ± 38 | 108 ± 41 * | 112 ± 36 | 133 ± 46 * | 0.668 |
| Blood Sample Indices b | |||||
| CRP (mg/dL) | 0.2 (0.1–0.4) | 0.1 (0–0.2) ** | 0.2 (0.1–0.5) | 0.1 (0–0.2) ** | 0.961 |
| IL-6 (pg/mL) | 17.4 (13.6–23.5) | 15.7 (12.9–21.2) | 16.4 (13.1–21.9) | 14.8 (11.6–19) | 0.253 |
| IL-10 (pg/mL) | 24.6 (23.5–26.1) | 27.5 (23.4–30.4) *** | 23.9 (23.5–25.1) | 24.3 (22.9–27.6) | 0.407 |
| VEGF (pg/mL) | 15 (13–21) | 27 (18–70) * | 14 (12–18) | 21 (17–27) * | 0.275 |
| EF (%) b | 30 (25–40) | 35 (29–44) ** | 35 (30–40) | 40 (34–45) * | 0.165 |
| EF < 40% 32 Patients | EF [40–49%] 12 Patients | p-Value between Groups | |||
|---|---|---|---|---|---|
| Before CR | After CR | Before CR | After CR | ||
| Endothelial Cellular Populations b | |||||
| CD34+/CD45−/CD133+ | 45 (22–75) | 88 (47–118) * | 53 (38–71) | 100 (79–118) ** | 0.618 |
| CD34+/CD45−/CD133+/VEGFR2 | 2 (1–3) | 5 (3–8) * | 2 (1–4) | 7 (4–8) ** | 0.743 |
| CD34+/CD133+/VEGFR2 | 12 (7–18) | 22 (14–37) ** | 12 (7–18) | 31 (20–45) ** | 0.773 |
| CD34+/CD45−/CD133− | 201 (149–266) | 437 (302–666) * | 227 (135–334) | 471 (253–772) ** | 0.858 |
| CD34+/CD45−/CD133−/VEGFR2 | 1 (1–2) | 5 (3–9) * | 1 (1–2) | 4 (4–8) ** | 0.596 |
| Cardiopulmonary Exercise Testing Indices a | |||||
| Peak VO2 (mL/kg/min) | 18.6 ± 4.3 | 20.6 ± 5.8 *** | 17.9 ± 4.6 | 21.1 ± 5.9 *** | 0.459 |
| Predicted peak VO2 (%) | 65 ± 15 | 72 ± 21 *** | 62 ± 18 | 74 ± 23 ** | 0.422 |
| VE/VCO2 slope | 29 ± 5 | 28 ± 6 | 28 ± 5 | 26 ± 5 | 0.906 |
| Peak WR (watts) | 100 ± 39 | 120 ± 44 * | 104 ± 39 | 121 ± 47 *** | 0.647 |
| Blood Sample Indices b | |||||
| CRP (mg/dL) | 0.2 (0.1–0.5) | 0.1 (0–0.2) * | 0.1 (0.1–0.4) | 0.1 (0–0.3) ** | 0.706 |
| IL-6 (pg/mL) | 16.8 (13.4–20.4) | 14.8 (12.2–18.1) | 16.6 (12.5–23.5) | 20.6 (12.8–22.8) | 0.067 |
| IL-10 (pg/mL) | 24.4 (23.6–26.3) | 25 (23.1–29.9) | 23.7 (23.5–24.7) | 24.8 (23.1–28.2) | 0.150 |
| VEGF (pg/mL) | 14 (13–20) | 22 (17–37) * | 15 (12–19) | 24 (16–65) ** | 0.886 |
| EF (%) b | 30 (25–35) | 35 (30–35) * | 44 (40–45) | 45 (44–50) *** | 0.726 |
| Peak VO2 | Predicted Peak VO2 | VE/VCO2 Slope | Ejection Fraction | Age | |
|---|---|---|---|---|---|
| Absolute Δθ | |||||
| CD34+/CD45−/CD133+ | −1.39 (−15.95, 13.15) | −0.20 (−4.53, 4.13) | 1.33 (−2.39, 5.07) | 0.54 (−1.55, 2.64) | −0.24 (−5.09, 4.59) |
| CD34+/CD45−/CD133+/VEGFR2 | −0.55 (−2.72, 1.62) | 0.13 (−0.51, 0.77) | 0.28 (−0.27, 0.84) | 0.10 (−0.21, 0.41) | −0.18 (−0.90, 0.53) |
| CD34+/CD133+/VEGFR2 | −0.97 (−8.98, 7.02) | 0.39 (−1.98, 2.78) | 0.73 (−1.31, 2.78) | −0.03 (−1.19, 1.11) | −0.42 (−3.08, 2.23) |
| CD34+/CD45−/CD133− | −60.91 (−224.99, 103.15) | 9.48 (−39.42, 58.39) | 18.40 (−23.67, 60.48) | −12.17 (−35.78, 11.44) | −23.83 (−78.43, 30.76) |
| CD34+/CD45−/CD133−/VEGFR2 | −0.09 (−2.06, 1.87) | 0.04 (−0.54, 0.63) | −0.02 (−0.52, 0.48) | −0.27 (−0.56, 0.01) | 0.09 (−0.55, 0.75) |
| Percentage Δθ | |||||
| CD34+/CD45−/CD133+ | −36.60 (−171.75, 98.53) | 5.45 (−34.83, 45.73) | 19.77 (−14.88, 54.43) | 3.81 (−15.64, 23.26) | −17.56 (−62.53, 27.40) |
| CD34+/CD45−/CD133+/VEGFR2 | −40.37 (−147.25, 66.50) | 7.83 (−24.02, 39.69) | 21.13 (−6.27, 48.54) | 2.84 (−12.54, 18.22) | −6.64 (−42.20, 28.92) |
| CD34+/CD133+/VEGFR2 | 33.03 (−206.57, 272.64) | −7.04 (−78.46, 64.37) | 26.14 (−35.30, 87.59) | −8.77 (−43.25, 25.71) | 9.60 (−70.12, 89.33) |
| CD34+/CD45−/CD133− | 18.27 (−386.84, 423.40) | −14.17 (−134.93, 106.58) | 62.95 (−40.95, 166.85) | 7.86 (−50.45, 66.17) | −29.44 (−164.25, 105.35) |
| CD34+/CD45−/CD133−/VEGFR2 | 12.24 (−79.94, 104.43) | −7.57 (−35.05, 19.90) | −13.93 (−37.57, 9.71) | −12.70 (−25.97, 0.56) | 14.78 (−15.89, 45.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourek, C.; Briasoulis, A.; Karatzanos, E.; Zouganeli, V.; Psarra, K.; Pratikaki, M.; Alevra-Prokopiou, A.; Skoularigis, J.; Xanthopoulos, A.; Nanas, S.; et al. The Effects of a Cardiac Rehabilitation Program on Endothelial Progenitor Cells and Inflammatory Profile in Patients with Chronic Heart Failure of Different Severity. J. Clin. Med. 2023, 12, 6592. https://doi.org/10.3390/jcm12206592
Kourek C, Briasoulis A, Karatzanos E, Zouganeli V, Psarra K, Pratikaki M, Alevra-Prokopiou A, Skoularigis J, Xanthopoulos A, Nanas S, et al. The Effects of a Cardiac Rehabilitation Program on Endothelial Progenitor Cells and Inflammatory Profile in Patients with Chronic Heart Failure of Different Severity. Journal of Clinical Medicine. 2023; 12(20):6592. https://doi.org/10.3390/jcm12206592
Chicago/Turabian StyleKourek, Christos, Alexandros Briasoulis, Eleftherios Karatzanos, Virginia Zouganeli, Katherina Psarra, Maria Pratikaki, Androula Alevra-Prokopiou, John Skoularigis, Andrew Xanthopoulos, Serafim Nanas, and et al. 2023. "The Effects of a Cardiac Rehabilitation Program on Endothelial Progenitor Cells and Inflammatory Profile in Patients with Chronic Heart Failure of Different Severity" Journal of Clinical Medicine 12, no. 20: 6592. https://doi.org/10.3390/jcm12206592
APA StyleKourek, C., Briasoulis, A., Karatzanos, E., Zouganeli, V., Psarra, K., Pratikaki, M., Alevra-Prokopiou, A., Skoularigis, J., Xanthopoulos, A., Nanas, S., & Dimopoulos, S. (2023). The Effects of a Cardiac Rehabilitation Program on Endothelial Progenitor Cells and Inflammatory Profile in Patients with Chronic Heart Failure of Different Severity. Journal of Clinical Medicine, 12(20), 6592. https://doi.org/10.3390/jcm12206592









