The Relationship between Anxiety, Visual Function, and Symptomatology in University Students
Abstract
:1. Introduction
2. Materials and Methods
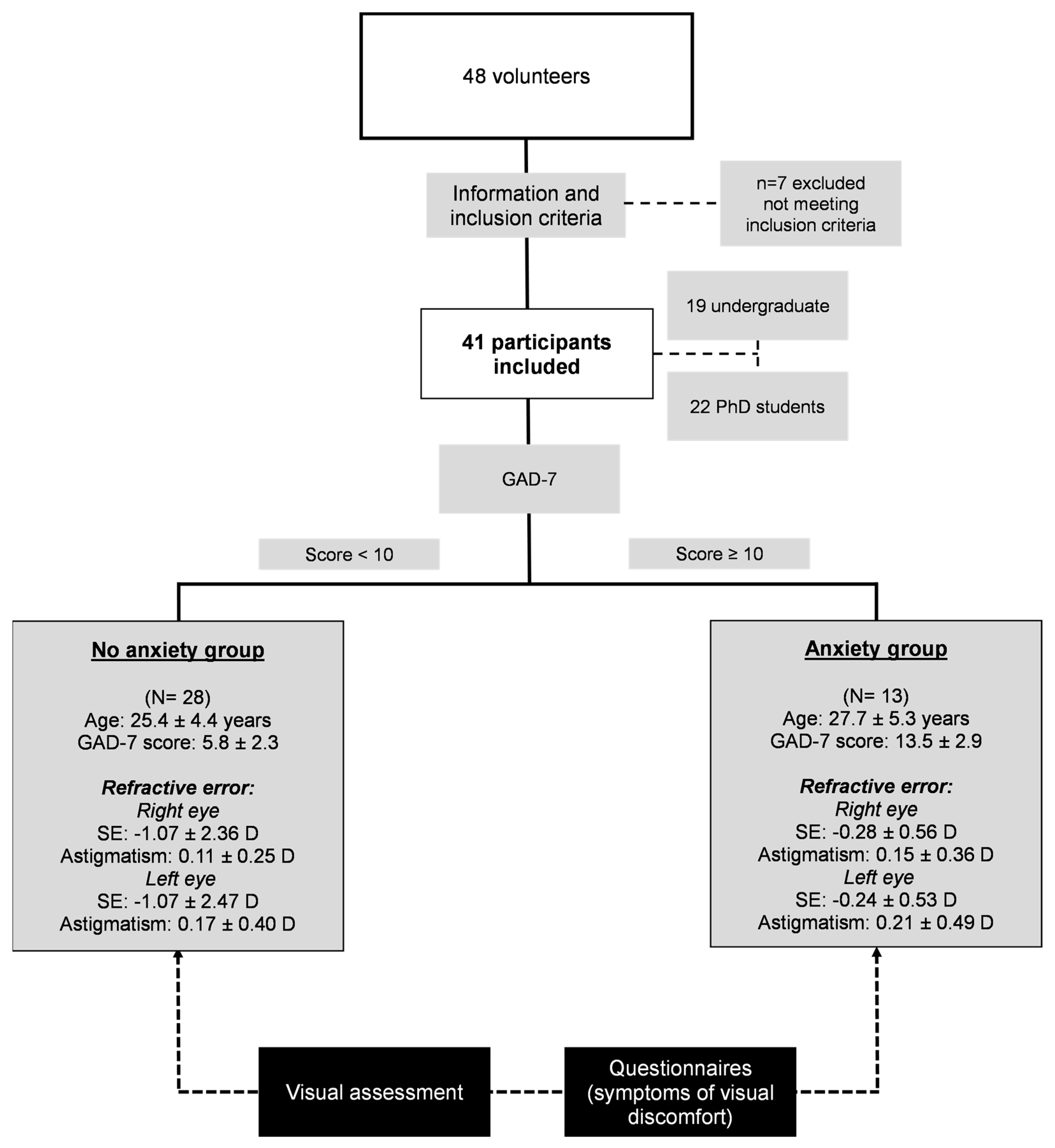
2.1. Participants
2.2. Visual Assessment
2.3. Questionnaires
2.4. Statistical Analysis
3. Results
3.1. Visual Performance Comparison between No Anxiety and Anxiety Groups
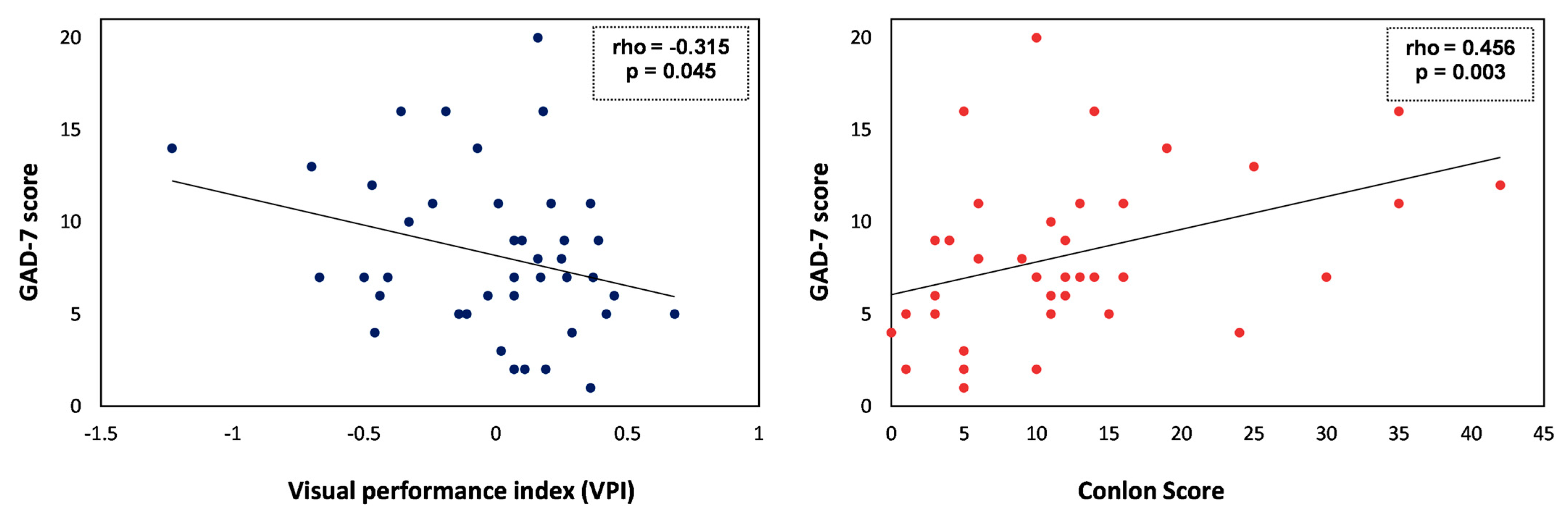
3.2. Associations between Anxiety Scores (GAD-7) and Demographics, Visual Performance Index (VPI), or Self-Perceived Visual Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levecque, K.; Anseel, F.; De Beuckelaer, A.; Van der Heyden, J.; Gisle, L. Work organization and mental health problems in PhD students. Res. Policy 2017, 46, 868–879. [Google Scholar] [CrossRef]
- Mattijssen, L.M.S.; Bergmans, J.E.; van der Weijden, I.C.M.; Teelken, J.C. In the eye of the storm: The mental health situation of PhD candidates. Perspect. Med. Educ. 2020, 10, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Woolston, C. PhDs: The tortuous truth. Nature 2019, 575, 403–406. [Google Scholar] [CrossRef]
- The mental health of PhD researchers demands urgent attention. Nature 2019, 575, 257–258. [CrossRef]
- Auerbach, R.P.; Alonso, J.; Axinn, W.G.; Cuijpers, P.; Ebert, D.D.; Green, J.G.; Hwang, I.; Kessler, R.C.; Liu, H.; Mortier, P.; et al. Mental disorders among college students in the World Health Organization World Mental Health Surveys. Psychol. Med. 2016, 46, 2955–2970. [Google Scholar] [CrossRef] [PubMed]
- Lipson, S.K.; Lattie, E.G.; Eisenberg, D. Increased rates of mental health service utilization by U.S. College students: 10-year population-level trends (2007–2017). Psychiatr. Serv. 2019, 70, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Arntz, A.; Ibañez, C.; Covarrubias, T. Unexplained ocular and visual symptoms: The need for a psychosomatic approach in Ophthalmology. Arch. Soc. Esp. Oftalmol. 2019, 94, 575–578. [Google Scholar] [CrossRef]
- Sabel, B.A.; Wang, J.; Cárdenas-Morales, L.; Faiq, M.; Heim, C. Mental stress as consequence and cause of vision loss: The dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. 2018, 9, 133–160. [Google Scholar] [CrossRef]
- Gilmartin, B.; Mallen, E.A.H.; Wolffsohn, J.S. Sympathetic control of accommodation: Evidence for inter-subject variation. Ophthalmic Physiol. Opt. 2002, 22, 366–371. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef]
- Pardhan, S.; López Sánchez, G.F.; Bourne, R.; Davis, A.; Leveziel, N.; Koyanagi, A.; Smith, L. Visual, hearing, and dual sensory impairment are associated with higher depression and anxiety in women. Int. J. Geriatr. Psychiatry 2021, 36, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; Drusian, A.; Ronchese, F.; Negro, C. Video display operator complaints: A 10-year follow-up of visual fatigue and refractive disorders. Int. J. Environ. Res. Public Health 2019, 16, 2501. [Google Scholar] [CrossRef] [PubMed]
- Mocci, F.; Serra, A.; Corrias, G.A. Psychological factors and visual fatigue in working with video display terminals. Occup. Environ. Med. 2001, 58, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Borsting, E.; Chase, C.H.; Ridder, W.H. Measuring visual discomfort in college students. Optom. Vis. Sci. 2007, 84, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Conlon, E.G.; Lovegrove, W.J.; Chekaluk, E.; Pattison, P.E. Measuring visual discomfort. Vis. Cogn. 1999, 6, 637–663. [Google Scholar] [CrossRef]
- Sheedy, J.E.; Hayes, J.; Engle, J. Is all asthenopia the same? Optom. Vis. Sci. 2003, 80, 732–739. [Google Scholar] [CrossRef]
- Tosha, C.; Borsting, E.; Ridder, W.H.; Chase, C. Accommodation response and visual discomfort. Ophthalmic Physiol. Opt. 2009, 29, 625–633. [Google Scholar] [CrossRef]
- Scheiman, M.; Wick, B. Clinical Management of Binocular Vision, 3rd ed.; Wolters Kluwer Health/Lippincott Williams & Wilins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Löwe, B.; Decker, O.; Müller, S.; Brähler, E.; Schellberg, D.; Herzog, W.; Herzberg, P.Y. Validation and Standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the General Population. Med. Care 2008, 46, 266–274. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Hashemi, H.; Nabovati, P.; Khabazkhoob, M.; Yekta, A.; Hassan Emamian, M.; Fotouhi, A. Does Hofstetter’s equation predict the real amplitude of accommodation in children? Clin. Exp. Optom. 2018, 101, 123–128. [Google Scholar] [CrossRef]
- Sheppard, A.L.; Davies, L.N. Clinical evaluation of the Grand Seiko Auto Ref/Keratometer WAM-5500. Ophthalmic Physiol. Opt. 2010, 30, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Peregrina, S.; Ortiz, C.; Martino, F.; Castro-Torres, J.J.; Anera, R.G. Dynamics of the accommodative response after smoking cannabis. Ophthalmic Physiol. Opt. 2021, 41, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Poltavski, D.V.; Biberdorf, D.; Petros, T.V. Accommodative response and cortical activity during sustained attention. Vis. Res. 2012, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Casares-López, M.; Castro-Torres, J.J.; Ortiz-Peregrina, S.; Ortiz, C.; Anera, R.G. Changes in accommodation dynamics after alcohol consumption, for two different doses. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 259, 919–928. [Google Scholar] [CrossRef]
- Alarcón, A.; Anera, R.G.; Villa, C.; del Barco, L.J.; Gutierrez, R. Visual quality after monovision correction by laser in situ keratomileusis in presbyopic patients. J. Cataract Refract. Surg. 2011, 37, 1629–1635. [Google Scholar] [CrossRef]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The development of an instrument to measure quality of vision: The quality of vision (QoV) questionnaire. Investig. Opthalmol. Vis. Sci. 2010, 51, 5537–5545. [Google Scholar] [CrossRef]
- Fernandes, P.; Amorim-de-Sousa, A.; Queirós, A.; Escandón-Garcia, S.; McAlinden, C.; González-Méijome, J.M. Light disturbance with multifocal contact lens and monovision for presbyopia. Contact Lens Anterior Eye 2018, 41, 393–399. [Google Scholar] [CrossRef]
- Armstrong, R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef]
- Woolston, C. Graduate survey: A love–hurt relationship. Nature 2017, 550, 549–552. [Google Scholar] [CrossRef]
- Debowska, A.; Horeczy, B.; Boduszek, D.; Dolinski, D. A repeated cross-sectional survey assessing university students’ stress, depression, anxiety, and suicidality in the early stages of the COVID-19 pandemic in Poland. Psychol. Med. 2020, 2, 1–4. [Google Scholar] [CrossRef]
- Mattijssen, L.; van Vliet, N.; van Doorn, T.; Kanbier, N.; Teelken, C. PNN PhD Survey Asking the Relevant Questions; Promovendi Netwerk Nederland: Utrecht, The Netherlands, 2020. [Google Scholar]
- Törnqvist, G. The relative importance of the parasympathetic and sympathetic nervous systems for accommodation in monkeys. Investig. Ophthalmol. 1967, 6, 612–617. [Google Scholar]
- Gilmartin, B.; Bullimore, M.A. Sustained near-vision augments sympathetic innvervation of the ciliary muscle. Clin. Vis. Sci. 1987, 1, 197–208. [Google Scholar]
- Gilmartin, B. Autonomic correlates of the near vision response in emmetropia and myopia. In Myopia and Nearwork; Butterworth Heinemann: London, UK, 1998; pp. 117–146. [Google Scholar]
- Gilmartin, B. Pharmacology of accommodative adaptation. In Accommodation and Vergence Mechanisms in the Visual System; Birkhäuser: Basel, Switzerland, 2000; pp. 141–150. [Google Scholar]
- Kiely, P.M.; Crewther, S.G.; Crewther, D.P. Is there an association between functional vision and learning to read? Clin. Exp. Optom. 2001, 84, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.; Tosha, C.; Borsting, E.; Ridder, W.H. Visual discomfort and objective measures of static accommodation. Optom. Vis. Sci. 2009, 86, 883–889. [Google Scholar] [CrossRef]

| No Anxiety Group (N = 28) | Anxiety Group (N = 13) | Statistic t/Z | p-Value | |
|---|---|---|---|---|
| Near point of convergence (cm) | 6.06 ± 1.32 | 6.76 ± 2.22 | −1.252 | 0.218 |
| Binocular AF (cycles/min) | 9.92 ± 3.46 | 7.14 ± 3.98 | 2.265 | 0.029 |
| Monocular AF (cycles/min) | 11.00 ± 3.97 | 7.56 ± 4.61 | 2.436 | 0.020 |
| Binocular AA relative to age (D) (AA− AA (age)) *,ª | 2.41 ± 2.05 | 0.95 ± 2.71 | −1.240 | 0.224 |
| Monocular AA relative to age (D) (AA− AA (age)) ª | 1.29 ± 1.93 | 0.72 ± 2.92 | 0.748 | 0.459 |
| Lag (2.5 D) * | −0.86 ± 0.50 | −1.00 ± 0.63 | −0.662 | 0.515 |
| Lag (3 D) | −0.97 ± 0.63 | −1.11 ± 0.68 | 0.628 | 0.534 |
| Lag (5 D) * | −1.40 ± 1.02 | −1.65 ± 1.25 | −0.492 | 0.628 |
| Binocular VA * (decimal scale) | 1.26 ± 0.15 | 1.17 ± 0.18 | −1.591 | 0.121 |
| Monocular VA * (decimal scale) | 1.14 ± 0.18 | 0.98 ± 0.17 | −2.499 | 0.012 |
| RMS total HOA * | 0.20 ± 0.06 | 0.17 ± 0.07 | −1.665 | 0.096 |
| RMS Spherical | 0.06 ± 0.07 | 0.02 ± 0.06 | 1.733 | 0.091 |
| RMS Coma | 0.12 ± 0.06 | 0.09 ± 0.06 | 1.845 | 0.073 |
| RMS Trefoil | 0.10 ± 0.05 | 0.09 ± 0.05 | 0.827 | 0.414 |
| General Visual Performance Index (VPI) * | 0.07 ± 0.32 | −0.20 ± 0.43 | −1.989 | 0.047 |
| QoV Symptom Frequency Subscale | 18.93 ± 12.44 | 27.18 ± 14.65 | −1.758 | 0.094 |
| QoV Symptom Severity Subscale * | 19.76 ± 13.24 | 29.23 ± 15.22 | −2.056 | 0.041 |
| QoV Symptom Bothersomeness Subscale | 14.88 ± 11.95 | 29.49 ± 17.15 | −2.774 | 0.013 |
| Conlon Total Score * | 9.44 ± 7.06 | 19.23 ± 11.75 | −2.849 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Peregrina, S.; Ortiz, C.; Casares-López, M.; Martino, F.; Granados-Delgado, P.; Anera, R.G. The Relationship between Anxiety, Visual Function, and Symptomatology in University Students. J. Clin. Med. 2023, 12, 6595. https://doi.org/10.3390/jcm12206595
Ortiz-Peregrina S, Ortiz C, Casares-López M, Martino F, Granados-Delgado P, Anera RG. The Relationship between Anxiety, Visual Function, and Symptomatology in University Students. Journal of Clinical Medicine. 2023; 12(20):6595. https://doi.org/10.3390/jcm12206595
Chicago/Turabian StyleOrtiz-Peregrina, Sonia, Carolina Ortiz, Miriam Casares-López, Francesco Martino, Pilar Granados-Delgado, and Rosario G. Anera. 2023. "The Relationship between Anxiety, Visual Function, and Symptomatology in University Students" Journal of Clinical Medicine 12, no. 20: 6595. https://doi.org/10.3390/jcm12206595
APA StyleOrtiz-Peregrina, S., Ortiz, C., Casares-López, M., Martino, F., Granados-Delgado, P., & Anera, R. G. (2023). The Relationship between Anxiety, Visual Function, and Symptomatology in University Students. Journal of Clinical Medicine, 12(20), 6595. https://doi.org/10.3390/jcm12206595







