Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review
Abstract
1. Introduction
2. Short Characteristics of Selected Immunosuppressive Drugs
2.1. Cyclosporin A
2.2. Tacrolimus
2.3. Sirolimus/Rapamycin
2.4. Mycophenolate Mofetil
2.5. Azathioprine
2.6. Glucocorticoids
2.7. Basiliximab and Anti-Thymocyte Globulin
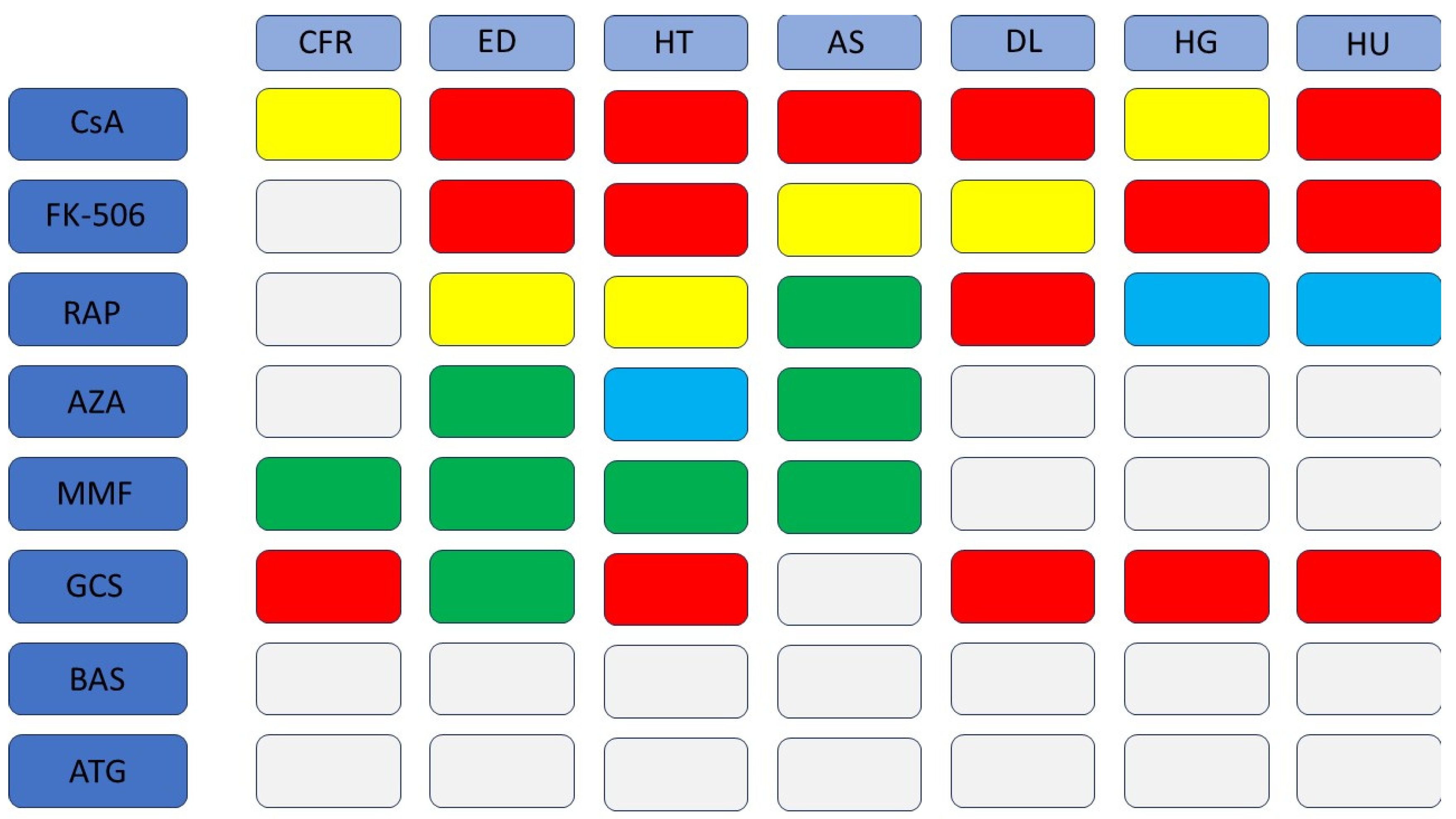
3. Effects of Immunosuppressive Drugs on Various Components of the Cardiovascular System
3.1. Fibrosis and Myocardial Remodeling
3.2. Endothelial Function
3.3. Hypertension
3.4. Atherosclerosis
3.5. Dyslipidemia
3.6. Hyperglycemia
3.7. Metabolic Syndrome
3.8. Hyperuricemia
3.9. Biological Drugs—Effects on Selected Metabolic and Cardiovascular Aspects
4. Summary
5. Conclusions
6. Clinical Implications
7. Recommendations for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suthanthiran, M.; Morris, R.E.; Strom, T.B. Immunosuppressants: Cellular and molecular mechanisms of action. Am. J. Kidney Dis. Off J. Natl. Kidney Found 1996, 28, 159–172. [Google Scholar] [CrossRef]
- Chang, D.H.; Youn, J.C.; Dilibero, D.; Patel, J.K.; Kobashigawa, J.A. Heart transplant immunosuppression strategies at cedars-sinai medical center. Int. J. Heart Fail. 2021, 3, 15–30. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Kuca, K.; Wu, W. Cyclosporine A: Chemistry and toxicity—A review. Curr. Med. Chem. 2021, 28, 3925–3934. [Google Scholar] [CrossRef]
- Dawar, F.U.; Xiong, Y.; Khattak, M.N.K.; Li, J.; Lin, L.; Mei, J. Potential role of cyclophilin A in regulating cytokine secretion. J. Leukoc. Biol. 2017, 102, 989–992. [Google Scholar] [CrossRef]
- Loeb, I.; Hermans, P. Cyclosporine A. Rev. Stomatol. Chir. Maxillofac. 2005, 106, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kuca, K. Metabolic Pathway of Cyclosporine A and Its Correlation with Nephrotoxicity. Curr. Drug Metab. 2019, 20, 84–90. [Google Scholar] [CrossRef]
- Letko, E.; Bhol, K.; Pinar, V.; Foster, C.S.; Ahmed, A.R. Tacrolimus (fk 506). Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 1999, 83, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kalt, D.A. Tacrolimus: A review of laboratory detection methods and indications for use. Lab. Med. 2017, 48, e62–e65. [Google Scholar] [CrossRef]
- Saunders, R.N.; Metcalfe, M.S.; Nicholson, M.L. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001, 59, 3–16. [Google Scholar] [CrossRef]
- Deng, L.; Chen, L.; Zhao, L.; Xu, Y.; Peng, X.; Wang, X.; Ding, L.; Jin, J.; Teng, H.; Wang, Y.; et al. Ubiquitination of Rheb governs growth factor-induced mTORC1 activation. Cell Res. 2019, 29, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B. Toxicity spectrum of inhibitors of mammalian target of rapamycin in organ transplantation: Etiology, pathogenesis and treatment. Expert Opin. Drug Saf. 2011, 10, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Nashan, B.; Citterio, F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: A critical review of the literature. Transplantation 2012, 94, 547–561. [Google Scholar] [CrossRef]
- Hood, K.A.; Zarembski, D.G. Mycophenolate mofetil: A unique immunosuppressive agent. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. 1997, 54, 285–294. [Google Scholar] [CrossRef]
- Moder, K.G. Mycophenolate mofetil: New applications for this immunosuppressant. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2003, 90, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.L.; Pirofski, L. Mycophenolate mofetil: Effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2009, 11, 290–297. [Google Scholar] [CrossRef]
- Villarroel, M.C.; Hidalgo, M.; Jimeno, A. Mycophenolate mofetil: An update. Drugs Today 2009, 45, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, O.; Azathioprine, K.T.A. StatPearls. Treasure Island (FL); StatPearls Publishing: Tampa, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542190/ (accessed on 18 July 2023).
- Van Scoik, K.G.; Johnson, C.A.; Porter, W.R. The pharmacology and metabolism of the thiopurine drugs 6-mercaptopurine and azathioprine. Drug Metab. Rev. 1985, 16, 157–174. [Google Scholar] [CrossRef]
- Broen, J.C.; van Laar, J.M. Mycophenolate mofetil, azathioprine and tacrolimus: Mechanisms in rheumatology. Nat. Rev. Rheumatol. 2020, 16, 167–178. [Google Scholar] [CrossRef]
- Chan, G.L.; Canafax, D.M.; Johnson, C.A. The therapeutic use of azathioprine in renal transplantation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1987, 7, 165–177. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Grzanka, A.; Jarząb, J. Niegenomowy mechanizm działania glikokortykosteroidów. Adv. Respir. Med. 2009, 77, 387–393. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Atluru, D.; Sierakowski, S.; Lianos, E.A. Mechanism of action of glucocorticosteroids. Inhibition of T cell proliferation and interleukin 2 production by hydrocortisone is reversed by leukotriene B4. J. Clin. Investig. 1986, 77, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.J.; McCormick, M.E. The mechanism of the anti-inflammatory activity of glucocorticosteroids. Agents Actions 1985, 16, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Samoliński, B.; Nowicka, A.; Wojas, O.; Lipiec, A.; Krzych-Fałta, E.; Tomaszewska, A. Intranasal glucocorticosteroids–not only in allergic rhinitis In the 40th anniversary of intranasal glucocorticosteroids’ introduction. Otolaryngol. Pol. 2014, 68, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Nashan, B.; Moore, R.; Amlot, P.; Schmidt, A.G.; Abeywickrama, K.; Soulillou, J.P. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet 1997, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Kapic, E.; Becic, F.; Kusturica, J. Basiliximab, mechanism of action and pharmacological properties. Med. Arh. 2004, 58, 373–376. [Google Scholar]
- Mohty, M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef]
- Yañez San Segundo, L.; Lopez Pereira, B.; Bermudez, M.A.; Insunza, A.; Romon, I.; Richard, C.; Conde, E. Differences of Antithymocyte Globulin (ATG) Side Effects during Allogeneic Stem Cell Transplantation. Blood 2015, 126, 5449. [Google Scholar] [CrossRef]
- Hughes, A.; Okasha, O.; Farzaneh-Far, A.; Kazmirczak, F.; Nijjar, P.S.; Velangi, P.; Akçakaya, M.; Martin, C.M.; Shenoy, C. Myocardial Fibrosis and Prognosis in Heart Transplant Recipients. Circ. Cardiovasc. Imaging 2019, 12, e009060. [Google Scholar] [CrossRef]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef]
- Surówka, A.; Prowans, P.; Żołnierczuk, M.; Miśkiewicz, M.; Wawrowski, T.; Skodda, M.; Markowska, M.; Kędzierska-Kapuza, K. The Effect of Calcineurin Inhibitors on MMPs Activity in Heart and Their Side Effects—A Review of Literature. Int. J. Mol. Sci. 2023, 24, 10291. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.; Rodella, L.; Rezzani, R. Cyclosporine A up-regulates expression of matrix metalloproteinase 2 and vascular endothelial growth factor in rat heart. Int. Immunopharmacol. 2003, 3, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Berthier, C.C.; Pally, C.; Weckbecker, G.; Raulf, F.; Rehrauer, H.; Wagner, U.; Le Hir, M.; Marti, H.P. Experimental heart transplantation: Effect of cyclosporine on expression and activity of metzincins. Swiss Med. Wkly. 2009, 139, 233–240. [Google Scholar] [CrossRef]
- Chi, J.; Wang, L.; Zhang, X.; Fu, Y.; Liu, Y.; Chen, W.; Liu, W.; Shi, Z.; Yin, X. Cyclosporin A induces autophagy in cardiac fibroblasts through the NRP-2/WDFY-1 axis. Biochimie 2018, 148, 55–62. [Google Scholar] [CrossRef]
- Kolář, F.; Papoušek, F.; MacNaughton, C.; Pelouch, V.; Milerová, M.; Korecky, B. Myocardial fibrosis and right ventricular function of heterotopically transplanted hearts in rats treated with cyclosporin. Mol. Cell. Biochem. 1996, 163, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Angoscini, P.; Rodella, L.F.; Bianchi, R. Alterations induced by cyclosporine A in myocardial fibers and extracellular matrix in rat. Histol. Histopathol. 2002, 17, 761–766. [Google Scholar]
- Stovin, P.G.; English, T.A. Effects of cyclosporine on the transplanted human heart. J. Heart Transplant. 1987, 6, 180–185. [Google Scholar]
- Karch, S.B.; Billingham, M.E. Cyclosporine induced myocardial fibrosis: A unique controlled case report. J. Heart Transplant. 1985, 4, 210–212. [Google Scholar]
- Yang, C.-H.; Sheu, J.-J.; Tsai, T.-H.; Chua, S.; Chang, L.-T.; Chang, H.-W.; Lee, F.-Y.; Chen, Y.-L.; Chung, S.-Y.; Sun, C.-K.; et al. Effect of tacrolimus on myocardial infarction is associated with inflammation, ROS, MAP kinase and Akt pathways in mini-pigs. J. Atheroscler. Thromb. 2013, 20, 9–22. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Tsujisaka, Y.; Li, V.Y.; Tani, K.; Lucena-Cacace, A.; Yoshida, Y. Immunosuppressants tacrolimus and sirolimus revert the cardiac antifibrotic properties of p38-MAPK inhibition in 3D-multicellular human iPSC-heart organoids. Front. Cell Dev. Biol. 2022, 10, 1001453. [Google Scholar] [CrossRef]
- Haller, S.T.; Yan, Y.; Drummond, C.A.; Xie, J.; Tian, J.; Kennedy, D.J.; Shilova, V.Y.; Xie, Z.; Liu, J.; Cooper, C.J.; et al. Rapamycin Attenuates Cardiac Fibrosis in Experimental Uremic Cardiomyopathy by Reducing Marinobufagenin Levels and Inhibiting Downstream Pro-Fibrotic Signaling. J. Am. Heart Assoc. 2016, 5, e004106. [Google Scholar] [CrossRef]
- Flynn, J.M.; O’Leary, M.N.; Zambataro, C.A.; Academia, E.C.; Presley, M.P.; Garrett, B.J.; Zykovich, A.; Mooney, S.D.; Strong, R.; Rosen, C.J.; et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 2013, 12, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Rud’, R.; Kogan, E.; Zaitsev, A.; Nedostup, A. Comparative Efficacy and Safety of Mycophenolate Mofetil and Azathioprine in Combination with Corticosteroids in the Treatment of Lymphocytic Myocarditis. J. Clin. Med. 2023, 12, 4913. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Ghose Roy, S.; Kar, D.; Bandyopadhyay, A. Excess of glucocorticoid induces myocardial remodeling and alteration of calcium signaling in cardiomyocytes. J. Endocrinol. 2011, 209, 105. [Google Scholar] [CrossRef]
- Tanaka, S.; Shibuya, H.; Suzuki, S.; Kanno, N.; Harada, Y.; Sato, A.; Soeta, S.; Hara, Y. Long-term administration of prednisolone: Effects on the myocardial tissue of healthy beagle dogs. J. Vet. Med. Sci. 2021, 83, 84–93. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Oakley, R.H.; Cidlowski, J.A. Glucocorticoid Signaling and the Aging Heart. Front. Endocrinol. 2020, 11, 347. Available online: https://www.frontiersin.org/articles/10.3389/fendo.2020.00347 (accessed on 13 September 2023). [CrossRef] [PubMed]
- Kelley, C.; Vander Molen, J.; Choi, J.; Bhai, S.; Martin, K.; Cochran, C.; Puthanveetil, P. Impact of Glucocorticoids on Cardiovascular System—The Yin Yang Effect. J. Pers. Med. 2022, 12, 1829. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. Glucocorticoid signaling in the heart: A cardiomyocyte perspective. J. Steroid Biochem. Mol. Biol. 2015, 153, 27–34. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef]
- Trepels, T.; Zeiher, A.M.; Fichtlscherer, S. The endothelium and inflammation. Endothel. J. Endothel. Cell Res. 2006, 13, 423–429. [Google Scholar] [CrossRef]
- Renner, B.; Klawitter, J.; Goldberg, R.; McCullough, J.W.; Ferreira, V.P.; Cooper, J.E.; Christians, U.; Thurman, J.M. Cyclosporine induces endothelial cell release of complement-activating microparticles. J. Am. Soc. Nephrol. JASN 2013, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Brodowski, L.; von Kaisenberg, C.; Schröder-Heurich, B.; von Versen-Höynck, F. Cyclosporine A and Tacrolimus Induce Functional Impairment and Inflammatory Reactions in Endothelial Progenitor Cells. Int. J. Mol. Sci. 2021, 22, 9696. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Diez, R.; González-Guerrero, C.; Ocaña-Salceda, C.; Rodrigues-Diez, R.R.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M.; Ramos, A.M. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci. Rep. 2016, 6, 27915. [Google Scholar] [CrossRef]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Reineke, D.C.; Müller-Schweinitzer, E.; Winkler, B.; Kunz, D.; Konerding, M.A.; Grussenmeyer, T.; Carrel, T.P.; Eckstein, F.S.; Grapow, M.T.R. Rapamycin impairs endothelial cell function in human internal thoracic arteries. Eur. J. Med. Res. 2015, 20, 59. [Google Scholar] [CrossRef]
- Rosner, D.; McCarthy, N.; Bennett, M. Rapamycin inhibits human in stent restenosis vascular smooth muscle cells independently of pRB phosphorylation and p53. Cardiovasc. Res. 2005, 66, 601–610. [Google Scholar] [CrossRef]
- Weigel, G.; Griesmacher, A.; DeAbreu, R.A.; Wolner, E.; Mueller, M.M. Azathioprine and 6-mercaptopurine alter the nucleotide balance in endothelial cells. Thromb. Res. 1999, 94, 87–94. [Google Scholar] [CrossRef]
- Marinkovic, G.; Hibender, S.; Hoogenboezem, M.; van Broekhoven, A.; Girigorie, A.F.; Bleeker, N.; Hamers, A.A.J.; Stap, J.; van Buul, J.D.; de Vries, C.J.M.; et al. Immunosuppressive drug azathioprine reduces aneurysm progression through inhibition of Rac1 and c-Jun-terminal-N-kinase in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2380–2388. [Google Scholar] [CrossRef]
- Fréguin-Bouilland, C.; Godin, M.; Bellien, J.; Richard, V.; Remy-Jouet, I.; Dautreaux, B.; Henry, J.P.; Compagnon, P.; Thuillez, C.; Plissonnier, D.; et al. Protective effect of mycophenolate mofetil on endothelial function in an aortic allograft model. Transplantation 2011, 91, 35–41. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Leckel, K.; Wittig, B.; Zenker, D.; Oppermann, E.; Harder, S.; Scholz, M.; Weber, S.; Schuldes, H.; Encke, A.; et al. Inhibition of endothelial receptor expression and of T-cell ligand activity by mycophenolate mofetil. Transpl. Immunol. 1998, 6, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, F.; Prati, C.; Maguin-Gaté, K.; Wendling, D.; Demougeot, C. Glucocorticoids and endothelial function in inflammatory diseases: Focus on rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Jahan, A.; Yadav, T.P.; Sachdev, N.; Chitkara, A.; Asare, R. Effect of glucocorticoids on serum lipid profile and endothelial function and arterial wall mechanics. Indian J. Pediatr. 2013, 80, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, K.A.; Van Moortel, L.; Opdenakker, G.; De Bosscher, K.; Van den Steen, P.E. Endothelial Response to Glucocorticoids in Inflammatory Diseases. Front. Immunol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, M.V.; Zivkovic, V.I.; Toncev, S.; Tasic, N.; Bogdanovic, V.; Djuric, D.M.; Jakovljevic, V.L. Carotid artery intima-media thickness and brachial artery flow-mediated vasodilatation in patients with rheumatoid arthritis. VASA Z Gefasskrankheiten. 2002, 41, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Chatzikyrkou, C.; Menne, J.; Gwinner, W.; Schmidt, B.M.; Lehner, F.; Blume, C.; Schwarz, A.; Haller, H.; Schiffer, M. Pathogenesis and management of hypertension after kidney transplantation. J. Hypertens. 2011, 29, 2283–2294. [Google Scholar] [CrossRef]
- El-Gowelli, H.M.; El-Mas, M.M. Central modulation of cyclosporine-induced hypertension. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 351–361. [Google Scholar] [CrossRef]
- Taler, S.J.; Textor, S.C.; Canzanello, V.J.; Schwartz, L. Cyclosporin-induced hypertension: Incidence, pathogenesis and management. Drug Saf. 1999, 20, 437–449. [Google Scholar] [CrossRef]
- Hošková, L.; Málek, I.; Kopkan, L.; Kautzner, J. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol. Res. 2017, 66, 167–180. [Google Scholar] [CrossRef]
- Marienhagen, K.; Lehner, F.; Klempnauer, J.; Hecker, H.; Borlak, J. Treatment of cyclosporine induced hypertension: Results from a long-term observational study using different antihypertensive medications. Vasc. Pharmacol. 2019, 115, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Tacrolimus-induced hypertension: What’s endothelial and hematopoietic FKBP12 got to do with it? Hypertens Dallas Tex. 1979, 2011, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, S.; Fei, L.; Dong, F.; Xie, L.; Qiu, X.; Lei, Y.; Guo, J.; Zhong, M.; Ren, X.; et al. Tacrolimus Causes Hypertension by Increasing Vascular Contractility via RhoA (Ras Homolog Family Member A)/ROCK (Rho-Associated Protein Kinase) Pathway in Mice. Hypertension 2022, 79, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.; Parada, B.; Teixeira de Lemos, E.; Garrido, P.; Dias, A.; Piloto, N.; Baptista, S.; Sereno, J.; Eufrásio, P.; Costa, E.; et al. Hypertension induced by immunosuppressive drugs: A comparative analysis between sirolimus and cyclosporine. Transpl. Proc. 2009, 41, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Arns, W.; Schwenger, V.; Mehrabi, A.; Fonouni, H.; Schmidt, J.; Zeier, M. Sirolimus in renal transplantation. Nephrol Dial Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2007, 22 (Suppl. S8), viii61–viii65. [Google Scholar] [CrossRef]
- Prüfer, J.; Schuchardt, M.; Tölle, M.; Prüfer, N.; Höhne, M.; Zidek, W.; van der Giet, M. Harmful effects of the azathioprine metabolite 6-mercaptopurine in vascular cells: Induction of mineralization. PLoS ONE 2014, 9, e101709. [Google Scholar] [CrossRef]
- Kalra, S.S.; Shanahan, C.M. Vascular calcification and hypertension: Cause and effect. Ann. Med. 2012, 44 (Suppl. S1), S85–S92. [Google Scholar] [CrossRef]
- Herrera, J.; Ferrebuz, A.; MacGregor, E.G.; Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 2006, 17 (Suppl. S3), S218–S225. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Fan, Q.; Li, X.; Liu, K.; Zhang, F. Effect of Mycophenolate Mofetil on Blood Pressure: A Meta-Analysi. 2019. Available online: https://assets.researchsquare.com/files/rs-6837/v1/b5daf893-ee7d-4b03-a991-76cdfb60748d.pdf?c=1631827856 (accessed on 28 May 2023).
- Brem, A.S. Insights into glucocorticoid-associated hypertension. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2001, 37, 1–10. [Google Scholar] [CrossRef]
- Isidori, A.M.; Graziadio, C.; Paragliola, R.M.; Cozzolino, A.; Ambrogio, A.G.; Colao, A.; Corsello, S.M.; Pivonello, R.; ABC Study Group. The hypertension of Cushing’s syndrome: Controversies in the pathophysiology and focus on cardiovascular complications. J. Hypertens. 2015, 33, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Nazareth, I.; Petersen, I. Synthetic Glucocorticoids and Early Variations of Blood Pressure: A Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2015, 100, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.E.; Yimer, B.B.; Roads, P.; Jani, M.; Dixon, W.G. Glucocorticoid use is associated with an increased risk of hypertension. Rheumatology 2021, 60, 132–139. [Google Scholar] [CrossRef]
- Mebrahtu, T.F.; Morgan, A.W.; West, R.M.; Stewart, P.M.; Pujades-Rodriguez, M. Oral glucocorticoids and incidence of hypertension in people with chronic inflammatory diseases: A population-based cohort study. CMAJ Can. Med. Assoc. J. 2020, 192, E295–E301. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927. [Google Scholar]
- Fuster, V.; Stein, B.; Ambrose, J.A.; Badimon, L.; Badimon, J.J.; Chesebro, J.H. Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation 1990, 82 (Suppl. S3), II47–II59. [Google Scholar]
- Kwiatkowska, M.; Oldakowska-Jedynak, U.; Wojtaszek, E.; Glogowski, T.; Malyszko, J. Potential Effects of Immunosuppression on Oxidative Stress and Atherosclerosis in Kidney Transplant Recipients. Oxidative Med. Cell. Longev. 2021, 2021, 6660846. [Google Scholar] [CrossRef]
- Moreno, J.M.; Ruiz, M.C.; Ruiz, N.; Gomez, I.; Vargas, F.; Asensio, C.; Osuna, A. Modulation factors of oxidative status in stable renal transplantation. Transpl. Proc. 2005, 37, 1428–1430. [Google Scholar] [CrossRef]
- Li, X.; Shang, X.; Sun, L. Tacrolimus reduces atherosclerotic plaque formation in ApoE-/-mice by inhibiting NLRP3 inflammatory corpuscles. Exp. Ther. Med. 2020, 19, 1393–1399. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Zou, S.; Qu, L. Rapamycin: A Bacteria-Derived Immunosuppressant That Has Anti-Atherosclerotic Effects and Its Clinical Application. Front. Pharmacol. 2019, 9, 1520. [Google Scholar] [CrossRef] [PubMed]
- Van Skike, C.E.; DeRosa, N.; Galvan, V.; Hussong, S.A. Rapamycin restores peripheral blood flow in aged mice and in mouse models of atherosclerosis and Alzheimer’s disease. GeroScience 2023, 45, 1987–1996. [Google Scholar] [CrossRef]
- Pols, T.W.H.; Bonta, P.I.; Pires, N.M.M.; Otermin, I.; Vos, M.; de Vries, M.R.; van Eijk, M.; Roelofsen, J.; Havekes, L.M.; Quax, P.H.A.; et al. 6-mercaptopurine inhibits atherosclerosis in apolipoprotein e* 3-leiden transgenic mice through atheroprotective actions on monocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1591–1597. [Google Scholar] [CrossRef]
- Romero, F.; Rodrıguez-Iturbe, B.; Pons, H.; Parra, G.; Quiroz, Y.; Rincon, J.; González, L. Mycophenolate mofetil treatment reduces cholesterol-induced atherosclerosis in the rabbit. Atherosclerosis 2000, 152, 127–133. [Google Scholar] [CrossRef]
- van Leuven, S.I.; van Wijk, D.F.; Volger, O.L.; de Vries, J.P.P.M.; van der Loos, C.M.; de Kleijn, D.V.P.; Horrevoets, A.J.G.; Tak, P.P.; van der Wal, A.C.; de Boer, O.J.; et al. Mycophenolate mofetil attenuates plaque inflammation in patients with symptomatic carotid artery stenosis. Atherosclerosis 2010, 211, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.N.; Magder, L.S.; Petri, M. Mycophenolate mofetil (MMF) does not slow the progression of subclinical atherosclerosis in SLE over 2 years. Rheumatol. Int. 2012, 32, 2701–2705. [Google Scholar] [CrossRef]
- van der Sluis, R.J.; Hoekstra, M. Glucocorticoids are active players and therapeutic targets in atherosclerotic cardiovascular disease. Mol. Cell. Endocrinol. 2020, 504, 110728. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.; Hadoke, P.W.; Nixon, M. Glucocorticoids: Fuelling the fire of atherosclerosis or therapeutic extinguishers? Int. J. Mol. Sci. 2021, 22, 7622. [Google Scholar] [CrossRef]
- Ross, I.L.; Marais, A.D. The influence of glucocorticoids on lipid and lipoprotein metabolism and atherosclerosis: Forum-clinical alert. South Afr. Med. J. 2014, 104, 671–674. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- De Giorgi, A.; Fabbian, F. Immunosuppression in renal transplantation and dyslipidemia, which factors should be considered? Nephro-Urol. Mon. 2013, 5, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzo, G.; Cuomo, G.; Di Lorenzo, A.; Tripaldella, M.; Mallardo, V.; Iaccarino Idelson, P.; Sagnelli, C.; Sica, A.; Creta, M.; Baltar, J.; et al. Dyslipidemia in Transplant Patients: Which Therapy? J. Clin. Med. 2022, 11, 4080. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, X.B.; Peng, L.K.; Peng, F.H.; Lan, G.B.; Wang, Y.; Yu, S.J.; Fang, C.H. Dyslipidemia in human kidney transplant recipients receiving cyclosporine and tacrolimus is associated with different expression of CD36 on peripheral blood monocytes. Transpl. Proc. 2011, 43, 1612–1615. [Google Scholar] [CrossRef]
- Kockx, M.; Glaros, E.; Leung, B.; Ng, T.W.; Berbée, J.F.P.; Deswaerte, V.; Nawara, D.; Quinn, C.; Rye, K.A.; Jessup, W.; et al. Low-Density Lipoprotein Receptor–Dependent and Low-Density Lipoprotein Receptor–Independent Mechanisms of Cyclosporin A–Induced Dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prasad, G.R. Post-transplant dyslipidemia: Mechanisms, diagnosis and management. World J. Transplant. 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, B.; Wei, Y.G.; Yan, L.N.; Wen, T.F.; Zhao, J.C.; Xu, M.Q.; Wang, W.T.; Ma, Y.K.; Yang, J.Y. Higher tacrolimus blood concentration is related to hyperlipidemia in living donor liver transplantation recipients. Dig. Dis. Sci. 2012, 57, 204–209. [Google Scholar] [CrossRef]
- Hakeam, H.A.; Al-Jedai, A.H.; Raza, S.M.; Hamawi, K. Sirolimus induced dyslipidemia in tacrolimus based vs. tacrolimus free immunosuppressive regimens in renal transplant recipients. Ann. Transpl. 2008, 13, 46–53. [Google Scholar]
- Morrisett, J.D.; Abdel-Fattah, G.; Hoogeveen, R.; Mitchell, E.; Ballantyne, C.M.; Pownall, H.J.; Opekun, A.R.; Jaffe, J.S.; Oppermann, S.; Kahan, B.D. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J. Lipid Res. 2002, 43, 1170–1180. [Google Scholar] [CrossRef]
- Akman, B.; Uyar, M.; Afsar, B.; Sezer, S.; Ozdemir, F.N.; Haberal, M. Lipid profile during azathioprine or mycophenolate mofetil combinations with cyclosporine and steroids. Transpl. Proc. 2007, 39, 135–137. [Google Scholar] [CrossRef]
- Subramanian, S.; Trence, D.L. Immunosuppressive agents: Effects on glucose and lipid metabolism. Endocrinol. Metab. Clin. North Am. 2007, 36, 891–905. [Google Scholar] [CrossRef]
- Miller, L.W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2002, 2, 807–818. [Google Scholar] [CrossRef]
- Arnaldi, G.; Scandali, V.M.; Trementino, L.; Cardinaletti, M.; Appolloni, G.; Boscaro, M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology 2010, 92 (Suppl. S1), 86–90. [Google Scholar] [CrossRef]
- Cattran, D.C.; Steiner, G.; Wilson, D.R.; Fenton, S.S.A. Hyperlipidemia after renal transplantation: Natural history and pathophysiology. Ann. Intern. Med. 1979, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Ettinger Jr, W.H.; Hazzard, W.R. Prednisone increases very low density lipoprotein and high density lipoprotein in healthy men. Metabolism 1988, 37, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-First Century? Int. J. Health Sci. 2007, 1, V–VIII. [Google Scholar]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246. [Google Scholar] [CrossRef] [PubMed]
- Fourtounas, C. Transplant options for patients with type 2 diabetes and chronic kidney disease. World J. Transplant. 2014, 4, 102. [Google Scholar] [CrossRef] [PubMed]
- Shivaswamy, V.; Boerner, B.; Larsen, J. Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr. Rev. 2016, 37, 37–61. [Google Scholar] [CrossRef]
- Tosur, M.; Viau-Colindres, J.; Astudillo, M.; Redondo, M.J.; Lyons, S.K. Medication-induced hyperglycemia: Pediatric perspective. BMJ Open Diabetes Res. Care 2020, 8, e000801. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Fasting and rapamycin: Diabetes versus benevolent glucose intolerance. Cell Death Dis. 2019, 10, 607. [Google Scholar] [CrossRef]
- Simmons, W.D.; Rayhill, S.C.; Sollinger, H.W. Preliminary risk-benefit assessment of mycophenolate mofetil in transplant rejection. Drug Saf. 1997, 17, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Montero, N.; Pascual, J. Immunosuppression and post-transplant hyperglycemia. Curr. Diabetes Rev. 2015, 11, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Clore, J.N.; Thurby-Hay, L. Glucocorticoid-induced hyperglycemia. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2009, 15, 469–474. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, J.G.; Mireles-Zavala, L.G.; Rodriguez-Gutierrez, R.; Gomez-Almaguer, D.; Lavalle-Gonzalez, F.J.; Tamez-Perez, H.E.; Gonzalez-Saldivar, G.; Villarreal-Perez, J.Z. Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol. Metab. Syndr. 2013, 5, 18. [Google Scholar] [CrossRef]
- Tamez-Pérez, H.E.; Quintanilla-Flores, D.L.; Rodríguez-Gutiérrez, R.; González-González, J.G.; Tamez-Peña, A.L. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J. Diabetes 2015, 6, 1073. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, Q.; Xu, Y.; Wang, W.; Zhang, X.; Hu, X. Effects of tacrolimus and cyclosporine treatment on metabolic syndrome and cardiovascular risk factors after renal transplantation: A meta-analysis. Chin. Med. J. 2014, 127, 2376–2381. [Google Scholar] [PubMed]
- Lo, A. Immunosuppression and metabolic syndrome in renal transplant recipients. Metab. Syndr. Relat. Disord. 2004, 2, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Steigerwalt, S.P.; Brar, N.; Dhungel, A.; Butcher, D.; Steigerwalt, S.; El-Ghouroury, M.; Provenzano, R. Improved 24-hour blood pressure control with sirolimus versus calcineurin inhibitor based immunosuppression in renal transplant recipients. Transpl. Proc. 2009, 41, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- den Hartigh, L.J.; Goodspeed, L.; Wang, S.A.; Kenerson, H.L.; Omer, M.; O’Brien, K.D.; Ladiges, W.; Yeung, R.; Subramanian, S. Chronic oral rapamycin decreases adiposity, hepatic triglycerides and insulin resistance in male mice fed a diet high in sucrose and saturated fat. Exp. Physiol. 2018, 103, 1469–1480. [Google Scholar] [CrossRef]
- Luijten, I.H.N.; Brooks, K.; Boulet, N.; Shabalina, I.G.; Jaiprakash, A.; Carlsson, B.; Fischer, A.W.; Cannon, B.; Nedergaard, J. Glucocorticoid-Induced Obesity Develops Independently of UCP1. Cell Rep. 2019, 27, 1686–1698. [Google Scholar] [CrossRef]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Harvey, I.; Stephenson, E.J.; Redd, J.R.; Tran, Q.T.; Hochberg, I.; Qi, N.; Bridges, D. Glucocorticoid-Induced Metabolic Disturbances are Exacerbated in Obese Male mice. Endocrinology 2018, 159, 2275–2287. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Nakagawa, T.; Kanbay, M.; Kuwabara, M.; Kumar, A.; Garcia Arroyo, F.E.; Roncal-Jimenez, C.; Sasai, F.; Kang, D.H.; Jensen, T.; et al. Hyperuricemia in Kidney Disease: A Major Risk factor for Cardiovascular Events, Vascular Calcification and Renal Damage. Semin. Nephrol. 2020, 40, 574–585. [Google Scholar] [CrossRef]
- Numakura, K.; Satoh, S.; Tsuchiya, N.; Saito, M.; Maita, S.; Obara, T.; Tsuruta, H.; Inoue, T.; Narita, S.; Horikawa, Y.; et al. Hyperuricemia at 1 year after renal transplantation, its prevalence, associated factors, and graft survival. Transplantation 2012, 94, 145–151. [Google Scholar] [CrossRef]
- Tumgor, G.; Arikan, C.; Kilic, M.; Aydogdu, S. Frequency of hyperuricemia and effect of calcineurin inhibitors on serum uric acid levels in liver transplanted children. Pediatr. Transplant. 2006, 10, 665–668. [Google Scholar] [CrossRef]
- Mazali, F.C.; Mazzali, M. Uric acid and transplantation. Semin. Nephrol. 2011, 31, 466–471. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Schold, J.D.; Vanrenterghem, Y.; Halloran, P.F.; Ekberg, H. Uric acid Levels Have No Significant Effect on Renal Function in Adult Renal Transplant Recipients: Evidence from the Symphony Study. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1655. [Google Scholar] [CrossRef]
- Rainey, H.; Baraf, H.S.B.; Yeo, A.; Lipsky, P. Thu0410 Companion Immunosuppression with Azathioprine Increases the Frequency of Persistent Responsiveness to Pegloticase in Patients with Chronic Refractory Gout. Ann. Rheum. Dis. 2020, 79 (Suppl. S1), 442–443. [Google Scholar] [CrossRef]
- Kelley, W.N.; Rosenbloom, F.M.; Seegmiller, J.E. The effects of azathioprine (Imuran) on purine synthesis in clinical disorders of purine metabolism. J. Clin. Investig. 1967, 46, 1518–1529. [Google Scholar] [CrossRef]
- Stamp, L.; Searle, M.; O’Donnell, J.; Chapman, P. Gout in solid organ transplantation: A challenging clinical problem. Drugs 2005, 65, 2593–2611. [Google Scholar] [CrossRef]
- Schlitt, H.J.; Barkmann, A.; Böker, K.H.; Schmidt, H.H.; Emmanouilidis, N.; Rosenau, J.; Bahr, M.J.; Tusch, G.; Manns, M.P.; Nashan, B.; et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: A randomised controlled study. Lancet 2001, 357, 587–591. [Google Scholar] [CrossRef]
- Jones, E.A.; Cain, G.D.; Dickinson, G. Corticosteroid-induced changes in urea metabolism in patients with hepatocellular disease. Gastroenterology 1972, 62, 612–617. [Google Scholar] [CrossRef]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Baroletti, S.; Bencivenga, G.A.; Gabardi, S. Treating gout in kidney transplant recipients. Prog. Transplant. 2004, 14, 143–147. [Google Scholar] [CrossRef]
- Wang, R.; Moura, L.A.Z.; Lopes, S.V.; da Costa, F.D.A.; Souza Filho, N.F.S.; Fernandes, T.L.; Salvatti, N.B.; Faria Neto, J.R. Reduced Progression of Cardiac Allograft Vasculopathy with Routine Use of Induction Therapy with Basiliximab. Arq. Bras. Cardiol. 2015, 105, 176–183. [Google Scholar] [CrossRef]
- Cunningham, M.W.; Amaral, L.M.; Campbell, N.E.; Cornelius, D.C.; Ibrahim, T.; Vaka, V.R.; LaMarca, B. Investigation of interleukin-2-mediated changes in blood pressure, fetal growth restriction, and innate immune activation in normal pregnant rats and in a preclinical rat model of preeclampsia. Biol. Sex Differ. 2021, 12, 4. [Google Scholar] [CrossRef]
- Prasad, N.; Gurjer, D.; Bhadauria, D.; Gupta, A.; Srivastava, A.; Kaul, A.; Jaiswal, A.; Yadav, B.; Yadav, S.; Sharma, R.K. Is basiliximab induction, a novel risk factor for new onset diabetes after transplantation for living donor renal allograft recipients? Nephrology Carlton Vic. 2014, 19, 244–250. [Google Scholar] [CrossRef]
- Masset, C.; Kerleau, C.; Blancho, G.; Hourmant, M.; Walencik, A.; Ville, S.; Kervella, D.; Cantarovich, D.; Houzet, A.; Giral, M.; et al. Very Low Dose Anti-Thymocyte Globulins Versus Basiliximab in Non-Immunized Kidney Transplant Recipients. Transpl. Int. 2023, 36, 10816. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Mildner, M.; Werba, G.; Beer, L.; Hoetzenecker, K.; Baumgartner, A.; Hasun, M.; Nickl, S.; Mitterbauer, A.; Zimmermann, M.; et al. Anti-thymocyte Globulin Induces Neoangiogenesis and Preserves Cardiac Function after Experimental Myocardial Infarction. PLoS ONE 2012, 7, e52101. [Google Scholar] [CrossRef]
- Mendes, B.; Figueiredo, C.; Cabral, M.; Borba, A.; Mineiro, A.; Cardoso, J.; Calvinho, P.; Semedo, L.; Fragata, J. Basiliximab vs. Antithymocyte Globulin as Initial Induction Therapy for Lung Transplantation: A National Two Years Review. Transplantology 2022, 3, 267–274. [Google Scholar] [CrossRef]
- Kyaw, T.; Winship, A.; Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Li, P.; Tipping, P.; Bobik, A.; Toh, B.H. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 2013, 127, 1028–1039. [Google Scholar] [CrossRef]
- Ducloux, D.; Courivaud, C.; Bamoulid, J.; Crepin, T.; Chalopin, J.M.; Tiberghien, P.; Saas, P. Polyclonal Antithymocyte Globulin and Cardiovascular Disease in Kidney Transplant Recipients. J. Am. Soc. Nephrol. JASN 2014, 25, 1349. [Google Scholar] [CrossRef] [PubMed]
- Azarbal, B.; Cheng, R.; Vanichsarn, C.; Patel, J.K.; Czer, L.S.; Chang, D.H.; Kittleson, M.M.; Kobashigawa, J.A. Induction Therapy with Antithymocyte Globulin in Patients Undergoing Cardiac Transplantation Is Associated with Decreased Coronary Plaque Progression as Assessed by Intravascular Ultrasound. Circ. Heart Fail. 2016, 9, e002252. [Google Scholar] [CrossRef]
- Kumar, V.; Prabhu, S.D.; Bansal, S.S. CD4+ T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 992653. [Google Scholar] [CrossRef]
- Matthia, E.L.; Setteducato, M.L.; Elzeneini, M.; Vernace, N.; Salerno, M.; Kramer, C.M.; Keeley, E.C. Circulating Biomarkers in Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2022, 11, e027618. [Google Scholar] [CrossRef]
- Becker, R.C.; Owens, A.P.; Sadayappan, S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J. Thromb. Thrombolysis 2020, 49, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lillo, R.; Graziani, F.; Franceschi, F.; Iannaccone, G.; Massetti, M.; Olivotto, I.; Crea, F.; Liuzzo, G. Inflammation across the spectrum of hypertrophic cardiac phenotypes. Heart Fail Rev. 2023, 28, 1065–1075. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Kumar, V.; Gupta, S.; Bermeo-Blanco, O.; Stratton, M.S.; Gumina, R.J.; Bansal, S.S. Estrogen receptor-β agonists modulate t-lymphocyte activation and ameliorate left ventricular remodeling during chronic heart failure. Circ. Heart Fail. 2022, 15, e008997. [Google Scholar] [CrossRef]
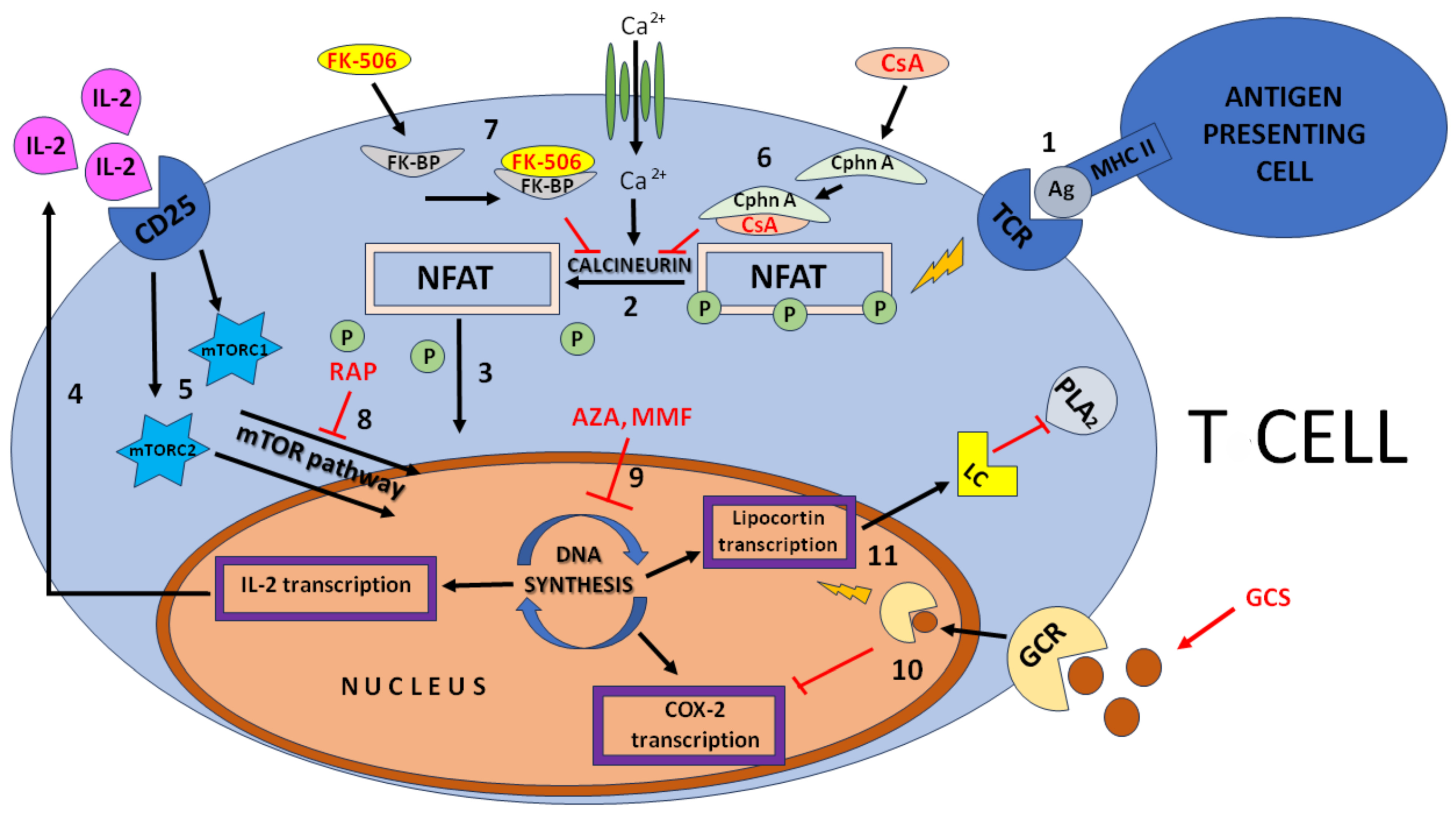
| Induction Therapy | Maintenance Therapy | |
|---|---|---|
| Calcineurin Inhibitors | FK-506 | CsA FK-506 |
| mTOR Inhibitors | – | RAP Everolimus |
| Anti-metabolites | MMF | MMF Enteric-coated MMF Mycophenolate sodium AZA |
| Glucocorticoids | High-dose GCSs | Methylprednisolone Prednisolone |
| Biologic Agents | ATG BAS | – |
| Disease Entity | Drug | Effect | Model | Reference |
|---|---|---|---|---|
| Fibrosis and Myocardial Remodeling | CsA | Increased cardiac fibrosis, increased expression of metalloproteinase 2, increased expression of vascular endothelial growth factor. | Rat | [34] |
| Reduced inflammatory response, increased fibrosis. | Rat | [35] | ||
| Profibrogenic effect. | Rat; CC * | [36,37,38] | ||
| Profibrogenic effect. | Human | [39,40] | ||
| FK-506 | Reduced infarct zone, reduced loss of left ventricular function, reduced fibrosis, and cardiac remodeling after infarction. | Pig | [41] | |
| Increased collagen deposition in the heart and increased fibrosis. | CC | [42] | ||
| RAP | Increased collagen deposition in the heart and increased fibrosis. | CC | [42] | |
| Reduced fibrotic zone in rats after nephrectomy, reduced expression of type I collagen. | Rat | [43] | ||
| No changes. | Rat | [44] | ||
| AZA | No reports. | --- | --- | |
| MMF | Decreased synthesis of pro-inflammatory cytokines and reduced fibrosis. | Human | [45] | |
| GCS | Increased weight of heart, left ventricular remodeling, increased fibrosis. | Rat | [46] | |
| Increased cardiac fibrosis, decreased levels of GCRs, and increased levels for the mineralocorticoid receptor. | Dog | [47] | ||
| Earlier cardiac aging, hypertrophy, fibrosis, diastolic dysfunction, increased wall stiffness, calcium imbalance in cardiomyocytes. | R/M ** | [48,49] | ||
| ATG | Reduced ischemia-induced cell damage, reduced remodeling and necrosis. | Rat | [152] | |
| Endothelial Disfunction | CsA | Activation of complement pathway, leading to endothelial damage. | Rat | [54] |
| Release of proinflammatory cytokines, causing damage and impaired endothelial function. | CC | [55,56] | ||
| Reduced vasodilatation. | Human | [57] | ||
| Modulation of vascular wall, increased oxidative stress and inflammation. | R/M | [32] | ||
| FK-506 | Release of proinflammatory cytokines, causing damage and impaired endothelial function. | CC | [55,56] | |
| Reduced vasodilatation, to a lesser extent than CsA. | Human | [57] | ||
| Modulation of vascular wall, increased oxidative stress and inflammation. | R/M | [32] | ||
| RAP | Reduced vasodilatation. | Human | [58] | |
| Antiproliferative effect. | Human | [59] | ||
| AZA | Antiproliferative effect. | CC | [60] | |
| Reduction in inflammation in the vessel walls and the inhibition of lymphocytes involved in the degradation of the integrity of the vessel walls. | Mouse | [61] | ||
| MMF | Increased availability of NO associated with improved functionality of endothelial nitric oxide synthase. | Rat | [62] | |
| Reduction in inflammatory infiltration in the transplanted organs. | CC | [63] | ||
| GCS | Reduced vasodilatation. | R/M | [64] | |
| No effect. | Human | [65] | ||
| Inhibition of immune response pathways in the inflammatory process and enhancement of the synthesis of protective factors for the vascular epithelium. | R/M | [66] | ||
| Improvement of endothelial function (low doses). | Human | [67] | ||
| BAS | Reduced coronary vessel volume and thinner intima layer. | Human | [148] | |
| Hypertension | CsA | Alteration of blood pressure. | R/M | [70] |
| Increased vasoconstriction. | R/M | [71] | ||
| Activation of renin–angiotensin–aldosterone system, increased oxidative stress, increased plasma sodium levels. | R/M | [72] | ||
| FK-506 | Activation of renin–angiotensin–aldosterone system, increased oxidative stress, increased plasma sodium levels. | R/M | [72] | |
| Inhibition of endothelial function related to the vasoconstrictor effect. | RM | [74] | ||
| Increased oxidative stress and activation of the angiotensin-II-dependent vasoconstriction pathway. | Mouse | [75] | ||
| RAP | Induction of hypertension. | Rat | [76] | |
| Induction of hypertension and nephrotoxicity to a lesser extent than CNIs. | R/M | [77] | ||
| AZA | Calcification of blood vessels, causing isolated hypertension in the elderly. | CC | [78] | |
| MMF | Reduction in systolic and diastolic blood pressure. | Human | [80] | |
| Reduction in diastolic blood pressure, though almost no effect on systolic blood pressure. | R/M | [81] | ||
| GCS | Development hypertension and other cardiovascular diseases. | R/M | [83] | |
| No effect on blood pressure (short-term use). | Human | [84] | ||
| Increased blood pressure (prednisolone > 7.5 mg/d), no changes in blood pressure (<7.5 mg/d). | Human | [85] | ||
| Significant increase in predisposition to the development of hypertension (cumulative doses of GCS). | Human | [86] | ||
| BAS | No effect. | Rat | [149] | |
| ATG | Risk of hypotension. | Human | [153] | |
| Atherosclerosis | CsA | Increased oxidative stress, contributing to oxygen free-radical-related vascular damage. | R/M | [90] |
| Extremely high risk of atherosclerosis. | Human | [91] | ||
| FK-506 | Similar profile of action on the oxidative status of the body to CsA, not a significant cause of dyslipidemia. | Human | [91] | |
| Inhibition of atherosclerotic plaque development. | Mouse | [92] | ||
| RAP | Stabilization of atherosclerotic plaque, reduced necrotic core of the plaque, and reduced vascular inflammation. | R/M | [93] | |
| Increased vascular blood flow in atherosclerotic vessels. | Mouse | [94] | ||
| AZA | Reduced inflammation in the plaque, reduced atherosclerotic lesion formation. | Mouse | [95] | |
| MMF | Reduction in diet-induced atherosclerotic potential. | Rabbit | [96] | |
| Reduced inflammatory cell infiltration into the plaques and reduced expression of pro-inflammatory genes. | Human | [97] | ||
| No decrease in carotid artery thickness (measure of atherosclerosis progression). | Human | [98] | ||
| GCS | Unknown, but more research should be conducted. | R/M | [100,101] | |
| ATG | Increased risk of atherosclerosis. | Human | [155] | |
| Reduced progression of atherosclerosis. | Human | [156] | ||
| Dyslipidemia | CsA | Significant increase in TC, LDL-C, and TG. | R/M | [104] |
| Abnormal lipoprotein clearance, decreased lipoprotein lipase activity, elevated apolipoprotein C-III, and impaired subtilisin/kexin type 9 protein converting activity. | Mouse | [106] | ||
| Increased risk of hypercholesterolaemia. | R/M | [107] | ||
| FK-506 | No significant changes in lipid profile. | Human | [105] | |
| No contribution to hypercholesterolemia. | R/M | [107] | ||
| Increased risk of dyslipidemia in patients taking high doses of FK-506. | Human | [108] | ||
| Increased levels of TG and TC. | Human | [109] | ||
| RAP | Increased levels of TG and TC. | Human | [109,110] | |
| AZA | No significant reports. | Human | [111] | |
| No significant reports | R/M | [112] | ||
| MMF | No significant reports. | Human | [111] | |
| No significant reports | R/M | [113] | ||
| GCS | Increased levels of TG an TC. | R/M | [114] | |
| Increased risk of hypertriglyceridemia (high dosages). | Human | [115] | ||
| Significant increase in VLDL-TG and VLDL-C with a concomitant increase in HDL-C. | Human | [116] | ||
| Hyperglycemia | CsA | Impaired glucose tolerance. | R/M | [120] |
| Increased risk of diabetes mellitus. | Human | [121] | ||
| FK-506 | Impaired glucose tolerance (more than CsA). | R/M | [120] | |
| Increased risk of diabetes mellitus (higher risk than CsA). | Human | [121] | ||
| RAP | Mild hyperglycemia in oncology patients, no increase in blood glucose in healthy patients. | R/M | [122] | |
| AZA | No significant reports. | R/M | [123,124] | |
| MMF | No significant reports. | R/M | [123,124] | |
| GCS | Dose-dependent risk of carbohydrate metabolism disorders. | R/M | [125] | |
| High potential to induce hyperglycemia. | Human | [126] | ||
| BAS | Greater predisposition to developing diabetes. | Human | [150,151] | |
| Metabolic Syndrome | CsA | Large contribution to development of MS. | N/A *** | [70,71,72,120,121,129,130] |
| FK-506 | Large contribution to development of MS. | N/A | [70,71,72,120,121,129,130] | |
| RAP | More studies needed. | N/A | [77,110,122,131,132] | |
| AZA | No increase in risk of MS development. | N/A | [78,79,80,81,111,112,113,123,124] | |
| MMF | No increase in risk of MS development. | N/A | [78,79,80,81,111,112,113,123,124] | |
| GCS | Dose- and time-depended risk of MS development. | N/A | [83,85,86,114,115,116,121,125,126,127,133,134,135] | |
| Hyperuricemia | CsA | Increased risk of developing hyperuricemia, increased endothelin-1 release, and reduced nitric oxide production. | Human | [137] |
| Increased risk of developing hyperuricemia. | Human | [138] | ||
| FK-506 | Increased risk of developing hyperuricemia, increased endothelin-1 release, and reduced nitric oxide production. | Human | [137] | |
| Increased risk of developing hyperuricemia. | Human | [138] | ||
| Lower incidence of hyperuricemia after FK-506 administration than after CsA. | R/M | [139] | ||
| RAP | No effect on uric acid metabolism. | Human | [140] | |
| AZA | Good effect with pegloticase on decreasing blood urate concentration. | Human | [141] | |
| MMF | No effect or decrease in the mean uric acid concentration. | Human | [144] | |
| GCS | Elevation in blood urate levels. | Human | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opałka, B.; Żołnierczuk, M.; Grabowska, M. Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. J. Clin. Med. 2023, 12, 6935. https://doi.org/10.3390/jcm12216935
Opałka B, Żołnierczuk M, Grabowska M. Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. Journal of Clinical Medicine. 2023; 12(21):6935. https://doi.org/10.3390/jcm12216935
Chicago/Turabian StyleOpałka, Bianka, Michał Żołnierczuk, and Marta Grabowska. 2023. "Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review" Journal of Clinical Medicine 12, no. 21: 6935. https://doi.org/10.3390/jcm12216935
APA StyleOpałka, B., Żołnierczuk, M., & Grabowska, M. (2023). Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. Journal of Clinical Medicine, 12(21), 6935. https://doi.org/10.3390/jcm12216935







