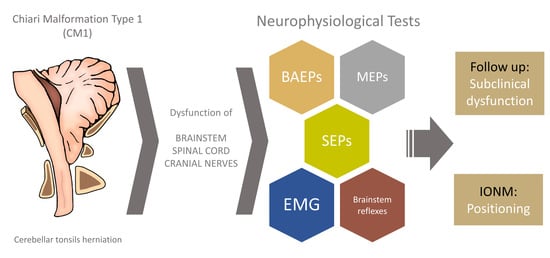
The Role of Neurophysiology in Managing Patients with Chiari Malformations
Abstract
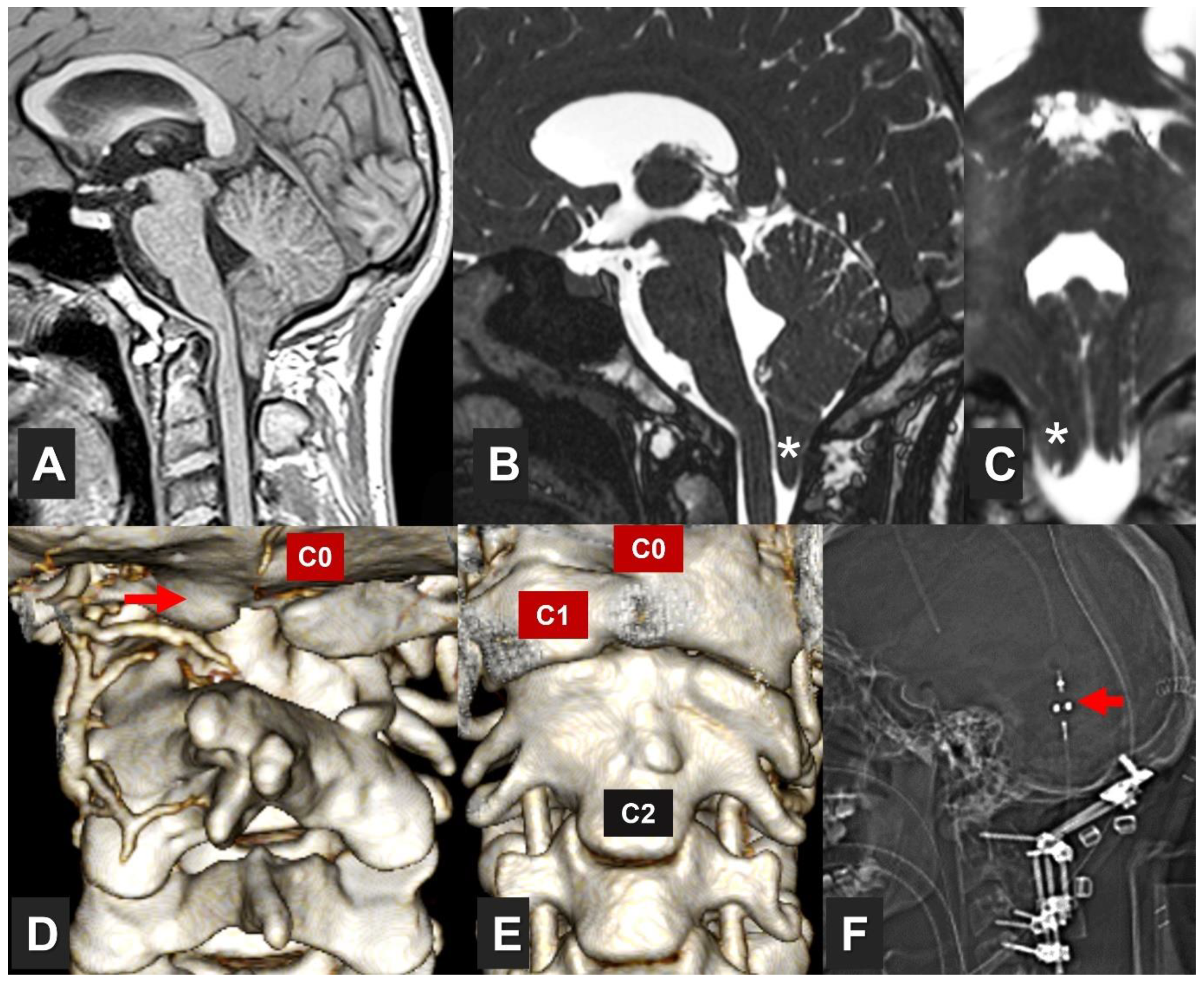
1. Introduction
2. Methods
3. Exploring Brainstem and Spinal Cord Functionality
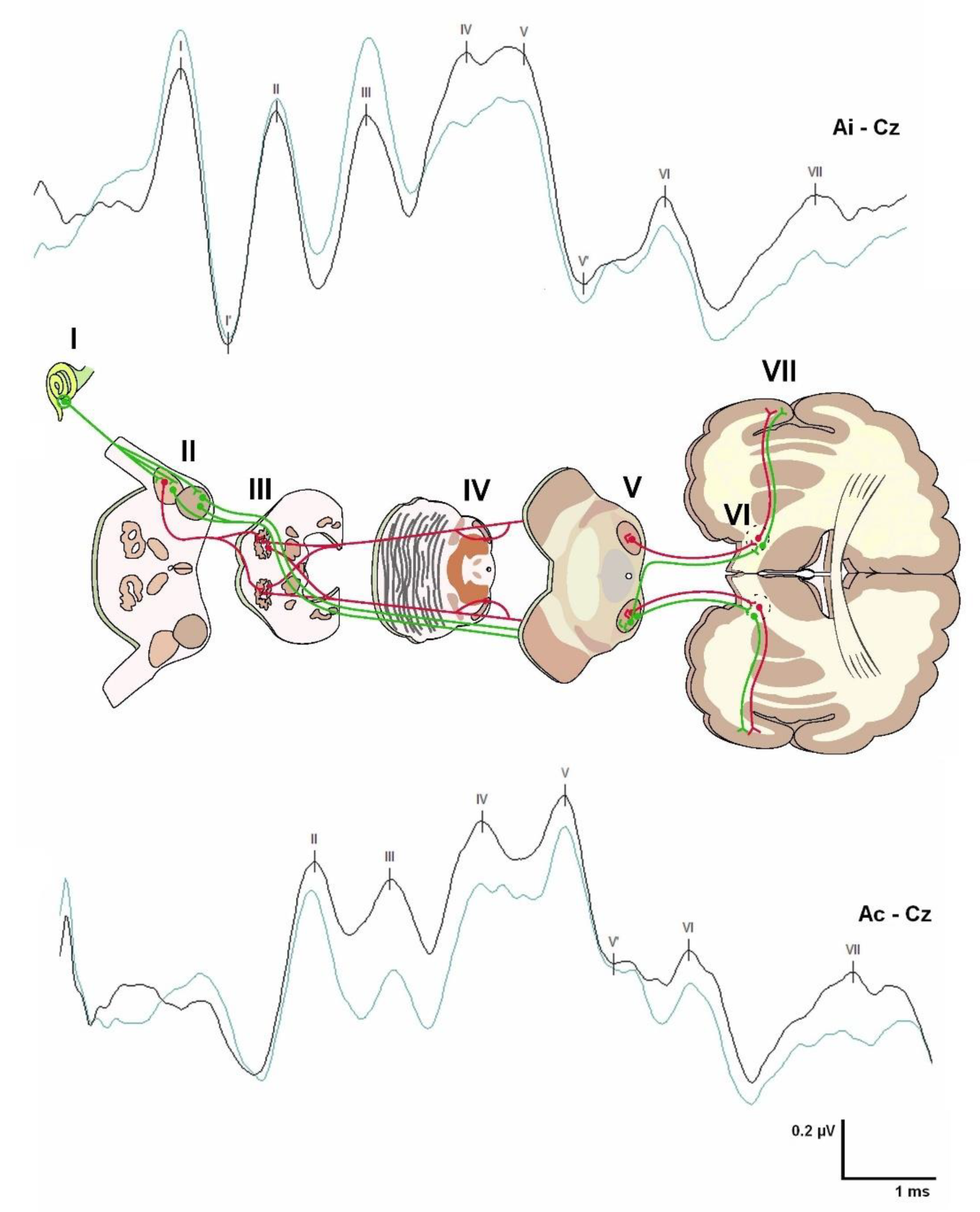
3.1. Brainstem Auditory Evoked Potentials (BAEPs)
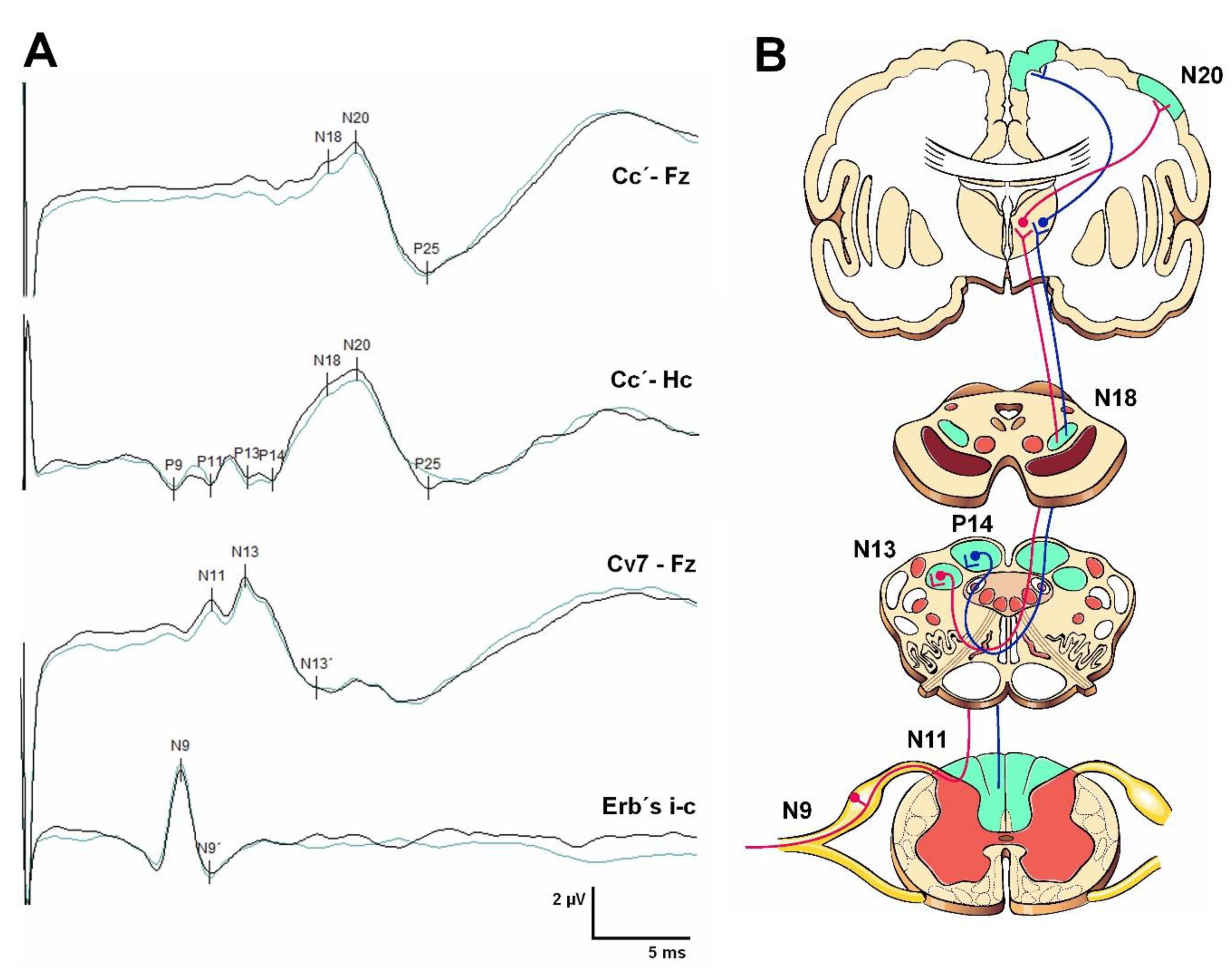
3.2. Somatosensory Evoked Potentials (SEPs)
3.3. Motor Evoked Potentials
3.4. Electromyography and Nerve Conduction Studies
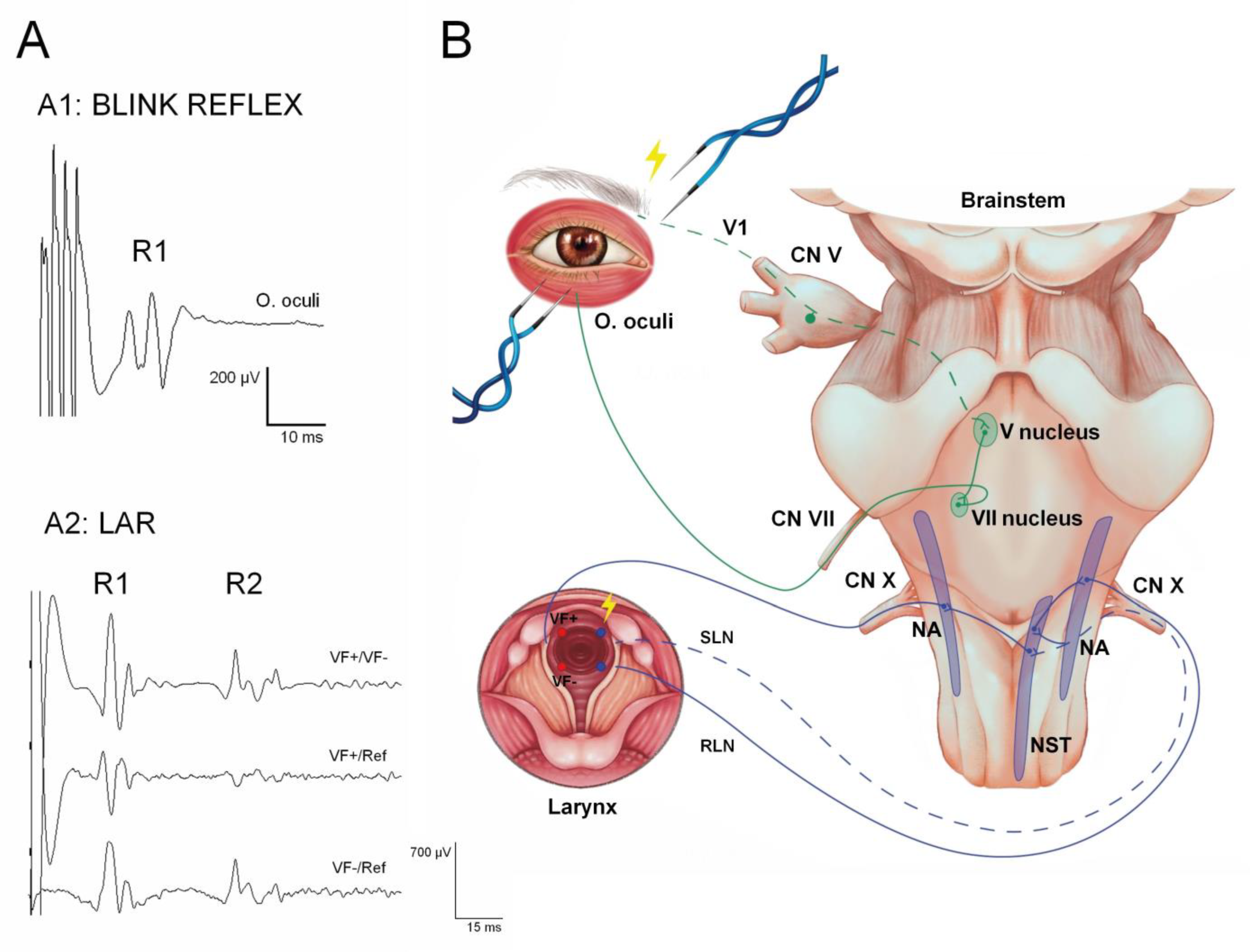
3.5. Brainstem Reflexes
4. Neurophysiological Limitations in the Developing Central Nervous System
5. Follow-Up Neurophysiological Studies in Patients with CM1
5.1. Brainstem Auditory Evoked Potentials
5.2. Somatosensory Evoked Potentials
5.3. Motor Evoked Potentials
5.4. NCS, EMG, and Other Peripheral Nerve Studies
5.5. Brainstem Reflexes
| Author | Year | N | Age/Sex | SYR | SCOL | BAEPs | SEPs | MEPs | EMG/NCS | Brainstem Reflexes |
|---|---|---|---|---|---|---|---|---|---|---|
| Cocito et al. [104] | 2022 | 100 Symptomatic CM1 = 34 CM1 + SYR = 53 SYR = 13 | 23–75-y-o 25♂/75♀ | 66 | ― | ― | ― | TMS of phrenic nerve: - 48%: ABN C5-MEP. - 20%: absence or delay of Cz-MEP Alterations of the Cz-MEP and C5-MEP were prevalent in patients with cervical SYR/syringobulbia, most associated with CM1 | ― | ― |
| Di Stefano et al. [90] | 2019 | Comparison between groups: IH = 18 CM1 = 18 SNHL = 20 Normal controls = 52 | CM1 49 ± 11-y-o 12♂/6♀ | ― | ― | Wave V, I–III, and III–V IPL were higher in CM1 than in controls | ― | ― | ― | ― |
| Guvenc et al. [98] | 2019 | T = 27 | 15–62-y-o 7♂/20♀ | ― | ― | ― | ABN SEP in 22.2% (PTN > MN). - Significant correlation between CSF flow disturbance, the degree of TE (p = 0.038), and the presence of SEP abnormality (p = 0.016). - CSF flow disturbance and SEP abnormality are more frequently seen in patients with platybasia | ― | ― | ― |
| Jayamanne et al. [111] | 2018 | T = 1 Presenting as left foot drop | 6-y-o ♀ | YES (holocord SYR) | ― | ― | ― | ― | NCS: CPN, PTN, and sural nerve were normal. EMG at left TA revealed fibrillations and scanty MUP. EMG of left medial gastrocnemius and right TA were normal | ― |
| Stancanelli et al. [102] | 2018 | T = 1 Presenting as excessive sweating on all the left side of the body | 22-y-o ♂ | ― | ― | Normal | Normal | Asymmetric response with increased CMCT on the right upper and lower limbs |
Sudoscan test: Asymmetric sweating with a higher electrochemical skin conductance on the left hand and foot | ― |
| Moncho et al. [19] | 2017 | 200 | 15–70-y-o 58♂/142♀ CM0 = 14 CM1 = 137 CM1.5 = 49 | YES (96) | ― | Only age, the degree of TE, and lower cranial nerve dysfunction had a statistically significant influence in predicting ABN BAEPs | Only age and the degree of TE were statistically significant at predicting ABN SEPs. BAEPs and SEPs play an essential role in clinically asymptomatic/oligosymptomatic patients | ― | ― | ― |
| Akakin et al. [96] | 2015 | T = 1 | 34-y-o ♂ | Large SYR from just below FM to T5 vertebral body. The spinal cord was thinned at these levels | ― | ― | Preoperatively SEPs were ABN: Increased N13-20 IPL of the MN SEP and reduced cortical AMP of the PTN SEP. After syringo-subarachnoid-peritoneal shunt insertion using a conventional lumbo-peritoneal shunt and a T-tub his SEP test turned to normal | ― | ― | ― |
| Awai and Curt [101] | 2015 | T = 7 SYR (1 CM1) | 32–53-y-o 6♂/1♀ MC1: 32-y-o ♂ | YES | ― | ― | Differently affected dSEPs and dCHEPs. dCHEPs in at least one dermatome were ABN in all patients | ― | All patients had normal NCS and MEPs of the upper limbs | ― |
| Moncho et al. [20] | 2015 | 50 | 16–67-y-o 11 ♂/39♀ | YES (20 patients) | ― | Altered in 52%. The most common finding was an increased I–V IPL and LAT of wave V (48%). A greater TE was observed in patients with pathological BAEPs compared to patients with normal BAEPs (not statistically significant, possible type II error) | Altered by 50%. The most frequent alteration of MN SEP was increased N13-N20 IPL; the most frequent alteration of PTN SEP was an increased N22-P37 IPL, sometimes associated with an alteration of the cervical potential. A greater TE was observed in patients with altered PTN SEPs, and MN SEPs compared to patients with normal SEPs (not statistically significant, possible type II error) | ― | ― | ― |
| Isik et al. [87] | 2013 | T = 44 SYR CM1 = 32 | 14–71-y-o 24♂/20♀ | YES | ― | Only pathological in 31.2% of patients with CM1. In 90% of cases improvement was seen and correlated with neurological and radiological improvement. This series suggested that BAEPs were more correlated with clinical and radiological findings than SEPs were | MN/PTN SEPs were ABN in 54.2% of patients (exact number of patients not known since the figures do not match). SEPs were not always correlated with the clinical findings | ― | ― | ― |
| Panda and Kaur [114] | 2013 | T = 1 Rapidly progressive right foot drop | 16-y-o ♂ | YES (holocord SYR) | ― | ― | ― | ― | NCS: CPN normal BIL with absent bilateral F waves. PTN, sural, superficial peroneal, and saphenous nerve were normal. EMG: fibrillation potentials and positive sharp waves in the right TA, peroneus longus, medial gastrocnemius, gluteus medius, and lumbar paraspinal muscles, confirming the lesion to be proximal (lumbar roots or anterior horn cell) | ― |
| Vidmer et al. [89] | 2011 | T = 66 (MC1 and 2) 1 MC1 | 3 months-60-y-o MC1: 9-y-o ♀ | ― | ― | Peripheral or cochlear alteration in a single pediatric patient with CM1 | SEP of MN ABN with increased LAT N20 and CCT UNIL Normal PTN SEP BIL | ― | ― | ― |
| Mc Millan et al. [113] | 2011 | T = 2 Abrupt onset UNIL foot drop | 5 and 4.5-y-o 2♀ | YES | ― | ― | Case (1) Not included or provided. Case (2) MN SEPs revealed prolonged cervical and cortical responses. PTN SEPs responses were poorly formed with normal latencies | ― | NCS: Case (1) CPN showed low motor AMP. EMG: fibrillation potentials and positive sharp waves confirming a proximal lesion. Case (2) EMG of the right TA revealed active denervation | ― |
| Saifudheen et al. [112] | 2011 | T = 1 Rapidly progressive BIL foot drop | 14-y-o ♀ | YES (holocord SYR) | ― | ― | ― | ― | NCS: Low AMP CMAP and normal LAT and velocity for both peroneal and left ulnar nerves. The F wave was normal. Sensory median, ulnar, and sural nerves were normal. EMG: On both sides, chronic neurogenic changes in the first dorsal interossei, TA, and medial gastrocnemius muscles | ― |
| Berciano et al. [100] | 2007 | T = 1 Lancinating left cervico-brachial pain provoked by coughing fits | 54-y-o ♀ | Cervico-dorsal SYR extending into the left posterolateral quadrant in axial sections passing through C7 and D1 vertebral levels | ― | ― | dSEPs left side: N20 of the C8 dermatome severely attenuated, both pre- and postoperatively. All other left-side dermatomes and right-hand dSEPs were normal Persistence of segmental hypoalgesia and altered SEPs despite the disappearance of SYR on MRI after PFD | ― | Bilateral motor and sensory conduction parameters of MN and UN, including F responses, were normal | ― |
| Henriques Filho and Pratesi [88] | 2006 | T = 75 MC1 = 27 MC2 = 48 | 27 patients = 19–70-y-o 48 patients = 2–16-y-o | ― | ― | In order of frequency: 1. Alteration of wave I or cochlear level (“segment 1”); 2. Alteration I-III or “between the acoustic nerve near the cochlea and the pontomedullary junction” (“segment 2”). -Two patients with an abnormality in the AMP V/I ratio | ― | ― | ― | ― |
| Brookler [82] | 2005 | T = 1 Dizziness | 63-y-o ♀ | ― | ― | Delay of wave V and III–V BIL, with normal audiology | ― | ― | ― | ― |
| Utzig et al. [95] | 2003 | T = 1 Headache, paresthesia, and SCOL | 15-y-o ♀ | YES | YES | ― | Left UN/PTN SEPs: Absence of cortical responses. After SUR: Cortical responses of reduced AMP for left extremities | ― | ― | ― |
| Hausmann et al. [123] | 2003 | T = 100 MC1 = 1 | 15.3 ± 2.2-y-o 20♂/80♀ | 3 | 100 | ― | Normality in PTN SEPs in the patient with MC1 | ― | ― | ― |
| Muhn et al. [110] | 2002 | T = 1 Rapidly progressive UNIL leg weakness | 5-y-o ♂ | YES | ― | ― | ― | ― | NCS showed a low-AMP CMAP for the left peroneal and normal sural nerve. EMG of the left TA, tibialis posterior, and medial gastrocnemius muscles showed fibrillation potentials at rest and reduced voluntary recruitment action potentials. Eight weeks after PFD, patient showed improvement in leg strength with a co-temporal resolution of the previously observed fibrillation potentials | ― |
| Paulig and Prosiegel [107] | 2002 | T = 1 Progressive dysphagia for a year | 78-y-o ♀ | ― | ― | ― | Normal | ― | -NRL examination: BIL paresis and atrophy of the tongue showed diffuse fibrillations. Further neurological examination was normal. -NCS and EMG of various muscles of the upper and lower limbs were normal | ― |
| Bagnato et al. [108] | 2001 | T = 1 Presenting as spinal myoclonus | 48-y-o ♂ | YES | ― | ― | Left MN SEPs revealed normal P14 and N20, while the N13, obtained after glottis reference, was nearly abolished | ― | EMG: (1) Chronic partial denervation in C8/D1 muscles; (2) rhythmic contractions in FDI and ABP muscles (spinal myoclonus); and (3) peripheral silent period after supramaximal electrical stimulation of UN at FDI muscle | ― |
| Jacome [122] | 2001 | T = 4 CM1 presented with blepharoclonus | 17–52-y-o 1♂/3♀ | 1 | ― | ― | ― | ― | Facial EMG: complex repetitive discharges of the right mentalis muscle in one patient | Blink reflexes were ABN in 3 patients |
| Scelsa [109] | 2000 | T = 1 Presenting as ulnar neuropathy at the elbow | 24-y-o ♀ | YES | YES | ― | ― | ― | NCS: Marked AMP reduction of the left ulnar CMAP without focal slowing or conduction block across the elbow. EMG: Fibrillations and high AMP MUP in left arm muscles innervated by C7-D1 spinal segments and the median and ulnar nerves | ― |
| Cheng et al. [99] | 1999 | T = 164 MC1 = 12 | 10–20-y-o (m₁ = 14.2) (m₂ = 13.6) 22♂/142♀ | 6 | 164 | ― | MN and PTN SEPs. Association between TE and SEP dysfunction (p < 0.001; c.c 0.672 Spearman). No differences in the degree of TE in patients with normal SEPs and those with ABN SEPs (p = 0.864; Mann–Whitney) | ― | ― | ― |
| Hort-Legrand and Emery [86] | 1999 | T = 79 SYR -Foraminal (64) -Meningitis (5) -Trauma (15) CM1 = 48 (CVJM = 11 BI = 7) | SYR foraminal 16–71-y-o 27♂/22♀ | YES | 11 | ABN in 13 of the 59 patients studied (total cohort), 6 with CVJM. The more frequent finding was I-V IPL PROL UNIL, less frequently BIL. (They did not specify how many patients with CM1 underwent BAEP or what the results were in this subgroup) | MN and PTN SEPs in all 79 patients. Abnormality (PTN or MN) was noted in 77 of the 79 patients. The most frequent findings for MN SEPs were altered cervical N13 response, an anomaly of the P14-N20 interval, or altered cortical response. The most frequent findings of PTN SEP were an absence of cortical waves or a decrease in their AMP. SEPs of the trigeminal nerve (V3) were recorded in 60 patients. Prolonged LAT UNIL (less frequently BIL). Much more sensitive than BAEPs: always altered when bulbar symptoms, while the MRI in no case showed syringobulbia. (They did not specify the results of these tests in the subgroup of CM1) | MEPs of the upper limbs in 60 patients. More frequent findings: PROL CCT, reduced AMP, or very polyphasic responses. (They did not specify the results of these tests in the subgroup of CM1) | ― | ― |
| Ahmmed et al. [85] | 1996 | T = 1 Tinnitus and mild hearing loss in the left ear | 13-y-o ♀ | ― | ― | Asymmetry in III, V LAT, and I-V IPL, more prolonged in the left ear that returned to normal values after PFD | ― | ― | ― | ― |
| Amoiridis et al. [121] | 1996 | T = 1 Dysesthesia and weakness with urinary retention | 25-y-o ♂ Mild hydrocephalus | YES | YES | ― | PTN SEPs: No lumbar potential (N24; Ll/iliac crest) could be obtained, whereas a high cervical potential (N33; C2/Fz) and a cortical (P40; Cz’/Fz) potential with a normal LAT were registered. MN SEPs were normal | ― | AMP of the H reflex in the soleus muscle was low bilaterally, and the H reflex LAT was prolonged on the right. Motor and sensory NCS were normal. F waves in AH, EDB, or hypothenar muscles, all on the left, were not elicited | BR: Rl was absent bilaterally, and R2 LAT with left side stimulation was prolonged BIL |
| Kaneko et al. [120] | 1996 | T = 5 | Mean age 18-y-o ♂ | YES (cervical) | ― | ― | Absent or reduced cervical N13 SEP with preserved cortical responses of the upper limb were observed in 3/5 patients | MEPs LAT and AMP were normal in all patients | CMAPs LAT and AMP were normal in all patients. On the symptomatic hand, all patients showed diminished cutaneous silent periods (CSPs) up to a stimulus intensity of 15 times of sensory threshold. Diminished CSP was the only ABN finding in 2 subjects. The decrease in CSPs was more sensitive to syringomyelic changes than ABN cervical N13 SEP | ― |
| Cristante et al. [105] | 1994 | T = 26 CM1 (8 with MEPs) | 35–65-y-o 13♂/13♀ (Data of patients studied with MEP N/A) | YES (5) | ― | ― | ― | Preoperative TMS MEPs: Muscles functionally impaired had their record ABN. The postoperative functional motor recovery was not reflected by improvement of the MEPs parameters | ― | ― |
| Johnson et al. [84] | 1994 | T = 1 Steadily progressive bilateral asymmetrical SNHL | 10-y-o ♂ | NO | ― | CCT or I-V BIL increased: Increased I–III in one ear and III–V in the other | ― | ― | ― | ― |
| Strowitzki et al. [97] | 1993 | T = 18 CM1 = 9 | YES | ― | ― | MN SEPs: ABN in 4 patients. PTN SEPs: ABN in 7 patients. No cortical responses were found in 6 patients and delayed responses in 2 (does not specify MN or PTN) | ― | ― | ― | |
| Morioka et al. [94] | 1992 | T = 11 (cervical SYR) CM1 = 10 | 24–56-y-o 3♂/7♀ | YES | ― | ― |
The most common MN SEP abnormality was the UNIL attenuation or absence of N13 with often normal N20 potentials. Spinal EPs provided information regarding abnormality responsible for the dorsal column dysfunction: the syrinx, the TE, or both | ― | ― | ― |
| Nogués et al. [103] | 1992 | T = 13 MC1 = 7 BI = 1 | 19–53-y-o (m = 37.4) 7♂/6♀ | T = 13 | ― | ― | Alteration of cortical PTN and reduction or absence of cervical potential of MN | The most frequent finding was increase in CMTC | ― | ― |
| Gerard et al. [106] | 1992 | T = 1 BI Presenting as velar insufficiency | 5-y-o ♀ | ― | ― | ― | ― | ― | Velar insufficiency: EMG BIL in levator palatini and anterior faucial pillars showed ample biphasic or polyphasic action potentials at rest; when the child cried, the frequency of these potentials increased poorly, and recruitment was impaired. Suspicion of denervation of the IX, X, and XI cranial nerves | ― |
| Hendrix et al. [83] | 1992 | T = 3 | 59, 34, 64-y-o | ― | ― | ABN in one patient: prolonged I-V IPL on the right side. The other two patients had normal BAEPs BIL | ― | ― | ― | ― |
| Restuccia and Maguière [39] | 1991 | T = 24 MC1 = 16 (9 *) | 20–74-y-o (m = 56) 10♂/14♀ | T = 24 | ― | ― | MN SEPs: ABN or absent N13 in 83% of patients with cervical SYR. Good correlation between loss of thermoalgesic sensation and absence of tendon reflexes. With associated CM: increased P14-N20 IPL | ― | ― | ― |
| Jabbari et al. [93] | 1990 | T = 22 MC1 = 4 | 15–69-y-o (m = 28) 15♂/7♀ | T = 22 | ― | ― | No significant relationship between SEPs in SYR when CM coexists: in 3 of 4 patients with SYR and CM, SEPs were normal | ― | ― | ― |
| Forcadas et al. [92] | 1988 | T = 18 MC1 = 17 | 12 | ― | ― | No significant relationship between SEPs in SYR when CM coexists: in 3 of 4 patients with SYR and CM, SEPs were normal | ― | ― | ― | |
| Anderson et al. [91] | 1986 | T = 9 MC1 = 8 | 16–65-y-o (m = 41) 1♂/8♀ | T = 9 | ― | ― | MN SEPs: AMP reduction or absence of the cervical potential, consistent with the clinically more affected side. 7/8 cases with CM1 had a prolonged or asymmetric CCT. One case with MC-1 presented normal MN SEPs | ― | ― | ― |
| Stone et al. [81] | 1983 | T = 1 Classified by the authors as MC2 (but without overt spinal defects), probably CM1.5 | 16-y-o ♂ With associated hydrocephalus | ― | ― | PROL I-III and I-V IPL in the left ear. Absence of wave III; I-V IPL more PROL in the right ear. Postoperative BAEPs showed BIL normalization | ― | ― | ― | ― |
6. Intraoperative Neurophysiological Monitoring in CM1
6.1. Posterior Fossa Surgery
6.2. Surgery for the Direct Treatment of Syringomyelia in MC1
6.3. CM1 in Scoliosis Surgery
6.4. Exploratory Research on the Subject
| Author | Year | No. of Cases | Study Type | Patient Characteristics | SEP | MEP | BAEP | BR or CN Mapping | Alarm Criteria | Alerts | Findings | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schaefer et al. [136] | 2022 | 93 | Retrospective | 17–76-y-o SYR = 53 | 93 | 92 | 83 | ― | - Decrease SEP/MEP AMP by 50% or increase SEP LAT by 10%. - BAEPs: decrease in wave V AMP by 50%, or increase in wave V LAT by 1 msec, or total loss of waves (I, III, and V) | - 1 (1.1%), which resolved spontaneously after 10 min (the patient had concomitant stenosis at C1–2) | - TE was significantly associated with unmonitorable MEPs but not with unmonitorable SEPs or BAEPs. - SYR characteristics were not significantly associated with unmonitorable MEPs, SEPs, or BAEPs. - Cerebellar symptoms were associated with unmonitorable MEPs and BAEPs but not SEPs | - PFD in CM1 may be performed safely without IONM. - In patients with additional occipitocervical pathology, IONM should be left as an option to be assessed by the surgeon on a case-by-case basis |
| Giampiccolo et al. [151] | 2021 | T = 10 CM1 = 1 | An exploratory and preliminary study of cerebello-cortical stimulation | 6–73-y-o 2 children | ― | 1 | ― | ― | - Electrical conditioning stimuli delivered to the exposed cerebellar cortex (cStim) alone did not produce any MEP. - Paired cortico-transcortical stimulation: M1 stimulation occurred after cerebellar stimulation at fixed intervals between 8 and 24 ms. They observed significant modulation of MEPs in 8/10 patients. 5 patients showed MEP inhibition, one patient MEP facilitation, and 2 patients showed both conditions at different interstimulus intervals. - cStim preceding Tc Stim produced a significant inhibition at 8 ms (p < 0.0001). | - Monitoring efferent cerebellar pathways to the motor cortex is feasible in intraoperative settings. - The study has promising implications for pediatric posterior fossa surgery to preserve the cerebello-cortical pathways and thus prevent cerebellar mutism | ||
| Heffez et al. [135] | 2020 | 488 | Observational, prospective | >18-y-o Patients divided into four groups depending on the position of the cerebellar tonsils: GR 1: 0–3 mm GR 2: 3–5 mm GR 3: 5–10 mm GR 4: >10 mm Some patients presented with tethered cord in GR 4 (CM2?) | ― | ― | 488 | ― | Any change of at least 0.1 msec from their initial BL | 35–49% of the patients had a reduction in III–V IPL of at least 0.1 msec | Reduction in III–V IPL was observed towards completing intradural dissection or during DP, with no statistical difference between groups | - Even with minimal TE, the function of at least one pathway within the brainstem may be impaired, similar to that seen with much more extreme TE. - This impairment improves after brainstem decompression. |
| Krishnakumar et al. [132] | 2020 | 1 | Case report | 13-y-o ♂ with atlantooccipital dislocation, BI, hypoplastic C1 arch, and CM1 | 1 | 1 | ― | ― | N/A | - Loss of MEP in all four limbs after prone position: neck flexion was reduced by 15°, which reverted the changes in MEP. - Loss of MEP in both the upper limbs following screw tightening: C1 arch excision was made and upper limb MEPs reappeared. | - IONM can contribute to safe surgical positioning and performance. - It is essential to promptly identify and rectify any changes in neuronal, structural, and vascular integrity to help minimize neurologic sequelae. | |
| Shi et al. [149] | 2020 | 270 | Retrospective and case-matched subgroup analysis | Scoliosis surgery 60 asymptomatic CMS patients (48 presented with SYR) vs. 210 IS patients. Case-matched patients: 60 CMS vs. 60 IS. - PFD was performed 8–12 months before correction surgery in whole patients with SYR | 270 | 258 | ― | ― | - Absent SEPs, UNIL or BIL prolonged LAT. - Asymmetric LAT when interside difference of LAT/AMP > 2.5 SD of normal control * LAT normalized with body height and > 2.5 SD (P37 LAT = 0.277 × height − 7.144, SD = 1.071) | - No significant difference was found between CMS and total IS patients in terms of the SEPs LAT and AMP as well as MEPs AMP. - There was no significant difference in SEPs LAT and AMP and MEPs AMP between CMS and matched IS patients. - CMS patients with SYR were correlated with lower SEPs amplitudes | - Neurologically asymptomatic CMS patients showed similar absolute values of IONM (SEPs LAT and AMP, and MEPs AMP) as compared with IS patients. - SYR in CMS patients indicated more severe curvature and a lower SEPs AMP even after PFD | |
| Tan et al. [148] | 2020 | 42 | Retrospective and case-matched | Scoliosis surgery 21 patients with SCOL secondary to CM1 and SYR matched with 21 patients with SCOL secondary to idiopathic SYR | ― | 42 | ― | ― | - Obvious MEP degeneration was defined as 40% to 80% MEP AMP loss. - Significant MEP loss was defined as >80% of MEP loss associated with high-risk surgical maneuvers | - Obvious MEP degeneration in 5 patients - Significant MEP loss in none | There were no differences in the successful recording of MEP BL | - Although patients with CM1 had longer SYR, their IONM signals during the operation were similar to those of the SCOL secondary to idiopathic SYR group. - The potential risk of SCOL surgery in patients with SYR-associated SCOL should not be ignored |
| Shah et al. [138] | 2019 | 20 | Observational, prospective | 7–52-y-o, with CM1 that were surgically treated by atlantoaxial stabilization surgery. No bone, dural, or neural decompression | 20 | 20 | ― | ― | N/A | - All the patients had an immediate intraoperative improvement from their BL MEPs after the fixation was complete, ranging from 20% to 35%. - No change in the SEPs during the entire surgery in any of the patients. | The improvement in electrophysiological parameters after atlantoaxial fixation fortifies their belief that atlantoaxial instability is the cause of Chiari formation and atlantoaxial fixation is the treatment | |
| Rasul et al. [130] | 2019 | 37 | Retrospective | <17-y-o undergoing PFD for CM1 SYR = 24 SCOL = 13 | 33 | ― | 19 | ― | N/A | SEP: 2/33 ↑ AMP 18/33 ↓ AMP 31/33 ↓ LAT BAEP: 13/19 ↓ LAT 4/19 ↑ LAT | - SEP LAT reduced in 93.9% of patients. - >50% of patients presented SEP AMP decrease - BAEPs decreased in 68.4% of patients | - PFD for CM1 is associated with changes in SEPs and BAEPs. - A definite link between clinical outcomes and IONM was not identified |
| Brînzeu and Sindou [150] | 2017 | T = 49 22 CM1 | Research study about the functional anatomy of the accessory nerve (XI CN) studied through IONM (mapping) | 20–73-y-o Far-lateral decompression of tonsils at the FM in addition to posterior decompression, followed by a Y-shaped dural incision without opening the arachnoid | Rootlet and cranial root stimulation in the majority of CM1 patients | - The CN XI pair has an organization around two components: a cranial root and a spinal root with several cervical rootlets. - The cranial component contributes at least to the motor innervation of the larynx. - The spinal component largely contributes to the innervation of the sternocleidomastoid and trapezius with a precise craniocaudal myotopic organization; the rootlets destined to innervate to the sternocleidomastoid are more cranial | ||||||
| Kawasaki et al. [131] | 2017 | 1 | Case report | Symptomatic 7-y-o boy who underwent surgery of PFD with tonsillectomy and DP for CM1 with cervicomedullary compression + pre-SYR state at the C3-4 dorsal spinal cord | 1 | 1 | ― | ― | A change of 50% in AMP or a 10% change in LAT from the BL value both in MEPs and SEPs | - MEPs improved, showing increased AMP and decreased LAT after craniotomy and durotomy - SEPs improved only after durotomy | The improvement in MEPs and SEPs observed during decompression may be a good indicator for the prediction of the clinical improvement seen postoperatively | |
| Roser et al. [134] | 2016 | 39 | Retrospective | 13–65-y-o patients with CM1, undergoing suboccipital decompression and DP SYR = 33 | 38 | 33 | ― | ― | N/A | - SEP deterioration during positioning 2/39 (↑ > 10% LAT in 4 recordings, ↓ or ↑ >50% AMP in 9 and 10 recordings) - MEP deterioration during positioning 2/39, (↓ and ↑ > 10% LAT in 11 and 10 recordings, ↓ and ↑ > 50% AMP in 14 and 17 recordings) | - No significant differences existed in the absolute BL and final LAT or AMP of MN and PTN SEPs. - There were no significant differences in the absolute BL and final LAT or AMP of APB and TA MEPs | - IONM during the primary treatment of CM1 shows only subtle non-significant changes in SEPs and MEPs without clinical correlation during suboccipital decompression |
| Barzilai et al. [152] | 2015 | 22 | Retrospective | 21 pediatric patients aged 2–17-y-o with CM1 SYR = 18 PFD + C1 (C2/C3) laminectomy (22 surgeries) | 22 | 22 | ― | ― | N/A | IONM positional-related alarms in 3 patients: 1 attenuation of SEPs, 1 attenuation of MEPs, and 1 attenuation in both SEPs and MEPs. | None of the 3 patients displayed new immediate post-operative deficits | - Multimodality IONM can be helpful during patient positioning. - MEP attenuations may occur independently of SEPs. - The clinical implications of these monitoring alerts have yet to be defined |
| Grossauer et al. [133] | 2015 | 1 | Case report | A 32-y-o woman who underwent surgery for CM1 associated with extensive cervicothoracic SYR | 1 | ― | ― | ― | A change of 50% in N20 amplitude from the BL value and a change of 10% in N20 LAT from the BL value | ― | - A few minutes after opening the IV ventricle, they observed a 230% ↑ in the N20 AMP and an 8% ↓ in the N20 LAT compared to the BL value. - This SEP improvement persisted until the end of the operation | - Conduction improvement in SEPs during CM1 decompression may not always occur after bone decompression or DP. It may also happen after opening the IV ventricle and establishing CSF flow at the level of the CVJ. - Additional studies are needed to adapt the degree of decompression to each CM1 patient based on the IONM data |
| Kennedy et al. [128] | 2015 | 156 | Retrospective | 7–20.6-y-o Suboccipital decompression without dural opening SYR = 68 SCOL = 18 m Cobb angle = 25° | 156? | ― | 156? | ― | N/A | N/A | - 78% (121) of patients exhibited at least UNIL improvement in I–V IPL after bony decompression, with a mean improvement of 0.26 ms. Once a preoperative neck position was established in the prone position with SEPs unchanged from BL, there were no adverse changes in the SEPs during any patient’s surgery | |
| Pencovich et al. [141] | 2013 | T = 13 CM1 = 6 | SYR Surgery 4–61-y-o → 1 CM1 = Syrinx drainage and PFD. Level T4-T5 → 5 CM1 = Syringo-subarachnoid shunt SYR approach: → 4 midline → 2 DREZ | 6 | 6 | ― | ― | SEP: non-linear AMP ↓ beyond 50% or LAT ↑ of over 10%. MEP: sudden attenuation beyond 85% AMP in at least two reproducible traces after ruling out technical and anesthetic considerations | - 1 patient with absent right leg MEP BL signal. - While draining the SYR before PFD, severe attenuation of the left leg MEP data was noted upon midline approach to the SYR: the catheter was removed, and the PFD was completed without SYR drainage. New neurologic deficit: transient worsening of right hemiparesis. | - Demonstration of the reversibility of intraoperative neurological damage identified by IONM. - An immediate response resulted in rapid recovery of cord functionality | - The data collected support routine usage of IONM in SYR surgeries. - IONM can inform the surgeon of potential intraoperative threats to the functional integrity of the spinal cord, providing a helpful adjunct to spinal cord surgeries for the treatment of SYR. - More extensive prospective studies are required to show that using IONM in these operations is advantageous conclusively. - The first study to address the benefits of multimodality IONM during SYR surgery, specifically | |
| Chen et al. [127] | 2012 | 13 | 2–17-y-o Suboccipital craniotomy for symptomatic CM1. In 3, the bone flap was not replaced (craniectomy), and in 9 cases, it was (craniotomy) SYR = 3 | 12 | ― | 9 | ― | N/A | ― | - MN or PTN SEP LAT improved in all patients. - BAEPs improved in 8 patients. - No significant SEP or BAEP deterioration was seen | IONM may be used to perform suboccipital decompression in a step-by-step fashion, enlarging the craniectomy or adding additional procedures (laminectomy, DP) until positive changes are observed | |
| Di [137] | 2009 | 26 | Endoscopic suboccipital decompression 18 months-16-y-o 0° and 30° endoscopes were adapted to perform the procedure of suboccipital craniectomy and upper cervical laminectomies SYR = 5 SCOL = 1 | 11 | ― | ― | ― | ― | ― | SEPs were monitored throughout the entire procedure for the first 11 patients, and it was then discontinued due to lack of significant benefit for the patients | SEP is still necessary, especially for the beginners of this procedure, to preclude the development of intraoperative spinal cord injury | |
| Zamel et al. [125] | 2009 | 80 | Retrospective | 2–36-y-o Group A: PFD Group B: PFD+ DP SYR = 33 | 80 | ― | 80 | ― | - BAEP’s IPLs of I–III, III–V, and I–V waves were compared at BL, after positioning, immediately after bony decompression, and at closure. - Neurophysiologic improvement in CCT was defined as a reduction in I–V IPL at closure compared with BL | ― | - A significant main interaction was found between the presence of SYR and the reduction of I–V IPL from BL to decompression. - Patients with SYR showed a significantly decreased I–V interval between BL and decompression compared with those without an SYR | - PFD with bone removal alone significantly improves conduction time in most pediatric patients with CM1. - DP allowed for further but a small improvement in conduction time in only 20% of patients, beyond that achieved by decompression alone. - None of the patients had any significant worsening of their BAEPs that would have alerted the neurosurgeon to modify the course of the surgery |
| Kim et al. [126] | 2004 | 11 | Retrospective | 1.5–17-y-o Significant BI and CM1 They underwent a novel treatment method involving decompression, manual reduction, and posterior instrumentation-augmented fusion. SYR = 3 | 11 | ― | ― | ― | N/A | ― | - SEPs remained stable in 10 cases and improved intraoperatively after decompression and manual reduction in 1 case. - SEPs improved significantly in 10 cases immediately after reduction and fusion. - SEPs remained unchanged from BL in 1 case | ― |
| Anderson et al. [124] | 2003 | 11 | Observational, prospective | 3–19-y-o Suboccipital decompressive procedure with DP SYR = 6 | 11 | ― | 11 | ― | N/A | 1: dramatically deteriorated of left MN SEP after turning the patient to the prone position with neck flexion. The patient was immediately repositioned in a neutral position, improving the left MN SEP. | - BAEPs: statistically significant decreased I-V IPLs after bone decompression but not after dural opening (compared to supine BL). -SEP for both sides in 10 patients remained stable throughout the procedure | - In pediatric CM1 patients, the most improvement in conduction through the brainstem occurs after bony decompression and division of the atlantooccipital membrane rather than after dural opening. - BAEPs and the SEPs indicate that these patients are at risk for neurologic injury during operative positioning with neck flexion |
| Milhorat et al. [140] | 1997 | T = 32 21 CM1 | 5–72-y-o In SYR with CM1: Patients with CM1 underwent suboccipital craniectomies without opening the dura + SYR shunting procedures | 21 | ― | ― | ― | N/A | - 8 patients had normal SEPs, and 6 had SEP abnormalities that were unchanged 30 min after SYR decompression - 18/24 patients with pre-drainage abnormalities showed a slight but consistent N20 LAT ↓ and less consistent ↑ of N20 AMP | Significant correlation between SEP findings and SYR morphology: 6/8 patients with normal SEPs had central core cavities, and 2/8 had central cavities that extended paracentral; these types of cavities were more likely to be associated with SEP improvements after shunting (Pearson Χ2 = 6.47, p = 0.039) | - N20 LAT improvement is indirect evidence of preexisting long tract compression, whereas the decompression of perisyrinx neurons presumably caused improvements of N20 amplitudes. - These conclusions were less certain for patients with CM1 in whom SEP improvements may have been caused, in part, by decompression of the cerebellar hernia | |
| Milhorat et al. [139] | 1996 | T = 13 11 CM1 | 12–72-y-o In SYR with CM1: PFD and SYR shunt to the PF cisterns (syringo-cisternostomy) | 11 | ― | ― | ― | Continuous BL values recorded 2–3 h before SYR shunting were compared with values obtained 30 min after shunting. SEP data were analyzed by the paired t-test | ― | 30 min after SYR decompression bilateral MN SEPs demonstrated a significant ↓ in N20 LAT and nonsignificant ↑ N20 AMP | - Findings suggest that distended spinal cord cavities can produce regional ischemia, possibly reflected by SEP abnormalities and reversed by SYR decompression. - Preliminary, the IONM of SEPs can provide useful information during surgical procedures for SYR | |
7. Conclusions
Author Contributions
Funding
Acknowledgments:
Conflicts of Interest
References
- Cleland. Contribution to the Study of Spina Bifida, Encephalocele, and Anencephalus. J. Anat. Physiol. 1883, 17, 257–292. [Google Scholar]
- Chiari, H. Über Veränderungen Des Kleinhirns Infolge von Hydrocephalie Des Grosshirns. Dtsch. Med. Wochenschr. 1891, 17, 1172–1175. [Google Scholar] [CrossRef]
- Chiari, H. Über veränderungen des Kleinhirns, des Pons un der Medulla Oblongata in folge von congenitaler Hydrocephalie des Grosshirns. Denkschr. Akad. Wiss 1896, 63, 71–116. [Google Scholar]
- Chiari, H. Concerning Alterations in the Cerebellum Resulting from Cerebral Hydrocephalus. 1891. Pediatr. Neurosci. 1987, 13, 3–8. [Google Scholar] [CrossRef]
- Cruveilhier, J. L’anatomie Pathologique Du Corps Humain; Descriptions Avec Figures Lithographiées et Coloriées; Diverses Altérations Morbides Dont Le Corps Humain et Susceptible; Bailliere: Paris, France, 1829. [Google Scholar]
- Mortazavi, M.M.; Tubbs, R.S.; Brockerhoff, M.A.; Loukas, M.; Oakes, W.J. The First Description of Chiari I Malformation with Intuitive Correlation between Tonsillar Ectopia and Syringomyelia. J. Neurosurg. Pediatr. 2011, 7, 257–260. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of Cerebellar Tonsillar Position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799. [Google Scholar] [PubMed]
- Bogdanov, E.I.; Heiss, J.D.; Mendelevich, E.G.; Mikhaylov, I.M.; Haass, A. Clinical and Neuroimaging Features of “Idiopathic” Syringomyelia. Neurology 2004, 62, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Botelho, R.V.; Bittencourt, L.R.A.; Rotta, J.M.; Tufik, S. A Prospective Controlled Study of Sleep Respiratory Events in Patients with Craniovertebral Junction Malformation. J. Neurosurg. 2003, 99, 1004–1009. [Google Scholar] [CrossRef]
- Meadows, J.; Kraut, M.; Guarnieri, M.; Haroun, R.I.; Carson, B.S. Asymptomatic Chiari Type I Malformations Identified on Magnetic Resonance Imaging. J. Neurosurg. 2000, 92, 920–926. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Nishikawa, M.; Kula, R.W.; Dlugacz, Y.D. Mechanisms of Cerebellar Tonsil Herniation in Patients with Chiari Malformations as Guide to Clinical Management. Acta Neurochir. 2010, 152, 1117–1127. [Google Scholar] [CrossRef]
- Sekula, R.F.; Jannetta, P.J.; Casey, K.F.; Marchan, E.M.; Sekula, L.K.; McCrady, C.S. Dimensions of the Posterior Fossa in Patients Symptomatic for Chiari I Malformation but without Cerebellar Tonsillar Descent. Cerebrospinal Fluid Res. 2005, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Iskandar, B.J.; Bartolucci, A.A.; Oakes, W.J. A Critical Analysis of the Chiari 1.5 Malformation. J. Neurosurg. 2004, 101, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Demerdash, A.; Vahedi, P.; Griessenauer, C.J.; Oakes, W.J. Chiari IV Malformation: Correcting an over One Century Long Historical Error. Child’s Nerv. Syst. 2016, 32, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and Treatment of Chiari Malformation and Syringomyelia in Adults: International Consensus Document. Neurol. Sci 2022, 43, 1327–1342. [Google Scholar] [CrossRef]
- Isik, N.; Elmaci, I.; Kaksi, M.; Gokben, B.; Isik, N.; Celik, M. A New Entity: Chiari Zero Malformation and Its Surgical Method. Turk. Neurosurg. 2011, 21, 264–268. [Google Scholar] [CrossRef]
- Kyoshima, K.; Kuroyanagi, T.; Oya, F.; Kamijo, Y.; El-Noamany, H.; Kobayashi, S. Syringomyelia without Hindbrain Herniation: Tight Cisterna Magna. Report of Four Cases and a Review of the Literature. J. Neurosurg. 2002, 96, 239–249. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Elton, S.; Grabb, P.; Dockery, S.E.; Bartolucci, A.A.; Oakes, W.J. Analysis of the Posterior Fossa in Children with the Chiari 0 Malformation. Neurosurgery 2001, 48, 1050–1054. [Google Scholar]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Cañas, V.; Sahuquillo, J. Are Evoked Potentials Clinically Useful in the Study of Patients with Chiari Malformation Type 1? J. Neurosurg. 2017, 126, 606–619. [Google Scholar] [CrossRef]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Rahnama, K.; Sahuquillo, J. Brainstem Auditory and Somatosensory Evoked Potentials in Relation to Clinical and Neuroimaging Findings in Chiari Type 1 Malformation. J. Clin. Neurophysiol. 2015, 32, 130–138. [Google Scholar] [CrossRef]
- Ferré, Á.; Poca, M.A.; de la Calzada, M.D.; Moncho, D.; Romero, O.; Sampol, G.; Sahuquillo, J. Sleep-Related Breathing Disorders in Chiari Malformation Type 1: A Prospective Study of 90 Patients. Sleep 2017, 40, zsx069. [Google Scholar] [CrossRef]
- Ferré, Á.; Poca, M.A.; de la Calzada, M.D.; Moncho, D.; Urbizu, A.; Romero, O.; Sampol, G.; Sahuquillo, J. A Conditional Inference Tree Model for Predicting Sleep-Related Breathing Disorders in Patients with Chiari Malformation Type 1: Description and External Validation. J. Clin. Sleep Med. 2019, 15, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Rahnama, K.; Sahuquillo, J. Brainstem auditory evoked potentials and somatosensory evoked potentials in Chiari malformation. Rev. Neurol. 2013, 56, 623–634. [Google Scholar]
- Sahuquillo, J.; Moncho, D.; Ferré, A.; López-Bermeo, D.; Sahuquillo-Muxi, A.; Poca, M.A. A Critical Update of the Classification of Chiari and Chiari-like Malformations. J. Clin. Med. 2023, 12, 4626. [Google Scholar] [CrossRef]
- Ferré Masó, A.; Poca, M.A.; de la Calzada, M.D.; Solana, E.; Romero Tomás, O.; Sahuquillo, J. Sleep Disturbance: A Forgotten Syndrome in Patients with Chiari I Malformation. Neurologia 2014, 29, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Jewett, D.L.; Romano, M.N.; Williston, J.S. Human Auditory Evoked Potentials: Possible Brain Stem Components Detected on the Scalp. Science 1970, 167, 1517–1518. [Google Scholar] [CrossRef]
- Stockard, J.J.; Rossiter, V.S. Clinical and Pathologic Correlates of Brain Stem Auditory Response Abnormalities. Neurology 1977, 27, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R. Monitoring Auditory Evoked Potentials. In Intraoperative Neurophysiological Monitoring; Humana Press: Totowa, NJ, USA, 2006; pp. 85–124. ISBN 978-1-59745-018-8. [Google Scholar]
- Jean-Michel, G. Les Potentiels Évoqués, 2nd ed.; Masson: Paris, France, 1993; Volume 1, ISBN 2-225-84107-1. [Google Scholar]
- Desmedt, J.E. Physiology and Physiopathology of Somatic Sensations Studied in Man by the Method of Evoked Potentials. J. Physiol. 1987, 82, 64–136. [Google Scholar]
- Cracco, R.Q.; Cracco, J.B. Somatosensory Evoked Potential in Man: Far Field Potentials. Electroencephalogr. Clin. Neurophysiol. 1976, 41, 460–466. [Google Scholar] [CrossRef]
- Cruccu, G.; Aminoff, M.J.; Curio, G.; Guerit, J.M.; Kakigi, R.; Mauguiere, F.; Rossini, P.M.; Treede, R.-D.; Garcia-Larrea, L. Recommendations for the Clinical Use of Somatosensory-Evoked Potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef]
- Nair, D.; Kumaraswamy, V.M.; Braver, D.; Kilbride, R.D.; Borges, L.F.; Simon, M.V. Dorsal Column Mapping via Phase Reversal Method: The Refined Technique and Clinical Applications. Neurosurgery 2014, 74, 437–446. [Google Scholar] [CrossRef]
- Simon, M.V.; Chiappa, K.H.; Borges, L.F. Phase Reversal of Somatosensory Evoked Potentials Triggered by Gracilis Tract Stimulation: Case Report of a New Technique for Neurophysiologic Dorsal Column Mapping. Neurosurgery 2012, 70, E783–E788. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; García-Larrea, L. Clinical Utility of Pain—Laser Evoked Potentials. Suppl. Clin. Neurophysiol. 2004, 57, 101–110. [Google Scholar] [CrossRef]
- Garcia-Larrea, L. Pain Evoked Potentials. In Handbook of Neurology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 81, pp. 439–462. [Google Scholar]
- Cruccu, G.; Anand, P.; Attal, N.; Garcia-Larrea, L.; Haanpää, M.; Jørum, E.; Serra, J.; Jensen, T.S. EFNS Guidelines on Neuropathic Pain Assessment. Eur. J. Neurol. 2004, 11, 153–162. [Google Scholar] [CrossRef]
- Kakigi, R.; Inui, K.; Tamura, Y. Electrophysiological Studies on Human Pain Perception. Clin. Neurophysiol. 2005, 116, 743–763. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Mauguière, F. The Contribution of Median Nerve SEPs in the Functional Assessment of the Cervical Spinal Cord in Syringomyelia. A Study of 24 Patients. Brain 1991, 114B Pt 1, 361–379. [Google Scholar] [CrossRef]
- Dotson, R.M.; Sandroni, P. Contact heat evoked potentials. In Clinical Neurophsyiology; Contemporary Neurology Series; OUP: New York, NY, USA, 2009; pp. 688–692. ISBN 978-0-19-538511-3. [Google Scholar]
- Leandri, M.; Marinelli, L.; Siri, A.; Pellegrino, L. Micropatterned Surface Electrode for Massive Selective Stimulation of Intraepidermal Nociceptive Fibres. J. Neurosci. Methods 2018, 293, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Chokroverty, S. (Eds.) Magnetic Stimulation in Clinical Neurophysiology, 2nd ed.; Elsevier Butterworth-Heinemann: Philadelphia, PA, USA, 2005; ISBN 978-0-7506-7373-0. [Google Scholar]
- Claus, D. Central Motor Conduction: Method and Normal Results. Muscle Nerve 1990, 13, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Strommen, J.A. Motor Evoked Potentials. In Clinical Neurophsyiology; Contemporary Neurology Series; OUP: New York, NY, USA, 2009; pp. 385–397. ISBN 978-0-19-538511-3. [Google Scholar]
- Kimura, J. Motor Evoked Potentials. In Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice; Oxford University Press: New York, NY, USA, 2013; pp. 526–551. ISBN 978-0-19-935316-3. [Google Scholar]
- Deletis, V.; Fernández-Conejero, I. Intraoperative Monitoring and Mapping of the Functional Integrity of the Brainstem. J. Clin. Neurol. 2016, 12, 262–273. [Google Scholar] [CrossRef]
- Deletis, V. Intraoperative Neurophysiology of the Corticospinal Tract of the Spinal Cord. Suppl. Clin. Neurophysiol. 2006, 59, 107–112. [Google Scholar] [CrossRef]
- Deletis, V.; Seidel, K.; Sala, F.; Raabe, A.; Chudy, D.; Beck, J.; Kothbauer, K.F. Intraoperative Identification of the Corticospinal Tract and Dorsal Column of the Spinal Cord by Electrical Stimulation. J. Neurol. Neurosurg. Psychiatry 2018, 89, 754–761. [Google Scholar] [CrossRef]
- Simon, M.V. Intraoperative Clinical Neurophysiology: A Comprehensive Guide to Monitoring and Mapping; Demos Medical: New York, NY, USA, 2010; ISBN 978-1-933864-46-4. [Google Scholar]
- Kugelberg, E. Facial reflexes. Brain 1952, 75, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.E. Cranial Reflexes and Related Techniques. In Clinical Neurophsyiology; OUP: New York, NY, USA, 2009; pp. 529–542. ISBN 978-0-19-538511-3. [Google Scholar]
- Deletis, V.; Urriza, J.; Ulkatan, S.; Fernandez-Conejero, I.; Lesser, J.; Misita, D. The Feasibility of Recording Blink Reflexes under General Anesthesia. Muscle Nerve 2009, 39, 642–646. [Google Scholar] [CrossRef]
- Aydinlar, E.I.; Kocak, M.; Soykam, H.O.; Mat, B.; Dikmen, P.Y.; Sezerman, O.U.; Elmaci, İ.; Pamir, M.N. Intraoperative Neuromonitoring of Blink Reflex During Posterior Fossa Surgeries and Its Correlation with Clinical Outcome. J. Clin. Neurophysiol. 2022, 39, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Conejero, I.; Ulkatan, S.; Deletis, V. Chapter 10—Monitoring Cerebellopontine Angle and Skull Base Surgeries. In Handbook of Clinical Neurology; Intraoperative Neuromonitoring; Nuwer, M.R., MacDonald, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 186, pp. 163–176. [Google Scholar]
- Karakis, I.; Simon, M.V. Neurophysiologic Mapping and Monitoring of Cranial Nerves and Brainstem. In Intraoperative Neurophysiology; Simon, M.V., Ed.; Springer Publishing Company: New York, NY, USA, 2018; pp. 345–388. ISBN 978-1-62070-117-1. [Google Scholar]
- Mirallave Pescador, A.; Téllez, M.J.; Sánchez Roldán, M.d.L.Á.; Samusyte, G.; Lawson, E.C.; Coelho, P.; Lejarde, A.; Rathore, A.; Le, D.; Ulkatan, S. Methodology for Eliciting the Brainstem Trigeminal-Hypoglossal Reflex in Humans under General Anesthesia. Clin. Neurophysiol. 2022, 137, 1–10. [Google Scholar] [CrossRef]
- Ishiwata, Y.; Ono, T.; Kuroda, T.; Nakamura, Y. Jaw-Tongue Reflex: Afferents, Central Pathways, and Synaptic Potentials in Hypoglossal Motoneurons in the Cat. J. Dent. Res. 2000, 79, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.A. The Neural Regulation of Tongue Movements. Prog. Neurobiol. 1980, 15, 295–344. [Google Scholar] [CrossRef]
- Morimoto, T.; Kawamura, Y. Properties of Tongue and Jaw Movements Elicited by Stimulation of the Orbital Gyrus in the Cat. Arch. Oral Biol. 1973, 18, 361–372. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, J.; Yang, R.; Pendlebury, W. Neuronal Circuitry and Synaptic Organization of Trigeminal Proprioceptive Afferents Mediating Tongue Movement and Jaw-Tongue Coordination via Hypoglossal Premotor Neurons. Eur. J. Neurosci. 2006, 23, 3269–3283. [Google Scholar] [CrossRef]
- Godaux, E.; Desmedt, J.E. Human Masseter Muscle: H- and Tendon Reflexes. Their Paradoxical Potentiation by Muscle Vibration. Arch. Neurol. 1975, 32, 229–234. [Google Scholar] [CrossRef]
- Kimura, J. H, T, and Masseter Reflexes and the Silent Period. In Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice; Oxford University Press: New York, NY, USA, 2013; pp. 216–218. ISBN 978-0-19-935316-3. [Google Scholar]
- Szentagothai, J. Anatomical Considerations on Monosynaptic Reflex Arcs. J. Neurophysiol. 1948, 11, 445–454. [Google Scholar] [CrossRef]
- Ulkatan, S.; Jaramillo, A.M.; Téllez, M.J.; Goodman, R.R.; Deletis, V. Feasibility of Eliciting the H Reflex in the Masseter Muscle in Patients under General Anesthesia. Clin. Neurophysiol. 2017, 128, 123–127. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Téllez, M.J.; Tapia, O.R.; Ulkatan, S.; Deletis, V. A Novel Methodology for Assessing Laryngeal and Vagus Nerve Integrity in Patients under General Anesthesia. Clin. Neurophysiol. 2017, 128, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.F.; Téllez, M.J.; Ulkatan, S. Noninvasive, Tube-Based, Continuous Vagal Nerve Monitoring Using the Laryngeal Adductor Reflex: Feasibility Study of 134 Nerves at Risk. Head Neck 2018, 40, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L.; Van Pelt, F.; Koda, J. Characteristics of Late Responses to Superior Laryngeal Nerve Stimulation in Humans. Ann. Otol. Rhinol. Laryngol. 1992, 101, 127–134. [Google Scholar] [CrossRef]
- Sasaki, C.T.; Suzuki, M. Laryngeal Reflexes in Cat, Dog, and Man. Arch. Otolaryngol. 1976, 102, 400–402. [Google Scholar] [CrossRef]
- Téllez, M.J.; Mirallave-Pescador, A.; Seidel, K.; Urriza, J.; Shoakazemi, A.; Raabe, A.; Ghatan, S.; Deletis, V.; Ulkatan, S. Neurophysiological Monitoring of the Laryngeal Adductor Reflex during Cerebellar-Pontine Angle and Brainstem Surgery. Clin. Neurophysiol. 2021, 132, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Kaneoka, A.; Pisegna, J.M.; Inokuchi, H.; Ueha, R.; Goto, T.; Nito, T.; Stepp, C.E.; LaValley, M.P.; Haga, N.; Langmore, S.E. Relationship Between Laryngeal Sensory Deficits, Aspiration, and Pneumonia in Patients with Dysphagia. Dysphagia 2018, 33, 192–199. [Google Scholar] [CrossRef]
- Tarantino, V.; Stura, M.; Vallarino, R. Development of Auditory Evoked Potentials of the Brainstem in Relation to Age. Pediatr. Med. Chir. 1988, 10, 73–76. [Google Scholar] [PubMed]
- Reroń, E.; Sekuła, J. Maturation of the Acoustic Path-Way in Brain Stem Responses (ABR) in Neonates. Otolaryngol. Pol. 1994, 48, 363–369. [Google Scholar] [PubMed]
- Boor, R.; Goebel, B. Maturation of Near-Field and Far-Field Somatosensory Evoked Potentials after Median Nerve Stimulation in Children under 4 Years of Age. Clin. Neurophysiol. 2000, 111, 1070–1081. [Google Scholar] [CrossRef]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial Magnetic and Direct Current Stimulation in Children. Curr. Neurol. Neurosci. Rep. 2017, 17, 11. [Google Scholar] [CrossRef]
- Garvey, M.A. Transcranial Magnetic Stimulation in Children. In Magnetic Stimulation in Clinical Neurophysiology; Hallett, M., Chokroverty, S., Eds.; Elsevier Butterworth-Heinemann: Philadelphia, PA, USA, 2005; pp. 429–433. ISBN 978-0-7506-7373-0. [Google Scholar]
- Kuntz, N.L. Chapter 6—Clinical Neurophysiology of the Motor Unit in Infants and Children. In Clinical Neurophysiology of Infancy, Childhood, and Adolescence; Holmes, G.L., Jones, H.R., Moshé, S.L., Eds.; Butterworth-Heinemann: Philadelphia, PA, USA, 2006; pp. 130–145. ISBN 978-0-7506-7251-1. [Google Scholar]
- Sasaki, C.T.; Levine, P.A.; Laitman, J.T.; Crelin, E.S. Postnatal Descent of the Epiglottis in Man. A Preliminary Report. Arch. Otolaryngol. 1977, 103, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, R.; Zhao, W.; Pilowsky, P.M. Medullary Mediation of the Laryngeal Adductor Reflex: A Possible Role in Sudden Infant Death Syndrome. Respir. Physiol. Neurobiol. 2016, 226, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gaglini, P.P.; Tavormina, P.; Ricci, F.; Peretta, P. A Method for Intraoperative Recording of the Laryngeal Adductor Reflex during Lower Brainstem Surgery in Children. Clin. Neurophysiol. 2018, 129, 2497–2498. [Google Scholar] [CrossRef]
- Ulkatan, S.; Téllez, M.J.; Sinclair, C. Laryngeal Adductor Reflex and Future Projections for Brainstem Monitoring. Reply to “A Method for Intraoperative Recording of the Laryngeal Adductor Reflex during Lower Brainstem Surgery in Children”. Clin. Neurophysiol. 2018, 129, 2499–2500. [Google Scholar] [CrossRef]
- Stone, J.L.; Bouffard, A.; Morris, R.; Hovsepian, W.; Meyers, H.L. Clinical and Electrophysiologic Recovery in Arnold-Chiari Malformation. Surg. Neurol. 1983, 20, 313–317. [Google Scholar] [CrossRef]
- Brookler, K.H. Vestibular Findings in a 62-Year-Old Woman with Dizziness and a Type I Chiari Malformation. Ear Nose Throat J. 2005, 84, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.A.; Bacon, C.K.; Sclafani, A.P. Chiari-I Malformation Associated with Asymmetric Sensorineural Hearing Loss. J. Otolaryngol. 1992, 21, 102–107. [Google Scholar] [PubMed]
- Johnson, G.D.; Harbaugh, R.E.; Lenz, S.B. Surgical Decompression of Chiari I Malformation for Isolated Progressive Sensorineural Hearing Loss. Am. J. Otol. 1994, 15, 634–638. [Google Scholar] [PubMed]
- Ahmmed, A.U.; Mackenzie, I.; Das, V.K.; Chatterjee, S.; Lye, R.H. Audio-Vestibular Manifestations of Chiari Malformation and Outcome of Surgical Decompression: A Case Report. J. Laryngol. Otol. 1996, 110, 1060–1064. [Google Scholar] [CrossRef]
- Hort-Legrand, C.; Emery, E. Motor and sensory evoked potentials in syringomyelia. Neurochirurgie 1999, 45, 95–104. [Google Scholar]
- Isik, N.; Elmaci, I.; Isik, N.; Cerci, S.; Basaran, R.; Gura, M.; Kalelioglu, M. Long-Term Results and Complications of the Syringopleural Shunting for Treatment of Syringomyelia: A Clinical Study. Br. J. Neurosurg. 2013, 27, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Henriques Filho, P.S.A.; Pratesi, R. Abnormalities in Auditory Evoked Potentials of 75 Patients with Arnold-Chiari Malformations Types I and II. Arq. Neuropsiquiatr. 2006, 64, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Vidmer, S.; Sergio, C.; Veronica, S.; Flavia, T.; Silvia, E.; Sara, B.; Valentini, L.G.; Daria, R.; Solero, C.L. The Neurophysiological Balance in Chiari Type 1 Malformation (CM1), Tethered Cord and Related Syndromes. Neurol. Sci. 2011, 32 (Suppl. 3), S311–S316. [Google Scholar] [CrossRef]
- Di Stefano, V.; Ferrante, C.; Telese, R.; Caulo, M.; Bonanni, L.; Onofrj, M.; Franciotti, R. Brainstem Evoked Potentials and Magnetic Resonance Imaging Abnormalities in Differential Diagnosis of Intracranial Hypotension. Neurophysiol. Clin. 2019, 49, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.E.; Frith, R.W.; Synek, V.M. Somatosensory Evoked Potentials in Syringomyelia. J. Neurol. Neurosurg. Psychiatr. 1986, 49, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Forcadas, I.; Hurtado, P.; Madoz, P.; Zarranz, J.J. Somatosensory Evoked Potentials in Syringomyelia and the Arnold-Chiari Anomaly. Clinical and Imaging Correlations. Neurologia 1988, 3, 172–175. [Google Scholar]
- Jabbari, B.; Geyer, C.; Gunderson, C.; Chu, A.; Brophy, J.; McBurney, J.W.; Jonas, B. Somatosensory Evoked Potentials and Magnetic Resonance Imaging in Syringomyelia. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 277–285. [Google Scholar] [CrossRef]
- Morioka, T.; Kurita-Tashima, S.; Fujii, K.; Nakagaki, H.; Kato, M.; Fukui, M. Somatosensory and Spinal Evoked Potentials in Patients with Cervical Syringomyelia. Neurosurgery 1992, 30, 218–222. [Google Scholar] [CrossRef]
- Utzig, N.; Burtzlaff, C.; Wiersbitzky, H.; Lauffer, H. Evoked Potentials in Chiari-Malformation Type I with Syringomyelia-a Case History. Klin. Padiatr. 2003, 215, 241–243. [Google Scholar] [CrossRef]
- Akakın, A.; Yılmaz, B.; Ekşi, M.Ş.; Kılıç, T. Treatment of Syringomyelia Due to Chiari Type I Malformation with Syringo-Subarachnoid-Peritoneal Shunt. J. Korean Neurosurg. Soc. 2015, 57, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, M.; Schwerdtfeger, K.; Donauer, E. The Value of Somato-Sensory Evoked Potentials in the Diagnosis of Syringomyelia. Acta Neurochir. 1993, 123, 184–187. [Google Scholar]
- Guvenc, G.; Kızmazoglu, C.; Aydın, H.E.; Coskun, E. Somatosensory Evoked Potentials and Cerebrospinal Fluid Flow in Chiari Malformation. Ann. Clin. Anal. Med. 2019, 10, 348–353. [Google Scholar] [CrossRef]
- Cheng, J.C.; Guo, X.; Sher, A.H.; Chan, Y.L.; Metreweli, C. Correlation between Curve Severity, Somatosensory Evoked Potentials, and Magnetic Resonance Imaging in Adolescent Idiopathic Scoliosis. Spine 1999, 24, 1679–1684. [Google Scholar] [CrossRef]
- Berciano, J.; Poca, M.-A.; García, A.; Sahuquillo, J. Paroxysmal Cervicobrachial Cough-Induced Pain in a Patient with Syringomyelia Extending into Spinal Cord Posterior Gray Horns. J. Neurol. 2007, 254, 678–681. [Google Scholar] [CrossRef]
- Awai, L.; Curt, A. Preserved Sensory-Motor Function despite Large-Scale Morphological Alterations in a Series of Patients with Holocord Syringomyelia. J. Neurotrauma 2015, 32, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Stancanelli, C.; Mazzeo, A.; Gentile, L.; Vita, G. Unilateral Hyperhidrosis as Persistently Isolated Feature of Syringomyelia and Arnold Chiari Type 1. Neurol. Sci. 2018, 39, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Nogués, M.A.; Pardal, A.M.; Merello, M.; Miguel, M.A. SEPs and CNS Magnetic Stimulation in Syringomyelia. Muscle Nerve 1992, 15, 993–1001. [Google Scholar] [CrossRef]
- Cocito, D.; Peci, E.; Garbossa, D.; Ciaramitaro, P. Neurophysiological Correlates in Patients with Syringomyelia and Chiari Malformation: The Cortico-Diaphragmatic Involvement. J. Clin. Med. 2022, 11, 5080. [Google Scholar] [CrossRef]
- Cristante, L.; Westphal, M.; Herrmann, H.D. Cranio-Cervical Decompression for Chiari I Malformation. A Retrospective Evaluation of Functional Outcome with Particular Attention to the Motor Deficits. Acta Neurochir. 1994, 130, 94–100. [Google Scholar] [CrossRef]
- Gerard, C.L.; Dugas, M.; Narcy, P.; Hertz-Pannier, J. Chiari Malformation Type I in a Child with Velopharyngeal Insufficiency. Dev. Med. Child Neurol. 1992, 34, 174–176. [Google Scholar] [CrossRef]
- Paulig, M.; Prosiegel, M. Misdiagnosis of Amyotrophic Lateral Sclerosis in a Patient with Dysphagia Due to Chiari I Malformation. J. Neurol. Neurosurg. Psychiatry 2002, 72, 270. [Google Scholar] [CrossRef]
- Bagnato, S.; Rizzo, V.; Quartarone, A.; Majorana, G.; Vita, G.; Girlanda, P. Segmental Myoclonus in a Patient Affected by Syringomyelia. Neurol. Sci. 2001, 22, 27–29. [Google Scholar] [CrossRef]
- Scelsa, S. Syringomyelia Presenting as Ulnar Neuropathy at the Elbow. Clin. Neurophysiol. 2000, 111, 1527–1530. [Google Scholar] [CrossRef]
- Muhn, N.; Baker, S.K.; Hollenberg, R.D.; Meaney, B.F.; Tarnopolsky, M.A. Syringomyelia Presenting as Rapidly Progressive Foot Drop. J. Clin. Neuromuscul. Dis. 2002, 3, 133–134. [Google Scholar] [CrossRef]
- Jayamanne, C.; Fernando, L.; Mettananda, S. Chiari Malformation Type 1 Presenting as Unilateral Progressive Foot Drop: A Case Report and Review of Literature. BMC Pediatr. 2018, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Saifudheen, K.; Jose, J.; Gafoor, V.A. Holocord Syringomyelia Presenting as Rapidly Progressive Foot Drop. J. Neurosci. Rural Pract. 2011, 2, 195–196. [Google Scholar] [CrossRef]
- McMillan, H.J.; Sell, E.; Nzau, M.; Ventureyra, E.C.G. Chiari 1 Malformation and Holocord Syringomyelia Presenting as Abrupt Onset Foot Drop. Child’s Nerv. Syst. 2011, 27, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Kaur, M. Rapidly Progressive Foot Drop: An Uncommon and Underappreciated Cause of Chiari I Malformation and Holocord Syrinx. BMJ Case Rep. 2013, 2013, bcr2013009644. [Google Scholar] [CrossRef]
- Leis, A.A.; Kofler, M.; Ross, M.A. The Silent Period in Pure Sensory Neuronopathy. Muscle Nerve 1992, 15, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Leis, A.A.; Stĕtkárová, I.; Berić, A.; Stokić, D.S. Spinal Motor Neuron Excitability during the Cutaneous Silent Period. Muscle Nerve 1995, 18, 1464–1470. [Google Scholar] [CrossRef]
- McLellan, D.L. The Electromyographic Silent Period Produced by Supramaximal Electrical Stimulation in Normal Man. J. Neurol. Neurosurg. Psychiatry 1973, 36, 334–341. [Google Scholar] [CrossRef][Green Version]
- Shefner, J.M.; Logigian, E.L. Relationship between Stimulus Strength and the Cutaneous Silent Period. Muscle Nerve 1993, 16, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Uncini, A.; Kujirai, T.; Gluck, B.; Pullman, S. Silent Period Induced by Cutaneous Stimulation. Electroencephalogr. Clin. Neurophysiol. 1991, 81, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Kawai, S.; Fuchigami, Y.; Morita, H.; Ofuji, A. Cutaneous Silent Period in Syringomyelia. Muscle Nerve 1997, 20, 884–886. [Google Scholar] [CrossRef]
- Amoiridis, G.; Meves, S.; Schols, L.; Przuntek, H. Reversible Urinary Retention as the Main Symptom in the First Manifestation of a Syringomyelia. J. Neurol. Neurosurg. Psychiatry 1996, 61, 407–408. [Google Scholar] [CrossRef]
- Jacome, D.E. Blepharoclonus and Arnold-Chiari Malformation. Acta Neurol. Scand. 2001, 104, 113–117. [Google Scholar] [CrossRef]
- Hausmann, O.N.; Böni, T.; Pfirrmann, C.W.A.; Curt, A.; Min, K. Preoperative Radiological and Electrophysiological Evaluation in 100 Adolescent Idiopathic Scoliosis Patients. Eur. Spine J. 2003, 12, 501–506. [Google Scholar] [CrossRef]
- Anderson, R.C.; Dowling, K.C.; Feldstein, N.A.; Emerson, R.G. Chiari I Malformation: Potential Role for Intraoperative Electrophysiologic Monitoring. J. Clin. Neurophysiol. 2003, 20, 65–72. [Google Scholar] [CrossRef]
- Zamel, K.; Galloway, G.; Kosnik, E.J.; Raslan, M.; Adeli, A. Intraoperative Neurophysiologic Monitoring in 80 Patients with Chiari I Malformation: Role of Duraplasty. J. Clin. Neurophysiol. 2009, 26, 70–75. [Google Scholar] [CrossRef]
- Kim, I.-K.; Wang, K.-C.; Kim, I.-O.; Cho, B.-K. Chiari 1.5 Malformation: An Advanced Form of Chiari I Malformation. J. Korean Neurosurg. Soc. 2010, 48, 375–379. [Google Scholar] [CrossRef]
- Chen, J.A.; Coutin-Churchman, P.E.; Nuwer, M.R.; Lazareff, J.A. Suboccipital Craniotomy for Chiari I Results in Evoked Potential Conduction Changes. Surg. Neurol. Int. 2012, 3, 165. [Google Scholar] [CrossRef][Green Version]
- Kennedy, B.; Kelly, K.; Phan, M.; Bruce, S.; McDowell, M.; Anderson, R.; Feldstein, N. Outcomes after Suboccipital Decompression without Dural Opening in Children with Chiari Malformation Type I. J. Neurosurg. Pediatr. 2015, 16, 150–158. [Google Scholar] [CrossRef]
- Barzilai, O.; Roth, J.; Korn, A.; Constantini, S. The Value of Multimodality Intraoperative Neurophysiological Monitoring in Treating Pediatric Chiari Malformation Type I. Acta Neurochir. 2016, 158, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Rasul, F.T.; Matloob, S.A.; Haliasos, N.; Jankovic, I.; Boyd, S.; Thompson, D.N.P. Intraoperative Neurophysiological Monitoring in Paediatric Chiari Surgery-Help or Hindrance? Child’s Nerv. Syst. 2019, 35, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Uchida, S.; Onishi, K.; Toyokuni, M.; Okanari, K.; Fujiki, M. Intraoperative Neurophysiologic Monitoring for Prediction of Postoperative Neurological Improvement in a Child with Chiari Type I Malformation. J. Craniofac. Surg. 2017, 28, 1837–1841. [Google Scholar] [CrossRef]
- Krishnakumar, M.; Ramesh, V.; Goyal, A.; Srinivas, D. Utility of Motor Evoked Potential in Identification and Treatment of Suboptimal Positioning in Pediatric Craniovertebral Junction Abnormalities: A Case Report. A A Pract. 2020, 14, e01323. [Google Scholar] [CrossRef]
- Grossauer, S.; Koeck, K.; Vince, G.H. Intraoperative Somatosensory Evoked Potential Recovery Following Opening of the Fourth Ventricle during Posterior Fossa Decompression in Chiari Malformation: Case Report. J. Neurosurg. 2015, 122, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Roser, F.; Ebner, F.H.; Liebsch, M.; Tatagiba, M.S.; Naros, G. The Role of Intraoperative Neuromonitoring in Adults with Chiari I Malformation. Clin. Neurol. Neurosurg. 2016, 150, 27–32. [Google Scholar] [CrossRef]
- Heffez, D.S.; Golchini, R.; Ghorai, J.; Cohen, B. Operative Findings and Surgical Outcomes in Patients Undergoing Chiari 1 Malformation Decompression: Relationship to the Extent of Tonsillar Ectopia. Acta Neurochir. 2020, 162, 1539–1547. [Google Scholar] [CrossRef]
- Schaefer, J.; Atallah, E.; Tecce, E.; Thalheimer, S.; Harrop, J.; Heller, J.E. Utility of Intraoperative Neuromonitoring for Decompression of Chiari Type I Malformation in 93 Adult Patients. J. Neurosurg. 2022, 137, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Di, X. Endoscopic Suboccipital Decompression on Pediatric Chiari Type I. Minim. Invasive Neurosurg. 2009, 52, 119–125. [Google Scholar] [CrossRef]
- Shah, A.; Patil, A.; Vutha, R.; Thakar, K.; Goel, A. Recovery of Transcranial Motor Evoked Potentials After Atlantoaxial Stabilization for Chiari Formation: Report of 20 Cases. World Neurosurg. 2019, 127, e644–e648. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Kotzen, R.M.; Capocelli, A.L.; Bolognese, P.; Bendo, A.A.; Cottrell, J.E. Intraoperative Improvement of Somatosensory Evoked Potentials and Local Spinal Cord Blood Flow in Patients with Syringomyelia. J. Neurosurg. Anesthesiol. 1996, 8, 208–215. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Capocelli, A.L.; Kotzen, R.M.; Bolognese, P.; Heger, I.M.; Cottrell, J.E. Intramedullary Pressure in Syringomyelia: Clinical and Pathophysiological Correlates of Syrinx Distension. Neurosurgery 1997, 41, 1102–1110. [Google Scholar] [CrossRef]
- Pencovich, N.; Korn, A.; Constantini, S. Intraoperative Neurophysiologic Monitoring during Syringomyelia Surgery: Lessons from a Series of 13 Patients. Acta Neurochir. 2013, 155, 785–791. [Google Scholar] [CrossRef]
- Sánchez-Roldán, M.A.; Moncho, D.; Rahnama, K.; Santa-Cruz, D.; Lainez, E.; Baiget, D.; Chocron, I.; Gandara, D.; Bescos, A.; Sahuquillo, J.; et al. Intraoperative Neurophysiological Monitoring in Syringomyelia Surgery: A Multimodal Approach. J. Clin. Med. 2023, 12, 5200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qiu, Y.; Wang, B.; Yu, Y.; Qian, B.; Zhu, F. Abnormal Spreading and Subunit Expression of Junctional Acetylcholine Receptors of Paraspinal Muscles in Scoliosis Associated with Syringomyelia. Spine 2007, 32, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Eule, J.M.; Erickson, M.A.; O’Brien, M.F.; Handler, M. Chiari I Malformation Associated with Syringomyelia and Scoliosis: A Twenty-Year Review of Surgical and Nonsurgical Treatment in a Pediatric Population. Spine 2002, 27, 1451–1455. [Google Scholar] [CrossRef]
- Zhu, Z.; Yan, H.; Han, X.; Jin, M.; Xie, D.; Sha, S.; Liu, Z.; Qian, B.; Zhu, F.; Qiu, Y. Radiological Features of Scoliosis in Chiari I Malformation Without Syringomyelia. Spine 2016, 41, E276–E281. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Beckman, J.; Naftel, R.P.; Chern, J.J.; Wellons, J.C.; Rozzelle, C.J.; Blount, J.P.; Oakes, W.J. Institutional Experience with 500 Cases of Surgically Treated Pediatric Chiari Malformation Type I. J. Neurosurg. Pediatr. 2011, 7, 248–256. [Google Scholar] [CrossRef]
- Sha, S.; Zhu, Z.; Lam, T.P.; Sun, X.; Qian, B.; Jiang, J.; Cheng, J.C.Y.; Qiu, Y. Brace Treatment versus Observation Alone for Scoliosis Associated with Chiari I Malformation Following Posterior Fossa Decompression: A Cohort Study of 54 Patients. Eur. Spine J. 2014, 23, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Lin, Y.; Rong, T.; Shen, J.; Zhang, J.; Feng, E.; Jiao, Y.; Liang, J.; Li, Z. Surgical Scoliosis Correction in Chiari-I Malformation with Syringomyelia Versus Idiopathic Syringomyelia. J. Bone Jt. Surg. Am. 2020, 102, 1405–1415. [Google Scholar] [CrossRef]
- Shi, B.; Qiu, J.; Xu, L.; Li, Y.; Jiang, D.; Xia, S.; Liu, Z.; Sun, X.; Shi, B.; Zhu, Z.; et al. Somatosensory and Motor Evoked Potentials during Correction Surgery of Scoliosis in Neurologically Asymptomatic Chiari Malformation-Associated Scoliosis: A Comparison with Idiopathic Scoliosis. Clin. Neurol. Neurosurg. 2020, 191, 105689. [Google Scholar] [CrossRef]
- Brinzeu, A.; Sindou, M. Functional Anatomy of the Accessory Nerve Studied through Intraoperative Electrophysiological Mapping. J. Neurosurg. 2017, 126, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Giampiccolo, D.; Basaldella, F.; Badari, A.; Squintani, G.M.; Cattaneo, L.; Sala, F. Feasibility of Cerebello-Cortical Stimulation for Intraoperative Neurophysiological Monitoring of Cerebellar Mutism. Child’s Nerv. Syst. 2021, 37, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Roth, J.; Korn, A.; Constantini, S. Letter to the Editor: Evoked Potentials and Chiari Malformation Type 1. J. Neurosurg. 2017, 126, 654–657. [Google Scholar] [CrossRef]
- Caldarelli, M.; Novegno, F.; Vassimi, L.; Romani, R.; Tamburrini, G.; Di Rocco, C. The Role of Limited Posterior Fossa Craniectomy in the Surgical Treatment of Chiari Malformation Type I: Experience with a Pediatric Series. J. Neurosurg. 2007, 106, 187–195. [Google Scholar] [CrossRef] [PubMed]
| Neurophysiological Tests | Pathway | Main Findings | Pitfalls and Limitations |
|---|---|---|---|
| SEP | Dorsal columns or lemniscal system | Increased N13-N20 interval. Reduced or absent cervical potential (N13) | - Associated neuropathy, other forms of cervical spinal stenosis, and cervical myelopathy - Requires patient collaboration (especially relaxation) |
| BAEP | Auditory from cochlea to superior pons | Increased I–V interval, suggesting central or retrocochlear involvement | - Associated hypoacusia of cochlear origin - Requires patient collaboration (especially relaxation) |
| MEP | Pyramidal system or corticospinal tract | Increased CMCT | - Variability related to maturative age - Requires patient collaboration |
| EMG/NCS | Peripheral nervous system (roots/anterior horn, nerves and muscles) | Preganglionic pattern at NCS (preserved sensory neurography) with acute or chronic denervation at EMG, compatible with the suspicion of anterior horn cell lesion if syringomyelia is present | - Concomitant neuropathy or radiculopathy secondary to spondylosis |
| Brainstem reflexes (Blink reflex) | Involved cranial nerves, nucleus, and pathways in the brainstem | Altered R1 and/or R2 | - Variability related to maturative age - Repetitive high frequencies stimuli cause adaptation phenomenon - Lack of published follow-up studies for CM1 and other brainstem reflexes |
| Intraoperative neurophysiological monitoring | All of the pathways described above | Evidence for benefit during positioning | Anesthetic considerations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncho, D.; Poca, M.A.; Rahnama, K.; Sánchez Roldán, M.Á.; Santa-Cruz, D.; Sahuquillo, J. The Role of Neurophysiology in Managing Patients with Chiari Malformations. J. Clin. Med. 2023, 12, 6472. https://doi.org/10.3390/jcm12206472
Moncho D, Poca MA, Rahnama K, Sánchez Roldán MÁ, Santa-Cruz D, Sahuquillo J. The Role of Neurophysiology in Managing Patients with Chiari Malformations. Journal of Clinical Medicine. 2023; 12(20):6472. https://doi.org/10.3390/jcm12206472
Chicago/Turabian StyleMoncho, Dulce, Maria A. Poca, Kimia Rahnama, M. Ángeles Sánchez Roldán, Daniela Santa-Cruz, and Juan Sahuquillo. 2023. "The Role of Neurophysiology in Managing Patients with Chiari Malformations" Journal of Clinical Medicine 12, no. 20: 6472. https://doi.org/10.3390/jcm12206472
APA StyleMoncho, D., Poca, M. A., Rahnama, K., Sánchez Roldán, M. Á., Santa-Cruz, D., & Sahuquillo, J. (2023). The Role of Neurophysiology in Managing Patients with Chiari Malformations. Journal of Clinical Medicine, 12(20), 6472. https://doi.org/10.3390/jcm12206472