Effects of Iliosacral Joint Immobilization on Walking after Iliosacral Screw Fixation in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Collective for Study
- (a)
- Medical conditions that may interfere with walking or impair gait (for example, significant soft tissue injury, infection, or pain; diagnosed movement disorder, such as Parkinson’s disease, essential tremor, or dystonia; stroke; and moderate to severe traumatic brain injury) or major limb amputation;
- (b)
- Diagnosed dementia;
- (c)
- Pregnancy;
- (d)
- Diagnosed substance dependence;
- (e)
- Inability to communicate with investigators;
- (f)
- Uncooperative behavior;
- (g)
- Inability to provide written informed consent;
- (h)
- Severe comorbidities that made participation hazardous (e.g., severely reduced bone mineral density).
2.2. Gait Laboratory
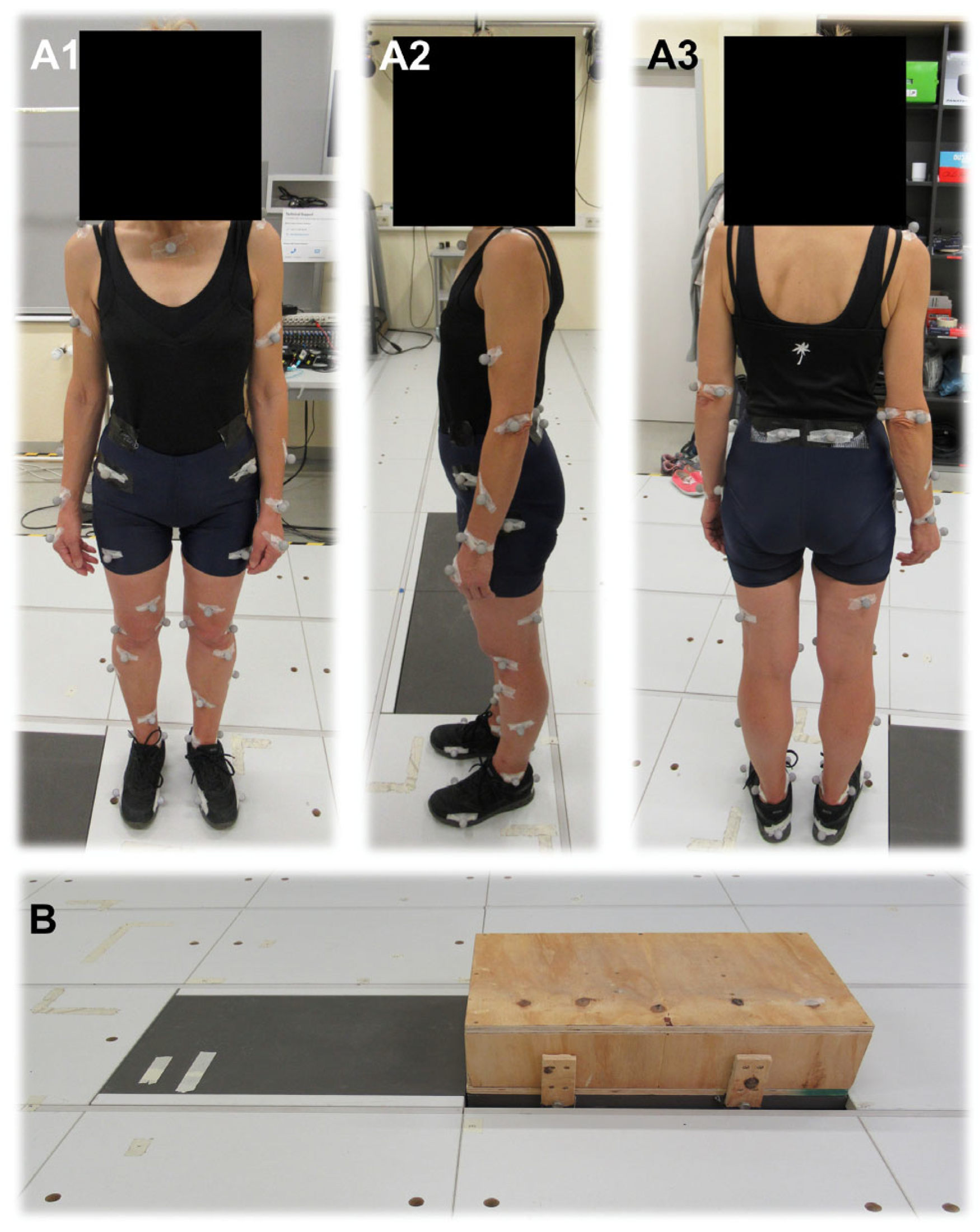
2.3. Selection of Markers
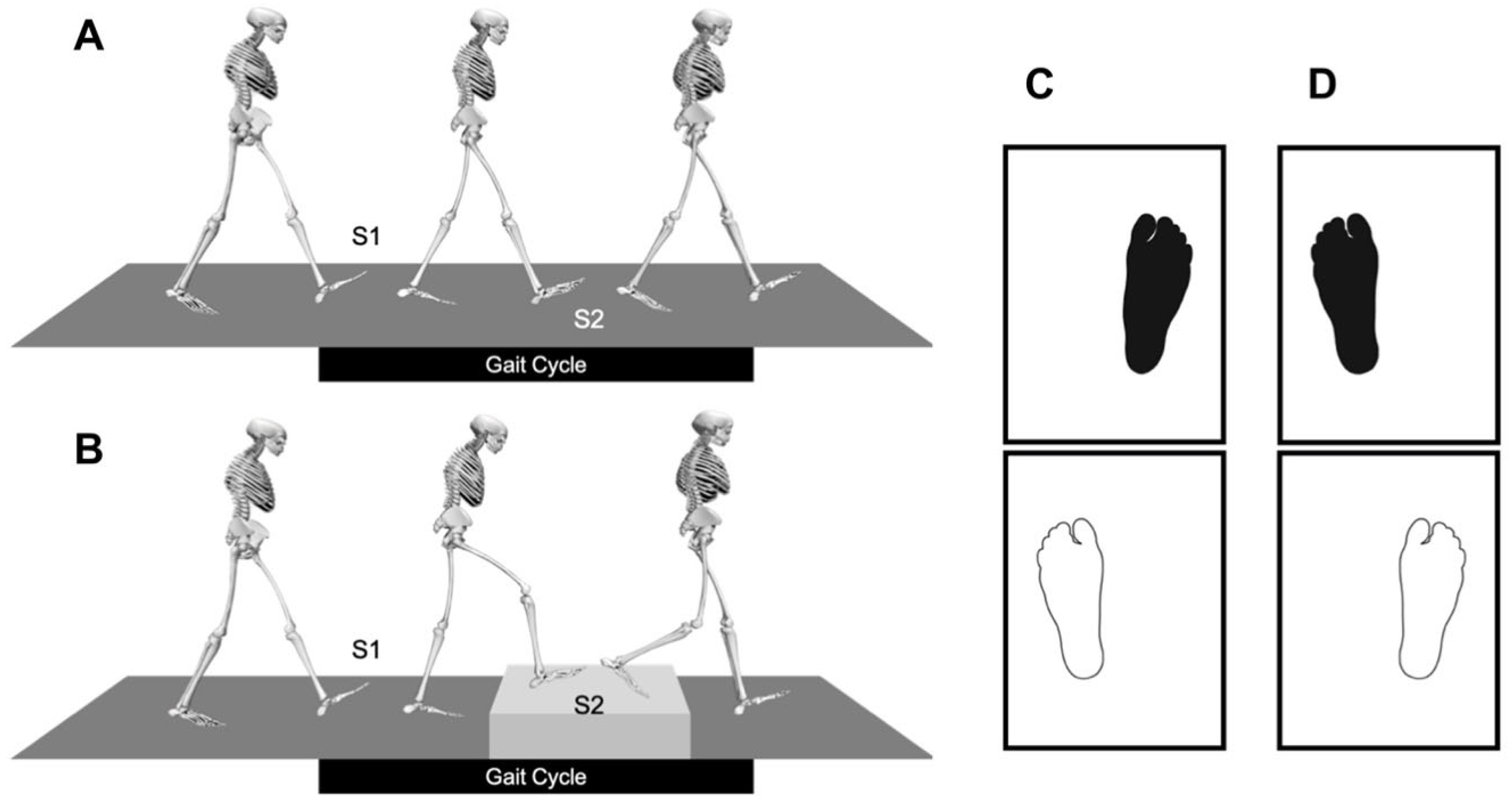
2.4. Gait Analysis
2.5. Preparation of the Gait Laboratory
2.6. Static Measurement
2.7. Dynamic Measurement
2.8. Measurement Systems and Data Processing
- 1.
- Motion measurement (motion capture)
- 2.
- Measurement of Ground Reaction Force
- 3.
- Data processing
2.9. Simulation with OpenSim
- The model
- 2.
- Scaling
- 3.
- Inverse Kinematics (IK)
- 4.
- Inverse dynamics (ID)
- 5.
- Residual Reduction Algorithm (RRA)
2.10. Statistics
- –
- Joint angles
- ○
- Peak mediolateral lumbar bending during S1 (LBA,S1)
- ○
- Peak mediolateral lumbar bending during S2 (LBA,S2)
- ○
- Hip flexion at the start of S1 (HFA,S1)
- ○
- Hip flexion at the start of S2 (HFA,S2)
- ○
- Peak hip adduction during S1 (HADA,S1)
- ○
- Peak hip adduction during S2 (HADA,S2)
- ○
- Knee flexion at the start of S1 (KFA,S1)
- ○
- Knee flexion at the start of S1 (KFA,S2)
- ○
- Peak ankle dorsiflexion during S1 (ADA,S1)
- ○
- Peak ankle dorsiflexion during S2 (ADA,S2)
- –
- Joint moments
- ○
- Peak mediolateral lumbar bending moment during S1 (LBM,S1)
- ○
- Peak mediolateral lumbar bending moment during S2 (LBM,S2)
- ○
- Peak hip extensor moment during S1 (HEM,S1)
- ○
- Peak hip extensor moment during S2 (HEM,S2)
- ○
- Peak hip flexor moment during S1 (HFM,S1)
- ○
- Peak hip flexor moment during S2 (HFM,S2)
- ○
- First peak hip abductor moment during S1 (HABM,S1)
- ○
- First peak hip abductor moment during S2 (HABM,S2)
- ○
- Peak hip external rotator moment during S1 (HRM,S1)
- ○
- Peak hip external rotator moment during S2 (HRM,S2)
- ○
- Peak knee extensor moment during S1 (KEM,S1)
- ○
- Peak knee extensor moment during S2 (KEM,S2)
- ○
- Peak ankle plantarflexor moment during S1 (APM,S1)
- ○
- Peak ankle plantarflexor moment during S2 (APM,S2)
- –
- Joint power
- ○
- Peak negative mediolateral lumbar bending power during S1 (LBP,S1)
- ○
- Peak negative mediolateral lumbar bending power during S2 (LBP,S2)
- ○
- Peak positive hip extensor power during S1 (HEP,S1)
- ○
- Peak positive hip extensor power during S2 (HEP,S2)
- ○
- Peak negative hip flexor power during S1 (HFP,S1)
- ○
- Peak negative hip flexor power during S2 (HFP,S2)
- ○
- Peak negative hip abductor power during S1 (HABP,S1)
- ○
- Peak negative hip abductor power during S2 (HABP,S2)
- ○
- Peak negative ankle plantarflexor power during S1 (APNP,S1)
- ○
- Peak negative ankle plantarflexor power during S2 (APNP,S2)
- ○
- Peak positive ankle plantarflexor power during S1 (APPP,S1)
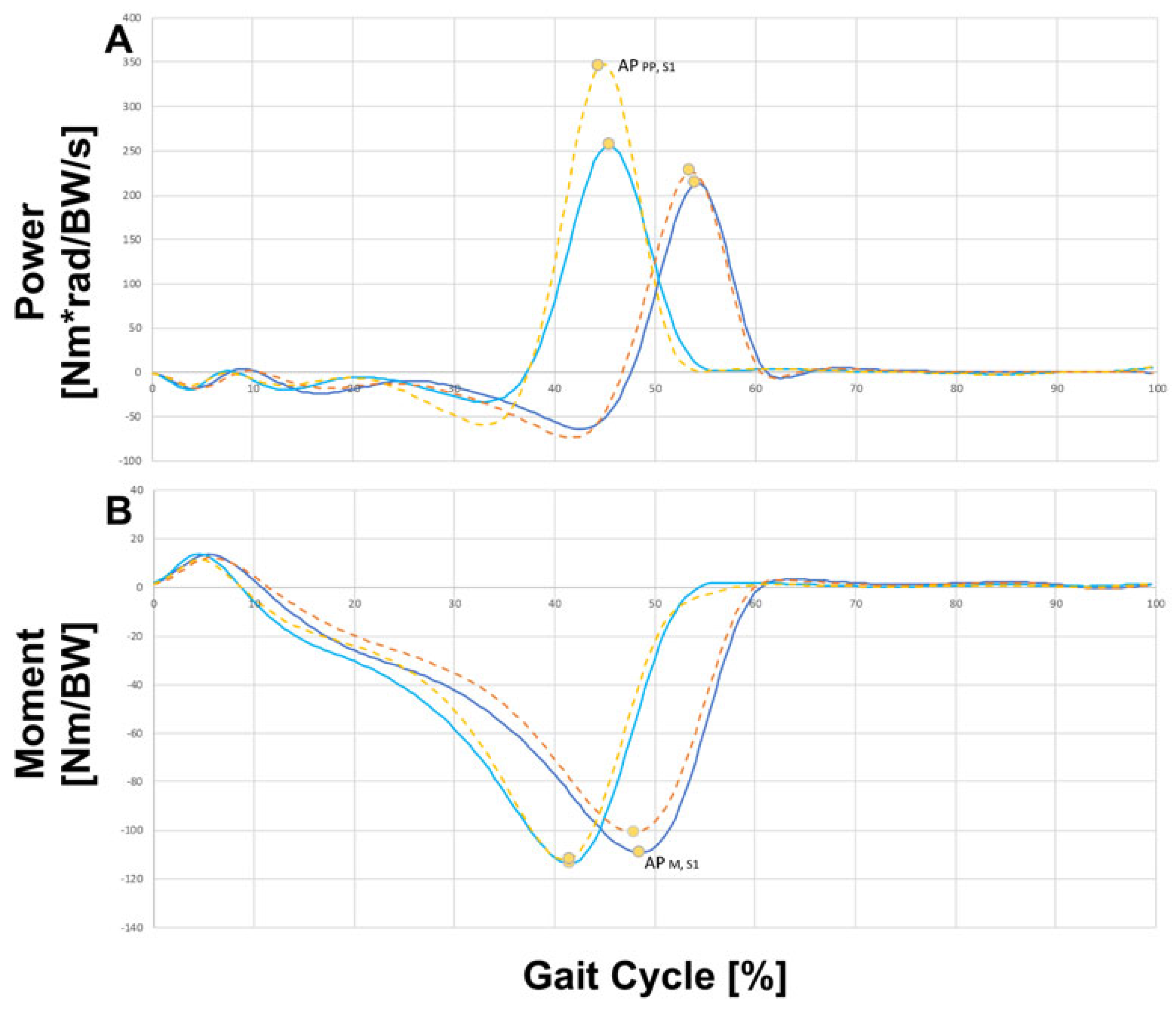
3. Results
Analyzed Joint Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascal-Moussellard, H.; Hirsch, C.; Bonaccorsi, R. Osteosynthesis in sacral fracture and lumbosacral dislocation. Orthop. Traumatol. Surg. Res. 2016, 102, S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pinto, R.; Kurd, M.F.; Schroeder, G.D.; Kepler, C.K.; Krieg, J.C.; Holstein, J.H.; Bellabarba, C.; Firoozabadi, R.; Oner, F.C.; Kandziora, F.; et al. Sacral fractures and associated injuries. Glob. Spine J. 2017, 7, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gruen, G.S.; Leit, M.E.; Gruen, R.J.; Garrison, H.G.; Auble, T.E.; Peitzman, A.B. Functional outcome of patients with unstable pelvic ring fractures stabilized with open reduction and internal fixation. J. Trauma 1995, 39, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Hakim, R.M.; Gruen, G.S.; Delitto, A. Outcomes of patients with pelvic-ring fractures managed by open reduction internal fixation. Phys. Ther. 1996, 76, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, T.A.; Ledoux, W.R.; Chapman, J.R.; Henley, M.B.; Tencer, A.F.; Routt, M.L.C. Triangular osteosynthesis and iliosacral screw fixation for unstable sacral fractures: A cadaveric and biomechanical evaluation under cyclic loads. J. Orthop. Trauma 2003, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Wagner, D.; Hofmann, A. Iliosacral, Screw, Osteosynthesis. In Fragility, Fractures of the Pelvis; Rommens, P.M., Hofmann, A., Eds.; McGraw-Hill: New York, NY, USA, 2017; pp. 121–137. [Google Scholar]
- Solonen, K.A. The sacroiliac joint in the light of anatomical, roentgenological and clinical studies. Acta Orthop. Scand. Suppl. 1957, 27, 1–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Instability of the sacroiliac joint and the consequences to gait. J. Man. Manip. Ther. 2013, 4, 22–29. [Google Scholar] [CrossRef]
- Lavignolle, B.; Vital, J.M.; Senegas, J.; Destandau, J.; Toson, B.; Bouyx, P.; Morlier, P.; Delorme, G.; Calabet, A. An approach to the functional anatomy of the sacroiliac joints in vivo. Anat. Clin. 1983, 5, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, E.W.; Van der Kleyn, R.; Hogervorst, M.; Vugt, A.B. Functional outcome of internal fixation for pelvic ring fractures. J. Trauma 1999, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, B.; Olerud, C.; Olerud, S. Anterior square-plate fixation of sacroiliac disruption. 2–8 years follow-up of 23 consecutive cases. Acta Orthop. Scand. 1993, 64, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sim, OpenSim User Manual: Release 2.4, 04 2012. Available online: https://www.google.de/search?q=OpenSim+user+manual+&ei=_h4CY_LuA7SKi-gPvpabkA0&ved=0ahUKEwjy5aDr8df5AhU0xQIHHT7LBtIQ4dUDCA0&uact=5&oq=OpenSim+user+manual+&gs_lcp=Cgdnd3Mtd2l6EAMyBggAEB4QFjIGCAAQHhAWMgYIABAeEBY6FAgAEOoCELQCEIoDELcDENQDEOUCOhEIABDqAhC0AhCKAxC3AxDlAjoRCC4Q6gIQtAIQigMQtwMQ5QI6FAguEOoCELQCEIoDELcDENQDEOUCSgQIQRgASgQIRhgAULoCWLoCYI8JaAFwAXgAgAGYAYgBmAGSAQMwLjGYAQCgAQGgAQKwAQrAAQE&sclient=gws-wiz (accessed on 29 April 2022).
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.C.; Pandy, M.G. A Dynamic Optimization Solution for Vertical Jumping in Three Dimensions. Comput. Methods Biomech. Biomed. Eng. 1999, 2, 201–231. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, G.T.; Zajac, F.E. A planar model of the knee joint to characterize the knee extensor mechanism. J. Biomech. 1989, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological 2; University of Waterloo Press: Waterloo, ON, Canada, 1991. [Google Scholar]
- Eng, J.J.; Winter, D.A. Kinetic analysis of the lower limbs during walking: What information can be gained from a three-dimensional model? J. Biomech. 1995, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Uchida, K.; Kokubo, Y.; Shimada, S.; Matsuo, H.; Yayama, T.; Miyazaki, T.; Sugita, D.; Watanabe, S.; Baba, H. Postoperative gait analysis and hip muscle strength in patients with pelvic ring fracture. Gait Posture 2013, 38, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.J.; Wretenberg, P.; Stark, A.; Palmblad, K.; Larsson, P.; Gröndal, L.; Broström, E. Gait pattern in rheumatoid arthritis. Gait Posture 2008, 28, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Olney, S.J.; Richards, C. Hemiparetic gait following stroke. Part I: Characteristics, Gait Posture 1996, 4, 136–148. [Google Scholar]
- Svehlík, M.; Zwick, E.B.; Steinwender, G.; Linhart, W.E.; Schwingenschuh, P.; Katschnig, P.; Ott, E.; Enzinger, C. Gait analysis in patients with Parkinson’s disease off dopaminergic therapy. Arch. Phys. Med. Rehabil. 2009, 90, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Sofuwa, O.; Nieuwboer, A.; Desloovere, K.; Willems, A.M.; Chavret, F.; Jonkers, I. Quantitative gait analysis in Parkinson’s disease: Comparison with a healthy control group. Arch. Phys. Med. Rehabil. 2005, 86, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Inman, V.T.; Eberhart, H.D. The major determinants in normal and pathological gait. J. Bone Jt. Surg. Am. 1953, 35, 543–558. [Google Scholar] [CrossRef]

| Description | ISF Group (n = 8) | Control Group (n = 8) |
|---|---|---|
| Number of patients | 8 | 8 |
| Age range [years] | 18–68 | 19–69 |
| Age mean [years] ± SD | 45.63 ± 23.19 | 46.50 ± 22.91 |
| Body height mean [cm] ± SD | 176.50 ± 12.99 | 170.50 ± 10.70 |
| Body weight mean [cm] ± SD | 71.88 ± 12.02 | 64.75 ± 12.83 |
| BMI mean [kg/m2] ± SD | 23.03 ± 2.45 | 22.51 ± 5.77 |
| Gender | ||
| Female [n] | 5 | 5 |
| Male [n] | 3 | 3 |
| Parameter | Main Effect | Comparison | 95% CI |
|---|---|---|---|
| LBA,S1 | Task: F1,14 = 34.2, p *** < 0.001 | LG > STEP | 1.02–2.20 degrees |
| LBA,S2 | Task: F1,14 = 54.0, p *** < 0.001 | LG < STEP | 2.98–5.46 degrees |
| LBM,S1 | Task: F1,14 = 23.5, p *** < 0.001 | LG < STEP | 0.004–0.010 Nm/BW |
| LBM,S2 | Task: F1,14 = 12.9, p ** = 0.003 Stance limb: F1,14 = 5.4, p * = 0.037 | LG < STEP Right < Left; Fixed > Free | 0.003–0.010 Nm/BW 4.30 × 10−4–0.012 Nm/BW |
| LBP,S1 | Stance limb: F1,14 = 5.0, p * = 0.043 | Right > Left; Fixed < Free | 2.26 × 10−4–0.013 Nm⋅rad/BW/s |
| LBP,S2 | Task: F1,14 = 22.5, p *** < 0.001 Stance limb: F1,14 = 5.8, p * = 0.032 | LG > STEP Right < Left; Fixed > Free | 0.006–0.015 Nm⋅rad/BW/s 4.93 × 10−4–0.009 Nm⋅rad/BW/s |
| HFA,S1 | Task: F1,14 = 10.5, p ** = 0.007 | LG < STEP | 0.45–2.27 degrees |
| HFA,S2 | Task: F1,14 = 822.3, p *** < 0.001 Stance limb: F1,14 = 6.4, p * = 0.025 | LG < STEP Right < Left; Fixed > Free | 32.40–37.68 degrees 0.11–1.43 degrees |
| HEM,S1 | Task: F1,14 = 4.8, p * = 0.047 | LG < STEP | 8.15 × 10−5–0.011 Nm/BW |
| HEM,S2 | Task: F1,14 = 114.0, p *** < 0.001 | LG < STEP | 0.040–0.060 Nm/BW |
| HFM,S1 | Task: F1,14 = 115.8, p *** < 0.001 | LG > STEP | 0.029–0.044 Nm/BW |
| HFM,S2 | Task: F1,14 = 124.7, p *** < 0.001 | LG < STEP | 0.041–0.060 Nm/BW |
| HEP,S2 | Task: F1,14 = 144.9, p *** < 0.001 Stance limb: F1,14 = 6.9, p * = 0.021 | LG < STEP Right < Left; Fixed > Free | 0.141–0.203 Nm⋅rad/BW/s 0.001–0.132 Nm⋅rad/BW/s |
| HFP,S1 | Task: F1,14 = 45.5, p *** < 0.001 | LG > STEP | 0.026–0.051 Nm⋅rad/BW/s |
| HFP,S2 | Task: F1,14 = 72.0, p *** < 0.001 | LG < STEP | 0.053–0.089 Nm⋅rad/BW/s |
| HADA,S1 | Task: F1,14 = 66.1, p *** < 0.001 | LG > STEP | 1.86–3.21 degrees |
| HADA,S2 | Task: F1,14 = 11.3, p ** = 0.005 | LG < STEP | 0.52–2.40 degrees |
| HABM,S1 | Task: F1,14 = 25.4, p *** < 0.001 | LG < STEP | 0.005–0.011 Nm/BW |
| HABM,S2 | Task: F1,14 = 196.1, p *** < 0.001 | LG > STEP | 0.021–0.029 Nm/BW |
| HABP,S1 | Stance limb: F1,14 = 7.3, p * = 0.018 | Right > Left; Fixed < Free | 0.002–0.017 Nm⋅rad/BW/s |
| HABP,S2 | Task: F1,14 = 101.4, p *** < 0.001 | LG > STEP | 0.038–0.059 Nm⋅rad/BW/s |
| HRM,S1 | Task: F1,14 = 7.5, p * = 0.017 | LG < STEP | 3.43 × 10−4–0.003 Nm/BW |
| HRM,S2 | Task: F1,14 = 55.6, p *** < 0.001 | LG < STEP | 0.005–0.009 Nm/BW |
| KFA,S1 | Task: F1,14 = 31.2, p *** < 0.001 | LG < STEP | 1.21–2.74 degrees |
| KFA,S2 | Task: F1,14 = 1560.3, p *** < 0.001 | LG < STEP | 42.56–47.48 degrees |
| KEM,S1 | Task: F1,14 = 13.5, p ** = 0.003 | LG < STEP | 0.004–0.017 Nm/BW |
| KEM,S2 | Task: F1,14 = 34.8, p *** < 0.001 | LG < STEP | 0.020–0.041 Nm/BW |
| ADA,S1 | Task: F1,14 = 20.2, p *** < 0.001 | LG > STEP | 1.73–4.93 degrees |
| ADA,S2 | Task: F1,14 = 358.4, p *** < 0.001 | LG < STEP | 14.69–18.48 degrees |
| APM,S1 | Group: F1,14 = 4.7, p * = 0.049 Task: F1,14 = 50.8, p *** < 0.001 | Control > ISF LG < STEP | 2.97 × 10−5–0.023 Nm/BW 0.015–0.029 Nm/BW |
| APM,S2 | Task: F1,14 = 574.8, p *** < 0.001 | LG > STEP | 0.041–0.049 Nm/BW |
| APNP,S1 | Task: F1,14 = 10.6, p ** = 0.006 Stance limb: F1,14 = 6.7, p * = 0.022 | LG > STEP Right > Left; Fixed < Free | 0.009–0.042 Nm⋅rad/BW/s 0.002–0.018 Nm⋅rad/BW/s |
| APNP,S2 | Task: F1,14 = 8.9, p * = 0.011 Stance limb: F1,14 = 6.7, p * = 0.023 | LG < STEP Right > Left; Fixed < Free | 0.009–0.056 Nm⋅rad/BW/s 0.002–0.027 Nm⋅rad/BW/s |
| APPP,S1 | Group: F1,14 = 14.2, p ** = 0.002 Task: F1,14 = 61.7, p *** < 0.001 | Control > ISF LG < STEP | 0.039–0.144 Nm⋅rad/BW/s 0.161–0.283 Nm⋅rad/BW/s |
| Task | Group | Speed [%height/s] | Step Length [%height] | ||
|---|---|---|---|---|---|
| Right/Fixed | Left/Free | Right/Fixed | Left/Free | ||
| Level ground | Control | 78 ± 6 | 78 ± 5 | 41 ± 2 | 41 ± 2 |
| ISF | 75 ± 8 | 75 ± 10 | 40 ± 2 | 40 ± 1 | |
| Step | Control | 67 ± 5 | 67 ± 7 | 40 ± 3 | 40 ± 2 |
| ISF | 64 ± 6 | 64 ± 7 | 39 ± 2 | 39 ± 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäckle, K.; Yoshida, T.; Neigefink, K.; Meier, M.-P.; Seitz, M.-T.; Hawellek, T.; von Lewinski, G.; Roch, P.J.; Weiser, L.; Schilling, A.F.; et al. Effects of Iliosacral Joint Immobilization on Walking after Iliosacral Screw Fixation in Humans. J. Clin. Med. 2023, 12, 6470. https://doi.org/10.3390/jcm12206470
Jäckle K, Yoshida T, Neigefink K, Meier M-P, Seitz M-T, Hawellek T, von Lewinski G, Roch PJ, Weiser L, Schilling AF, et al. Effects of Iliosacral Joint Immobilization on Walking after Iliosacral Screw Fixation in Humans. Journal of Clinical Medicine. 2023; 12(20):6470. https://doi.org/10.3390/jcm12206470
Chicago/Turabian StyleJäckle, Katharina, Takashi Yoshida, Kira Neigefink, Marc-Pascal Meier, Mark-Tilmann Seitz, Thelonius Hawellek, Gabriela von Lewinski, Paul Jonathan Roch, Lukas Weiser, Arndt F. Schilling, and et al. 2023. "Effects of Iliosacral Joint Immobilization on Walking after Iliosacral Screw Fixation in Humans" Journal of Clinical Medicine 12, no. 20: 6470. https://doi.org/10.3390/jcm12206470
APA StyleJäckle, K., Yoshida, T., Neigefink, K., Meier, M.-P., Seitz, M.-T., Hawellek, T., von Lewinski, G., Roch, P. J., Weiser, L., Schilling, A. F., & Lehmann, W. (2023). Effects of Iliosacral Joint Immobilization on Walking after Iliosacral Screw Fixation in Humans. Journal of Clinical Medicine, 12(20), 6470. https://doi.org/10.3390/jcm12206470







