Hemodynamic and Hydrodynamic Pathophysiology in Chiari Type 1 Malformations: Towards Understanding the Genesis of Syrinx
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
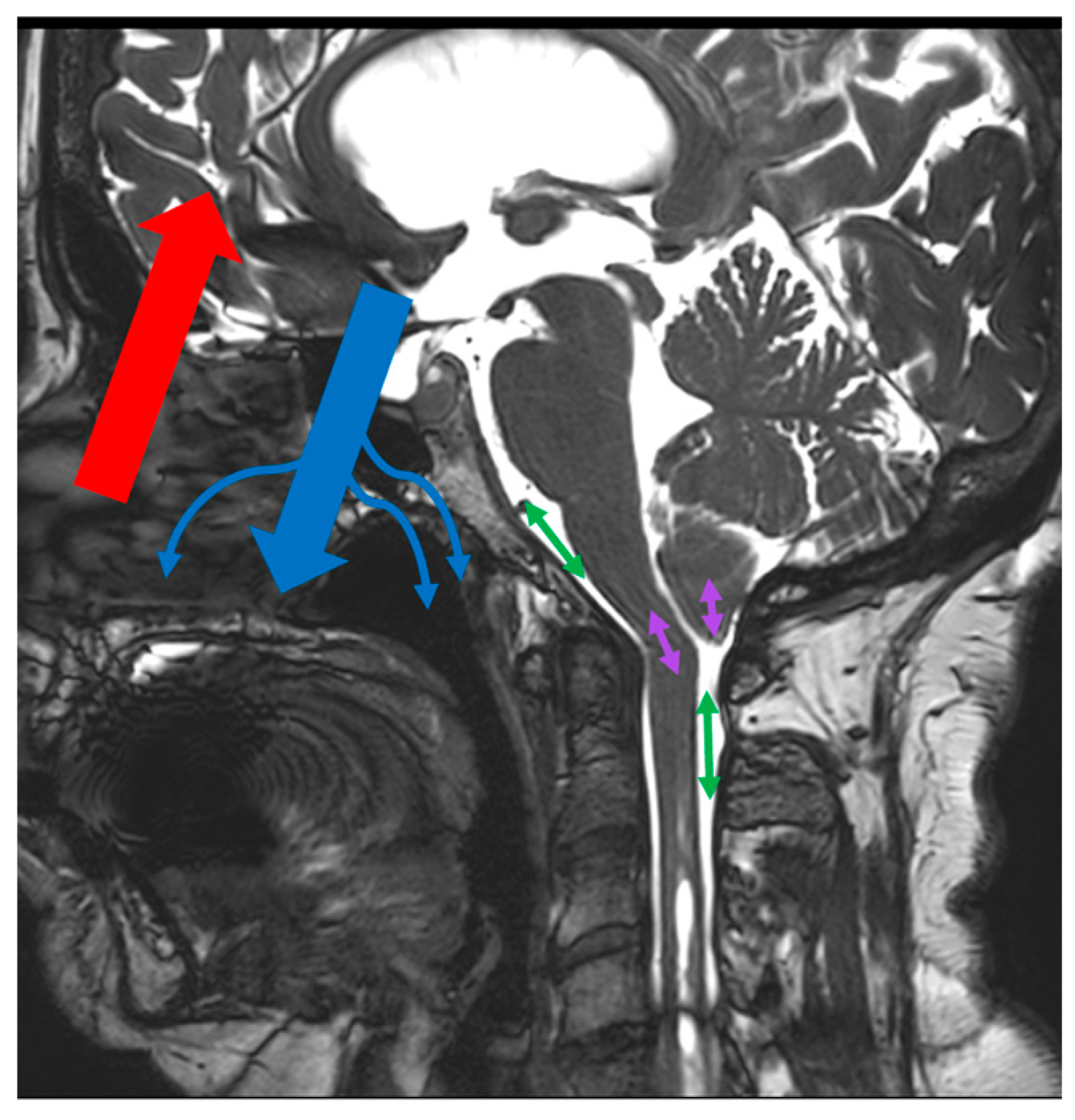
2.2. PCMRI Acquisition
2.3. Data Analysis
- -
- SVCSF-aqu, which corresponding to the stroke volume of CSF oscillating through the aqueduct during one cardiac cycle.
- -
- SVCSF-FM, which corresponds to the stroke volume of CSF oscillating through the foramen magnum during one cardiac cycle.
- -
- SVtonsils, which corresponds to the stroke volume of the tonsils that oscillates through the foramen magnum during one cardiac cycle. This is due to the pulsatility of the tonsils.
- -
- SVCSF-PPC, which corresponds to the stroke volume of CSF oscillating through the prepontine cistern (PPC) during one cardiac cycle.
- -
- SVCSF-cerv, which corresponds to the stroke volume of CSF oscillating through the upper cervical SAS at the level of the C2C3 intervertebral disc during one cardiac cycle.
- -
- SVsyrinx, which corresponds to the stroke volume of CSF oscillating into the syrinx during one cardiac cycle.
- -
- CBFa, which represents mean cerebral arterial blood flow during one cardiac cycle.
- -
- SVblood, which represents the volume of blood oscillating into the cranial compartment during one cardiac cycle.
- -
- Venous correction factor α, which is the ratio between CBFa and mean venous flow. It corresponds to the cerebral venous drainage repartition. When the α factor is 1, there is exclusive sinus drainage. When the α factor is greater than one, it means that the accessory venous drainage pathway contributes to the sinuses to drain all cerebral blood flow. According to the example, if the α factor equals 2, that means that the sinuses participate in only 1/2 (or 50%) of total cerebral drainage.
2.4. Statistic Analysis
3. Results
3.1. Patients
3.2. Comparison of Hemoydrodynamic Parameters in the Syrinx and Non-Syrinx Groups
3.3. Hemodynamic and Hydrodynamic Interactions
4. Discussion
4.1. Comparison of Hydrodynamic Parameters in the Syrinx and Non-Syrinx Groups
4.1.1. Tonsil Pulsatility and Compliance Preservation
4.1.2. CSF Pulsatility Compensation
4.2. Hemodynamic and Hydrodynamic Interactions
4.2.1. Hemodynamic and Hydrodynamics Parameter Interactions
4.2.2. Venous Drainage and Regulation of Intracranial Volume Changes
4.2.3. Arteriovenous Delay and Venous Drainage Alteration
4.3. Limits and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aboulezz, A.O.; Sartor, K.; Geyer, C.A.; Gado, M.H. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach with MR imaging. J. Comput. Assist. Tomogr. 1985, 9, 1033–1036. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of cerebellar tonsillar position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799. [Google Scholar]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; Speer, M.C. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.; Loftus, J.R.; Oliu, C.J.; Bagci, A.M.; Lee, S.H.; Ertl-Wagner, B.; Green, B.; Sekula, R. Magnetic resonance imaging measures of posterior cranial fossa morphology and cerebrospinal fluid physiology in Chiari malformation type I. Neurosurgery 2014, 75, 515–522; discussion 522. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef]
- Frič, R.; Eide, P.K. Chiari type 1-a malformation or a syndrome? A critical review. Acta Neurochir. 2020, 162, 1513–1525. [Google Scholar] [CrossRef]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef]
- Frič, R.; Lindstrøm, E.K.; Ringstad, G.A.; Mardal, K.-A.; Eide, P.K. The association between the pulse pressure gradient at the cranio-cervical junction derived from phase-contrast magnetic resonance imaging and invasively measured pulsatile intracranial pressure in symptomatic patients with Chiari malformation type 1. Acta Neurochir. 2016, 158, 2295–2304. [Google Scholar] [CrossRef]
- Greitz, D.; Wirestam, R.; Franck, A.; Nordell, B.; Thomsen, C.; Ståhlberg, F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology 1992, 34, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.; Vikingstad, E.M.; Gomez-Anson, B.; Levin, D.N. Hemodynamically independent analysis of cerebrospinal fluid and brain motion observed with dynamic phase contrast MRI. Magn. Reson. Med. 1996, 35, 741–754. [Google Scholar] [CrossRef]
- Bateman, G.A. Vascular compliance in normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2000, 21, 1574–1585. [Google Scholar] [PubMed]
- Balédent, O.; Henry-Feugeas, M.C.; Idy-Peretti, I. Cerebrospinal fluid dynamics and relation with blood flow: A magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Investig. Radiol. 2001, 36, 368–377. [Google Scholar] [CrossRef]
- Lokossou, A.; Metanbou, S.; Gondry-Jouet, C.; Balédent, O. Extracranial versus intracranial hydro-hemodynamics during aging: A PC-MRI pilot cross-sectional study. Fluids Barriers CNS 2020, 17, 1. [Google Scholar] [CrossRef]
- Feinberg, D.A.; Mark, A.S. Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology 1987, 163, 793–799. [Google Scholar] [CrossRef]
- Greitz, D. Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta Radiol. Suppl. 1993, 386, 1–23. [Google Scholar]
- Shaffer, N.; Martin, B.; Loth, F. Cerebrospinal fluid hydrodynamics in type I Chiari malformation. Neurol. Res. 2011, 33, 247–260. [Google Scholar] [CrossRef]
- Haughton, V.M.; Korosec, F.R.; Medow, J.E.; Dolar, M.T.; Iskandar, B.J. Peak systolic and diastolic CSF velocity in the foramen magnum in adult patients with Chiari I malformations and in normal control participants. AJNR Am. J. Neuroradiol. 2003, 24, 169–176. [Google Scholar]
- Quigley, M.F.; Iskandar, B.; Quigley, M.E.; Nicosia, M.; Haughton, V. Cerebrospinal fluid flow in foramen magnum: Temporal and spatial patterns at MR imaging in volunteers and in patients with Chiari I malformation. Radiology 2004, 232, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sakas, D.E.; Korfias, S.I.; Wayte, S.C.; Beale, D.J.; Papapetrou, K.P.; Stranjalis, G.S.; Whittaker, K.W.; Whitwell, H.L. Chiari malformation: CSF flow dynamics in the craniocervical junction and syrinx. Acta Neurochir. 2005, 147, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Magnussen, J.S.; Stoodley, M.A.; Bilston, L.E. Cerebellar and hindbrain motion in Chiari malformation with and without syringomyelia. J. Neurosurg. Spine 2016, 24, 546–555. [Google Scholar] [CrossRef]
- Heiss, J.D.; Patronas, N.; DeVroom, H.L.; Shawker, T.; Ennis, R.; Kammerer, W.; Eidsath, A.; Talbot, T.; Morris, J.; Eskioglu, E.; et al. Elucidating the pathophysiology of syringomyelia. J. Neurosurg. 1999, 91, 553–562. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Migliaretti, G.; Ferraris, M.; Garnero, A.; Morana, G.; Carucci, P.; Stura, I.; Massaro, F.; Garbossa, D. Syringomyelia Associated with Chiari 1 Malformation in Adults: Positive Outcome Predictors after Posterior Fossa Decompression with Duraplasty. J. Clin. Med. 2023, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S.C.; Dawson, J.D.; Magnotta, V.; Menezes, A.H.; Dlouhy, B.J. Fourth Ventricle Enlargement in Chiari Malformation Type I. World Neurosurg. 2020, 133, e259–e266. [Google Scholar] [CrossRef] [PubMed]
- Capel, C.; Padovani, P.; Launois, P.-H.; Metanbou, S.; Balédent, O.; Peltier, J. Insights on the Hydrodynamics of Chiari Malformation. J. Clin. Med. 2022, 11, 5343. [Google Scholar] [CrossRef] [PubMed]
- Hurth, M.; Parker, F. History, controversy and pathogenesis. Neurochirurgie 1999, 45 (Suppl. S1), 138–157. [Google Scholar]
- Gardner, W.J. Hydrodynamic Mechanism of Syringomyelia: Its Relationship to Myelocele. J. Neurol. Neurosurg. Psychiatry 1965, 28, 247–259. [Google Scholar] [CrossRef]
- Williams, B. On the pathogenesis of syringomyelia: A review. J. R. Soc. Med. 1980, 73, 798–806. [Google Scholar] [CrossRef]
- Pinna, G.; Alessandrini, F.; Alfieri, A.; Rossi, M.; Bricolo, A. Cerebrospinal fluid flow dynamics study in Chiari I malformation: Implications for syrinx formation. Neurosurg. Focus. 2000, 8, E3. [Google Scholar] [CrossRef]
- Enzmann, D.R.; Pelc, N.J. Cerebrospinal fluid flow measured by phase-contrast cine MR. AJNR Am. J. Neuroradiol. 1993, 14, 1301–1307; discussion 1309–1310. [Google Scholar]
- Alperin, N.; Sivaramakrishnan, A.; Lichtor, T. Magnetic resonance imaging-based measurements of cerebrospinal fluid and blood flow as indicators of intracranial compliance in patients with Chiari malformation. J. Neurosurg. 2005, 103, 46–52. [Google Scholar] [CrossRef]
- Capel, C.; Baroncini, M.; Gondry-Jouet, C.; Bouzerar, R.; Czosnyka, M.; Czosnyka, Z.; Balédent, O. Cerebrospinal Fluid and Cerebral Blood Flows in Idiopathic Intracranial Hypertension. Acta Neurochir. Suppl. 2018, 126, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Balédent, O.; Gondry-Jouet, C.; Meyer, M.-E.; De Marco, G.; Le Gars, D.; Henry-Feugeas, M.-C.; Idy-Peretti, I. Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Investig. Radiol. 2004, 39, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A. The pathophysiology of idiopathic normal pressure hydrocephalus: Cerebral ischemia or altered venous hemodynamics? AJNR Am. J. Neuroradiol. 2008, 29, 198–203. [Google Scholar] [CrossRef]
- ElSankari, S.; Balédent, O.; van Pesch, V.; Sindic, C.; de Broqueville, Q.; Duprez, T. Concomitant analysis of arterial, venous, and CSF flows using phase-contrast MRI: A quantitative comparison between MS patients and healthy controls. J. Cereb. Blood Flow. Metab. 2013, 33, 1314–1321. [Google Scholar] [CrossRef] [PubMed]


| PCMRI | |
|---|---|
| FOV (cm2) | 14 × 14 or 16 × 16 |
| Resolution (mm2) | 0.6 × 0.6 |
| Thickness (mm2) | 2 |
| Flip angle (degree) | 30 |
| SENSE | 1.5 |
| TE (ms) | Minimum |
| TR (ms) | Minimum |
| Number of images | 32 |
| Acquisition time (s) | 50–115 |
| Number of images per cycle | 32 |
| Patients Parameters | |
|---|---|
| Sex ratio (M/F) | 2/26 |
| Syrinx ratio (s/wo.s) | 9/19 |
| Age (years) | 37 ± 12 |
| Flow Parameters | CS with a Syrinx | CS without a Syrinx | p Value |
|---|---|---|---|
| SVCSF-aqu (µL/CC) | 47 ± 35 | 39 ± 26 | 0.55 |
| SVCSF-FM (µL/CC) | 484 ± 193 | 464 ± 208 | 0.50 |
| SVtonsils (µL/CC) | 181 ± 106 | 181 ± 128 | 0.99 |
| SVCSF-PPC (µL/CC) | 365 ± 169 | 309 ± 212 | 0.55 |
| SVCSF-cerv (µL/CC) | 587 ± 167 | 584 ± 162 | 0.96 |
| SVsyrinx (µL/CC) | 47 ± 7 | / | / |
| CBF (mL/min) | 728 ± 192 | 685 ± 133 | 0.52 |
| SVblood (mL/CC) | 0.83 ± 0.19 | 0.81 ± 0.27 | 0.79 |
| α-factor | 1.46 ± 0.26 | 1.49 ± 0.17 | 0.72 |
| CS with a Syrinx R2 (p-Value) | CS without a Syrinx R2 (p-Value) | Overall CS R2 (p-Value) | |
|---|---|---|---|
| SVblood vs. SVCSF-FM | 0.32 (0.39) | 0.38 (0.10) | 0.36 (0.15) |
| SVblood vs. SVCSF-PPC | 0.48 (0.18) | 0.15 (0.51) | 0.23 (0.22) |
| SVblood vs. SVtonsils | 0.17 (0.64) | −0.22 (0.34) | −0.14 (0.47) |
| SVblood vs. SVCSF-cerv | 0.04 (0.89) | 0.67 (<0.01) | 0.50 (<0.01) |
| CS with a Syrinx | CS without a Syrinx | Overall CS | |
|---|---|---|---|
| α factor vs. CBFa | 0.46 (0.21) | 0.36 (0.13) | 0.40 (0.03) |
| α factor vs. SVCSF-FM | −0.61 (0.04) | −0.23 (0.32) | −0.37 (0.04) |
| α factor vs. SVtonsils | 0.47 (0.20) | 0.30 (0.20) | 0.34 (0.06) |
| α factor vs. SVCSF-PPC | −0.40 (0.28) | −0.31 (0.24) | −0.17 (0.37) |
| α factor vs. SVCSF-cerv | −0.80 (<0.01) | −0.05 (0.81) | −0.37 (0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capel, C.; Lantonkpode, R.; Metanbou, S.; Peltier, J.; Balédent, O. Hemodynamic and Hydrodynamic Pathophysiology in Chiari Type 1 Malformations: Towards Understanding the Genesis of Syrinx. J. Clin. Med. 2023, 12, 5954. https://doi.org/10.3390/jcm12185954
Capel C, Lantonkpode R, Metanbou S, Peltier J, Balédent O. Hemodynamic and Hydrodynamic Pathophysiology in Chiari Type 1 Malformations: Towards Understanding the Genesis of Syrinx. Journal of Clinical Medicine. 2023; 12(18):5954. https://doi.org/10.3390/jcm12185954
Chicago/Turabian StyleCapel, Cyrille, Romaric Lantonkpode, Serge Metanbou, Johann Peltier, and Olivier Balédent. 2023. "Hemodynamic and Hydrodynamic Pathophysiology in Chiari Type 1 Malformations: Towards Understanding the Genesis of Syrinx" Journal of Clinical Medicine 12, no. 18: 5954. https://doi.org/10.3390/jcm12185954
APA StyleCapel, C., Lantonkpode, R., Metanbou, S., Peltier, J., & Balédent, O. (2023). Hemodynamic and Hydrodynamic Pathophysiology in Chiari Type 1 Malformations: Towards Understanding the Genesis of Syrinx. Journal of Clinical Medicine, 12(18), 5954. https://doi.org/10.3390/jcm12185954





