1. Introduction
IgA nephropathy (IgAN), also known as Berger’s disease, is the most common glomerulonephritis worldwide, characterized by the predominance of immunoglobulin A (IgA) deposits in a renal biopsy [
1]. The prevalence rate of IgAN in Europe is 2.53/10,000, making the disease a rare one [
2].
IgAN might lead to end-stage renal disease (ESRD) in 20–30% of patients after 20 years of disease [
3]. In contrast, other studies report that 30% of patients with proteinuria 0.44–0.89 g/g and 20% of patients with proteinuria < 0.44 g/g developed renal failure within 10 years of disease onset [
4].
Risk factors for progression of IgA nephropathy include decreased glomerular filtration rate (GFR), sustained hypertension, or substantial proteinuria [
5]. Many studies found relevant connections between ESRD and baseline kidney function. They highlighted, i.e., high risk for ESRD among the patients with IgA nephropathy and impaired kidney function at the onset of the disease. Decreased GFR might occur suddenly as acute kidney injury (AKI) and be a signal of insufficiency of the compensation mechanism. It may also emerge at advanced stages of renal damage when damage processes are already irreversible [
6]. In the VALIGA study, the multivariate analysis of the 1130 study population, eGFR, MAP, and proteinuria at the time of renal biopsy were significantly associated with the rate of renal function loss [
7].
Consequently, not every factor has the same value. While an impaired GFR tends to be a sign of far-progressed kidney damage, proteinuria or hematuria can signal damage in progress. All the above-mentioned factors are closely related to the histopathological lesions found in the renal biopsy, which we rate using the Oxford MEST-C classification [
8]. All parameters assessed by this scale are also relevant risk factors.
Worldwide recommendations for the treatment of IgAN in children are not yet established.
The 2021 KDIGO recommendations mainly apply to the adult group, due to the lack of randomized controlled trials in children, which may determine management in the pediatric group [
9].
Considering the importance of impaired renal function at the onset of the disease for the prognosis of children with IgA nephropathy, this particular group may be a useful source of knowledge about the natural course of the disease and its treatment.
We aimed to examine factors that may contribute to disease progression in children that present with impaired eGFR at the beginning of IgAN.
2. Materials and Methods
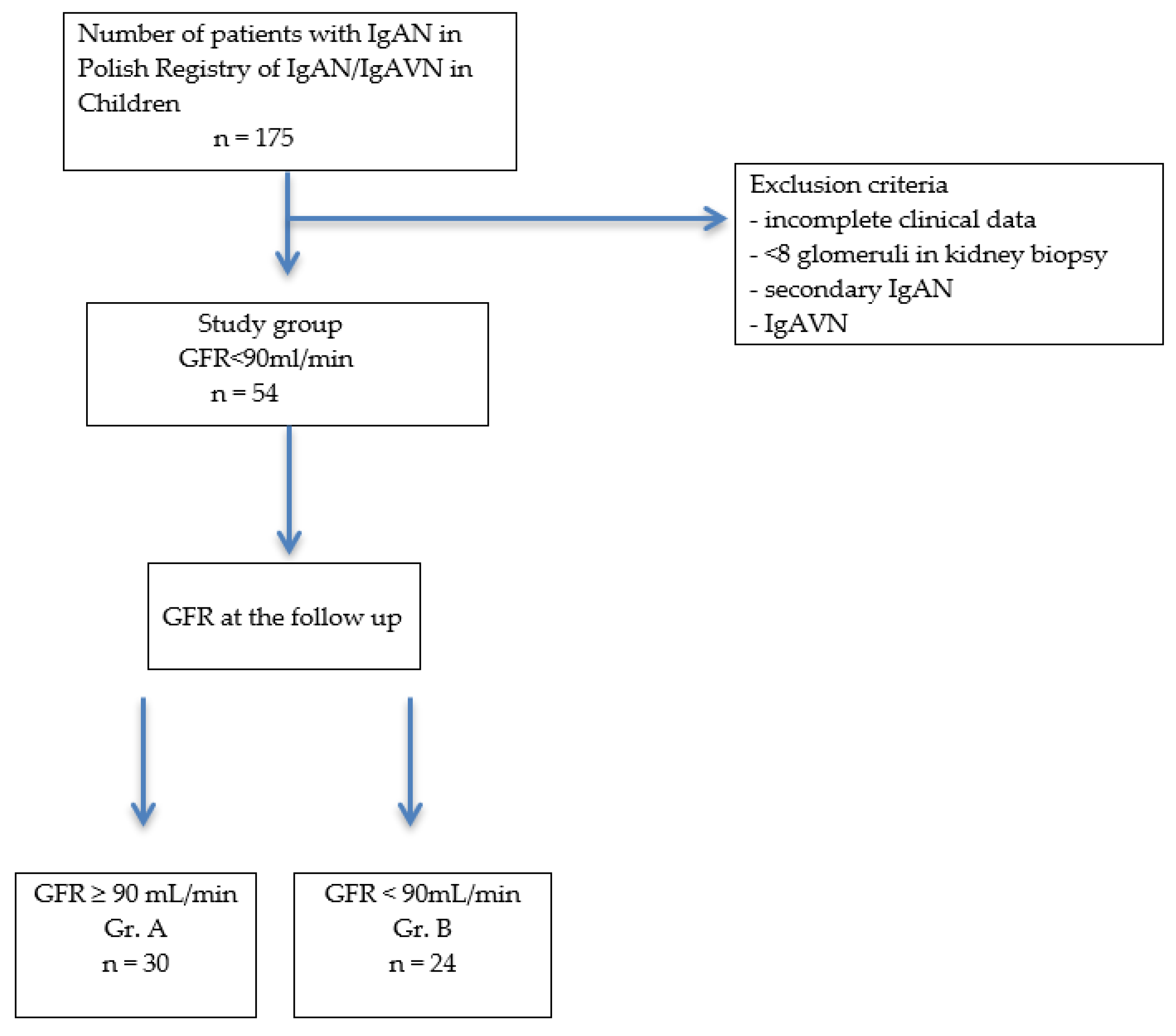
Of the 175 patients with IgAN from the Polish Pediatric Registry of IgAN and IgAVN between 2000 and 2020, 54 patients with IgAN were included in the study. They had an onset of disease with impaired renal function (GFR < 90 mL/min), which was considered as acute kidney injury (AKI). All patients had a diagnosis of IgA nephropathy based on renal biopsy with histological confirmation of the predominance of IgA deposits in the mesangium. All biopsy samples were examined by light, electron, and immunofluorescence microscopy. We analyzed the initial parameters and the results of the renal biopsy, with assessment via the Oxford classification, the treatment used, and the follow-up. Based on the GFR score obtained at the follow-up, patients were divided into 2 groups: A—GFR > 90 mL/min and B—GFR < 90 mL/min.
2.1. Clinical Features and Biochemical Parameters
Parameters assessed at the start of the disease were age, duration of follow-up, protein in 24-h urine collection, protein and erythrocytes in urinalysis, eGFR, creatinine, protein, IgA, and complement components C3 and C4. After treatment, parameters were measured again.
A level of protein in the urine ≥ 50 mg/kg/day was used as the definition of nephrotic proteinuria and non-nephrotic proteinuria was defined as <50 mg/kg/day. The concentration in the urine sample was measured using the Exton method. Hematuria was determined by more than 5 erythrocytes per high power field in the microscopic urine sediment. Hematuria was indicated by the presence of a change in urine color. The dry chemistry test (Vitro, Ortho Clinical Diagnostic) was used to measure serum creatinine concentration, expressed in mg/dL. GFR (mL/min/1.73 m2) was calculated using the Schwartz formula. The concentration of IgA and complement components C3 and C4 were measured by nephelometry at five clinical centers and by turbidimetry at three centers, but the age-dependent normal ranges did not differ significantly between each other. The reason for using two laboratory methods in the assessment of IgA and C3 and C4 concentrations was due to the retrospective nature of the study, which was conducted at several different clinical centers.
2.2. Histological Parameters
A renal biopsy was performed to establish the diagnosis. We obtained the diagnostic material from a large-needle percutaneous biopsy performed under ultrasound guidance and evaluated 3 bioptates under the light/electron microscope or in the immunofluorescence tests. We evaluated 25–50 serial slices 2–5 μm thick and performed direct immunofluorescence with fluorescein-conjugated antibodies (FITC). Retrospectively, renal biopsy was assessed using the Oxford MEST-C classification and the overall score was calculated as the sum of M, E, S, T, and C. Assessment criteria: M0 > 50%, M1 < 50%; E—endocapillary hypercellularity: 0—absent, 1—present; S—segmental sclerosis/adhesion: 0—absent, 1—present; T—tubular atrophy/interstitial fibrosis: T0 0–25%, T1 26–50%, T2 > 50%; C—crescents: C0 0%, C1 0–25%, C2 > 25%.
2.3. Treatment
Once the disease was diagnosed, patients received various treatments. These were R—renoprotection (ACEI/ARB), P—prednisone+R (renoprotection), Aza—azathioprine+P+R, Cyc—cyclophosphamide+P+R, CsA—cyclosporine+P+R, or MMF—mycophenolate mofetil+P+R. Treatment with Aza, Cyc, CsA, or MMF +P+R was analyzed as I (immunosuppressive treatment).
2.4. Follow Up
At the follow-up, blood and urine tests were repeated. Data on the disease progression were submitted anonymously to the Polish Pediatric Registry of IgAN and IgAVN.
Based on the GFR result obtained at the end, patients were divided into 2 groups: A—GFR > 90 mL/min and B—GFR < 90 mL/min.
The study was approved by the Bioethics Committee of the Medical University of Warsaw (No. KB/147/2017).
Figure 1 presents a flow diagram of the study.
3. Statistics
Statistical analysis was performed using Dell Statistica 13.0 PL software. Results were presented as mean and standard deviation (SD) for normally distributed variables and as median and range for non-normally distributed variables. The normality of the distribution was checked using the Lilliefors and Shapiro–Wolf test. The statistical significance of differences between mean values was tested using ANOVA for variables with a normal distribution and the Kruskal–Wallis test for variables with a non-normal distribution. The statistical significance of differences between the two groups was calculated using the Student’s t-test (for variables with normal distributions) and the Mann–Whitney test (for variables with non-normal distributions). Student’s t-test and Wilcoxon test (for normal and non-normal distributions, respectively) were used to test for differences between baseline and follow-up values. A value of p < 0.05 was considered statistically significant.
4. Results
Patients qualified for the study group represented 31% of the children included in the Polish Pediatric Registry of IgAN and IgAVN (35 boys, 19 girls).
The characteristics of the study group are shown in
Table 1.
The mean age of the patient at the onset of IgAN was 12.87 ± 3.57 years, proteinuria was 18 (0–967) mg/kg/d, and mean GFR was 66.12 ± 17.32 mL/min. The follow-up time in the study group was 2.16 (0.05–11) years.
In 72% of children, treatment was P+R or I+P+R (prednisone+renoprotection or immunosuppression+prednisone+renoprotection, respectively).
The prevalence of particular urinary findings is shown in
Table 2.
The most common symptom of disease onset was non-nephrotic proteinuria and erythrocyturia, which was observed in 55% of the patients. Gross hematuria was detected in 33% of the patients.
Study parameters were consecutively analyzed in Groups A (GFR > 90 mL/min at the follow-up, 30 pts: 16 boys, 14 girls) and B (GFR < 90 mL/min at the follow-up, 24 pts: 19 boys, 5 girls).
Analysis of clinical signs at the onset showed that the prevalence of nephrotic proteinuria, non-nephrotic proteinuria, hematuria, and hypertension were not significantly different between groups, as shown in
Table 2.
There were neither significant differences in any of the other biochemical parameters studied nor differences in proteinuria levels between the groups. Median proteinuria and mean creatinine levels were higher, and GFR was lower in Group A, although not significantly, as shown in
Table 3.
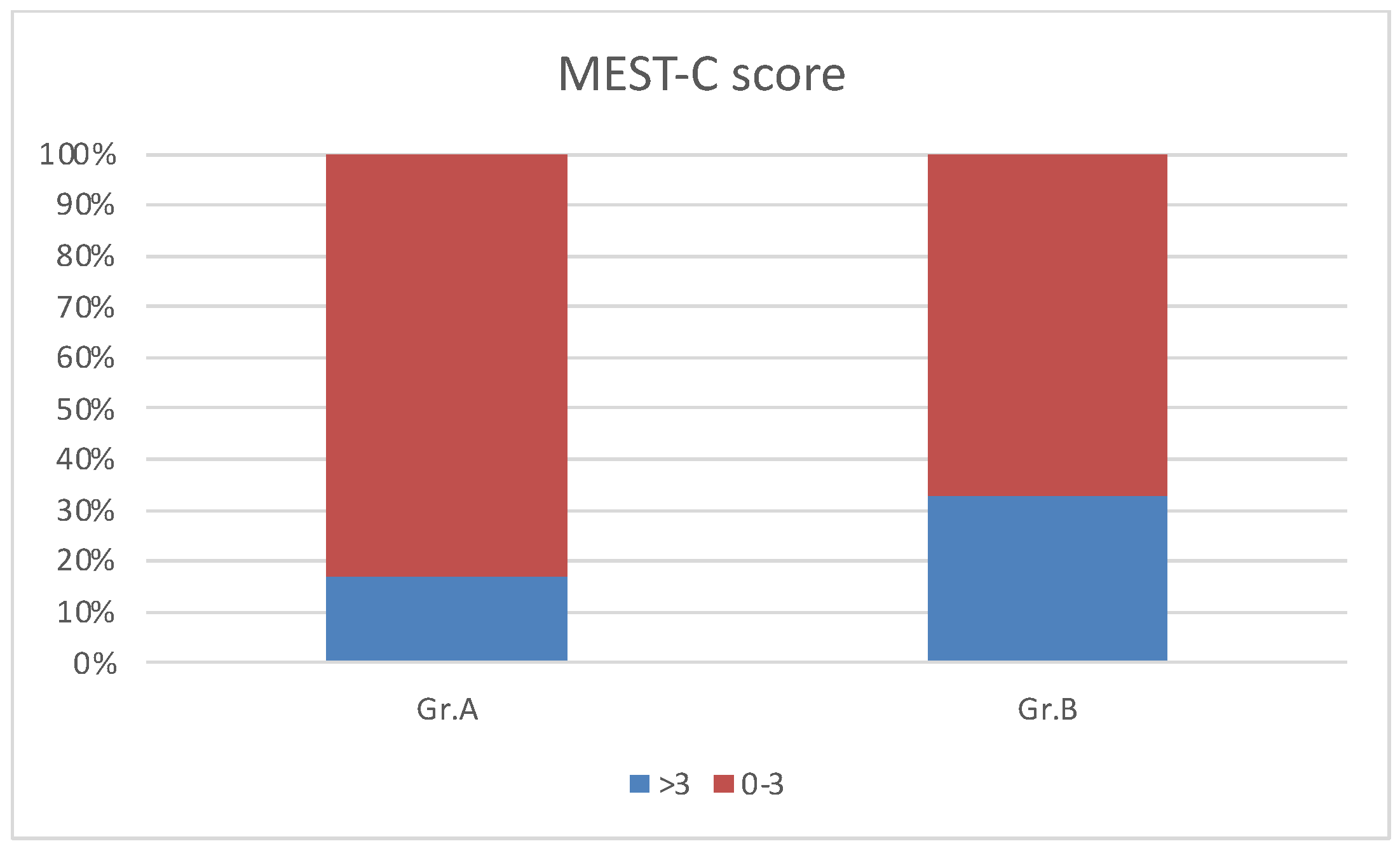
We analyzed the Oxford kidney biopsy classification findings in Groups A and B. There were no significant differences in the mean MESTCscore in Groups A and B, but a MESTCscore > 3 was present in 33% of Group B patients and 17% of children in Group A, as shown in
Figure 2.
We also reviewed the methods of treatment used in Groups A and B in the evaluation of long-time follow-up.
Patients with normal GFR at the follow-up (Group A) were significantly more likely to receive prednisone with renoprotection and/or immunosuppressive treatment with renoprotection than those in Group B (
p < 0.05), as shown in
Table 4.
5. Discussion
Our retrospective study shows the outcome of pediatric patients with IgAN whose disease started with acute kidney injury. Such a group of children with IgAN was not previously described in the medical literature. This may relate to a situation of acute onset, but also to an incidentally recognized abnormal GFR combined with urinary alterations. This is a result of the natural course of the disease, which can remain undiagnosed for several years.
According to Japanese evidence and the authors’ own research, the average age of diagnosis of the disease in pediatric population is around 11 years, although in Chinese studies diagnosis is even below 16 years of age [
10,
11,
12]. A greater age of diagnosis may be a risk factor for poor prognosis. In our previous studies, such an age was >13.9 years [
11]. In the current study, the age of diagnosis in Group B patients (GFR < 90 mL/min at follow-up) was also above 13 years.
In a Polish study group, AKI was found in 31% of children with IgAN, while in the Chinese cohort, AKI with hematuria and massive proteinuria was detected in 9.7% of 196 children with IgAN [
13], C1 was found in 80%, E1 in 60%, and T1-2 in 70% [
12], whereas in our group C1-2 was 37%, E1 39%, and T1-2 in 30% of patients.
Proteinuria at the onset of the disease was a median of 15 mg/kg/day (0–220) and was not significantly different from that in Group A of 28 mg/kg/day (0–967). At the end of follow-up, a reduction in proteinuria was obtained in both groups (with no significant statistical difference in median values), but the GFR in Group B remained abnormal after a follow up period of 2.16 (0.05–11) years.
According to the findings of Pitchner et al., even a small persistent proteinuria < 0.44 g/g may impair renal function at distant follow-up [
4]. Nevertheless, the authors of this article looked for factors differentiating these two groups of patients with an unfavorable prognosis, due to the presence of reduced GFR at the onset of the disease as an independent factor of poor prognosis.
Among the analyzed treatment methods, the use of prednisone in combination with renoprotection or immunosuppression in 72% of patients in the study group may be an indicator of disease severity.
Noteworthy is the fact that patients in Group A (with normal renal function at the follow-up) had, at the start of the disease, higher proteinuria, higher creatinine, and lower GFR, and although these differences were not statistically significant, they were marked in relatively small groups of patients.
According to Coppo et al., in children with IgAN, contrary to adults, it is not common to find slowly progressive cases of IgAN that can only be treated with renoprotective therapy for 6 months, as recommended by KDIGO. Pediatric nephrologists are aware of the possibility of progression that is not completely blocked by renin-angiotensin system blocking (RASB) drugs and require more aggressive anti-inflammatory treatment with CS/IS (corticosteroids/immunosuppressive) drugs [
3].
In the VALIGA Study in 261 children and young adults, the use of corticosteroid and/or immunosuppressive therapy (CS/IS) was confirmed in more than 50% of children. The study also showed that children < 16 years old with M0 and normal renal function at the onset of disease had a high probability of resolution of proteinuria at the end of treatment and the benefits of CS/IS therapy were statistically significance [
14].
In our work, we showed that a significant factor distinguishing the group with a good outcome from the group with a poor outcome was the use of prednisone in combination with renoprotection or immunosuppression which occurred significantly more often in Group A. This could confirm the thesis that immunosuppressive treatment/steroid therapy should be started earlier in children than in adults, especially in the group of children with impaired renal function at the beginning of the disease, with the presence of non-nephrotic proteinuria.
Similar conclusions were reached by Japanese authors who, in a randomized control trial, demonstrated the efficacy of two years of CS/IS therapy in children with IgAN combined with antiplatelet treatment [
15].
Cabier et al. confirmed positive results of corticosteroids and/or immunosuppressive treatment in an uncontrolled study group of children from France of 6 months duration. This group included patients with acute kidney injury, macroscopic hematuria, or acute nephritic syndrome [
16].
The use of CS/IS treatment in the pediatric population is still a subject of discussion, but it seems that it might be beneficial for this group.
6. Limitation
The main limitation of the study is the relatively small study group. Nevertheless, considering the rarity of the disease, obtaining data from a national registry, and inclusion of the patients with AKI only, it still represents a large group. The results need to be confirmed in a larger, international group of children. Other limitations of the study are the retrospective design and lack of a control group. A prospective group-controlled study with predefined treatment and follow-up criteria would be needed to provide stronger conclusions.
Our research does not evaluate treatment methods, but in the absence of treatment standards in children, these data were considered important in the analysis of outcome in children. It is also important to mention that only patients with renal dysfunction were included, which is not representative of all pediatric patients with IgA nephropathy. We are also aware that, in addition to the treatment we mentioned, there may have been other disturbing factors and effects on renal function.
7. Conclusions
In the Polish pediatric population with IgAN and impaired renal function at the onset of the disease, a normal GFR in the follow-up is observed in 56% of the cases.
The use of immunosuppressive/corticosteroid treatment in this group may contribute to the normalization of GFR in long-term follow-up, which requires confirmation in a larger group of pediatric patients.
Author Contributions
Conceptualization, M.M.-W. and E.P.; Data curation, M.M., D.D., A.F.-A., R.S., B.B., P.S., A.R.-S., A.W., M.S., M.D.-D., A.Ż., A.P.-M., M.T. and M.P.-T.; Investigation, M.M., B.B. and M.P.-T.; Methodology, M.M.-W., E.P., J.M. and M.P.-T.; Project administration, M.M.-W.; Resources, M.M.-W., M.M., D.D., A.W., M.S., A.Ż., A.P.-M., D.Z. and M.T.; Visualization, M.M.-W.; Writing—original draft, M.M.-W. and E.P. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Medical University of Warsaw (protocol code No. KB/147/2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Willey, C.J.; Coppo, R.; Schaefer, F.; Mizerska-Wasiak, M.; Mathur, M.; Schultz, M.J. The incidence and prevalence of IgA nephropathy in Europe. Nephrol. Dial. Transplant. 2023, gfad082. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R. Pediatric IgA Nephropathy in Europe. Kidney Dis. 2019, 5, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, D.; Braddon, F.; Hendry, B.; Mercer, A.; Osmaston, K.; Saleem, M.A.; Steenkamp, R.; Wong, K.; Turner, A.N.; Wang, K.; et al. Long-Term Outcomes in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2023, 18, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Radford, M.G., Jr.; Donadio, J.V., Jr.; Bergstralh, E.J.; Grande, J.P. Predicting renal outcome in IgA nephropathy. J. Am. Soc. Nephrol. 1997, 8, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Reich, H.N. Risk stratification of patients with IgA nephropathy. Am. J. Kidney Dis. 2012, 59, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; D’Arrigo, G.; Tripepi, G.; Russo, M.L.; Roberts, I.S.D.; Bellur, S.; Cattran, D.; Cook, T.H.; Feehally, J.; Tesar, V.; et al. Is there long-term value of pathology scoring in immunoglobulin A nephropathy? A validation study of the Oxford Classification for IgA Nephropathy (VALIGA) update. Nephrol. Dial. Transplant. 2020, 35, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Zhang, H. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.; Blanchette, E.; Kendrick, J. A lack of KDIGO guidelines for adolescents and young adults with IgA nephropathy. Pediatr. Nephrol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shibano, T.; Takagi, N.; Maekawa, K.; Mae, H.; Hattori, M.; Takeshima, Y.; Tanizawa, T. Epidemiological survey and clinical investigation of pediatric IgA nephropathy. Clin. Exp. Nephrol. 2015, 20, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Wasiak, M.; Turczyn, A.; Such, A.; Cichoń-Kawa, K.; Małdyk, J.; Miklaszewska, M.; Pietrzyk, J.; Rybi-Szumińska, A.; Wasilewska, A.; Firszt-Adamczyk, A.; et al. IgA Nephropathy in Children: A Multicenter Study in Poland. Adv. Exp. Med. Biol. 2016, 952, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, F.; Meng, H.; Zhang, L.; Jin, X. Comparison of clinicopathological features between children and adults with IgA nephropathy. Pediatr. Nephrol. 2012, 27, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.G.; Ye, X.H.; Liang, H.Y.; Yang, Q. Clinical and pathological analysis of IgA nephropathy with acute kidney injury. Zhonghua Er Ke Za Zhi Chin. J. Pediatr. 2016, 54, 610–613. [Google Scholar]
- Coppo, R.; Lofaro, D.; Camilla, R.R.; Bellur, S.; Cattran, D.; Cook, H.T.; Roberts, I.S.D.; Peruzzi, L.; Amore, A.; Emma, F.; et al. Risk factors for progression in children and young adults with IgA nephropathy: An analysis of 261 cases from the VALIGA European cohort. Pediatr. Nephrol. 2017, 32, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Ito, H.; Sakai, T.; Takekoshi, Y.; Honda, M.; Awazu, M.; Ito, K.; Iitaka, K.; Koitabashi, Y.; Yamaoka, K.; et al. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J. Am. Soc. Nephrol. 1999, 10, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Cambier, A.; Rabant, M.; Peuchmaur, M.; Hertig, A.; Deschenes, G.; Couchoud, C.; Kolko, A.; Salomon, R.; Hogan, J.; Robert, T. Immunosuppressive treatment in children with IgA nephropathy and the clinical value of podocytopathic features. Kidney Int. Rep. 2018, 3, 916–925. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).