Microcirculation and Mitochondria: The Critical Unit
Abstract
:1. Introduction
2. The Key Role of Microcirculation/Endothelium in Critical Illness
2.1. Critical Illness—Microcirculation
2.2. Critical Illness—Endothelium
3. The Important Role of Mitochondria in Critical Illness
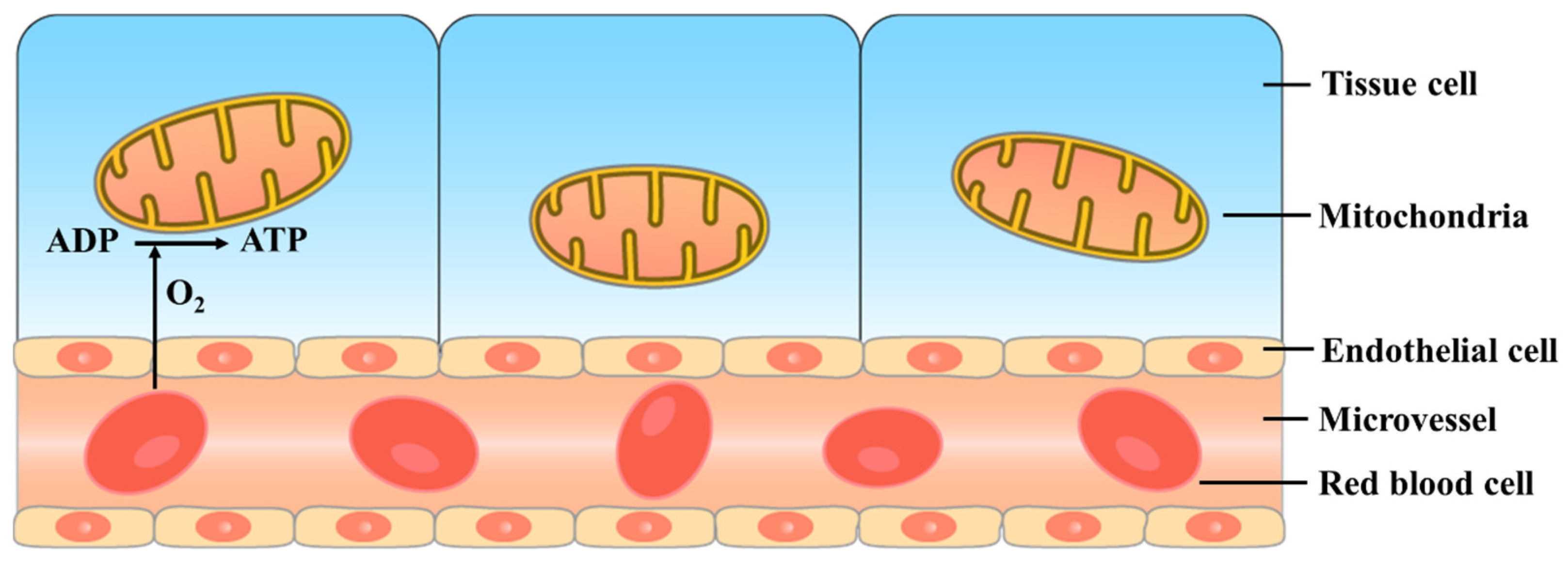
4. The Critical Unit Concept
4.1. The Microcirculation–Mitochondria Relationship
4.2. The Endothelium–Mitochondria Relationship
5. Monitoring the Critical Unit
5.1. PpIX-TSLT and COMET
5.2. FMSF and FMSF-PORH
5.3. CritiView
6. Intervention and Protection of the Critical Unit
6.1. Microcirculation-Guided Treatments
6.2. Mitochondria-Guided Treatments
6.3. Regulation of the Autonomic Nervous System (ANS)
6.4. Importance of the Awareness of Reinjury in Critical Unit-Guided Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.L. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit. Care Med. 2013, 41, 791–799. [Google Scholar] [CrossRef]
- Preau, S.; Vodovar, D.; Jung, B.; Lancel, S.; Zafrani, L.; Flatres, A.; Oualha, M.; Voiriot, G.; Jouan, Y.; Joffre, J.; et al. Energetic dysfunction in sepsis: A narrative review. Ann. Intensive Care 2021, 11, 104. [Google Scholar] [CrossRef]
- Damiani, E.; Carsetti, A.; Casarotta, E.; Domizi, R.; Scorcella, C.; Donati, A.; Adrario, E. Microcirculation-guided resuscitation in sepsis: The next frontier? Front. Med. 2023, 10, 1212321. [Google Scholar] [CrossRef]
- Ince, C.; De Backer, D.; Mayeux, P.R. Microvascular Dysfunction in the Critically Ill. Crit. Care Clin. 2020, 36, 323–331. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Creteur, J.; Preiser, J.C.; Dubois, M.J.; Vincent, J.L. Microvascular blood flow is altered in patients with sepsis. Am. J. Respir. Crit. Care Med. 2002, 166, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.S.; Enrico, C.; Laviolle, B.; Vazquez, A.R.; Ince, C.; Dubin, A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit. Care Med. 2012, 40, 1443–1448. [Google Scholar] [CrossRef]
- Ince, C.; Mik, E.G. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J. Appl. Physiol. 2016, 120, 226–235. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Machin, D.R.; Bloom, S.I.; Campbell, R.A.; Phuong, T.T.; Gates, P.E.; Lesniewski, L.A.; Rondina, M.T.; Donato, A.J. Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H531–H539. [Google Scholar] [CrossRef]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- van Iterson, M.; Bezemer, R.; Heger, M.; Siegemund, M.; Ince, C. Microcirculation follows macrocirculation in heart and gut in the acute phase of hemorrhagic shock and isovolemic autologous whole blood resuscitation in pigs. Transfusion 2012, 52, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Piotti, A.; Novelli, D.; Meessen, J.M.T.A.; Ferlicca, D.; Coppolecchia, S.; Marino, A.; Salati, G.; Savioli, M.; Grasselli, G.; Bellani, G.; et al. Endothelial damage in septic shock patients as evidenced by circulating syndecan-1, sphingosine-1-phosphate and soluble VE-cadherin: A substudy of ALBIOS. Crit. Care 2021, 25, 113. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.R.; Jiang, J.; Damiano, E.R. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ. Res. 2009, 104, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.T.; Xiong, S.; Ye, Z.; Hong, Z.; Di, A.; Tsang, K.M.; Gao, X.; An, S.; Mittal, M.; Vogel, S.M.; et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Investig. 2017, 127, 4124–4135. [Google Scholar] [CrossRef]
- Vincent, J.L.; Ince, C.; Pickkers, P. Endothelial dysfunction: A therapeutic target in bacterial sepsis? Expert. Opin. Ther. Targets 2021, 25, 733–748. [Google Scholar] [CrossRef]
- Colbert, J.F.; Schmidt, E.P. Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin. Chest Med. 2016, 37, 263–275. [Google Scholar] [CrossRef]
- Otifi, H.M.; Adiga, B.K. Endothelial Dysfunction in COVID-19 Infection. Am. J. Med. Sci. 2022, 363, 281–287. [Google Scholar] [CrossRef]
- Rongvaux, A. Innate immunity and tolerance toward mitochondria. Mitochondrion 2018, 41, 14–20. [Google Scholar] [CrossRef]
- Merz, T.; Denoix, N.; Huber-Lang, M.; Singer, M.; Radermacher, P.; McCook, O. Microcirculation vs. Mitochondria-What to Target? Front. Med. 2020, 7, 416. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; King, M.S.; Zögg, T.; Aleksandrova, A.A.; Pardon, E.; Crichton, P.G.; Steyaert, J.; Kunji, E.R.S. The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier. Cell 2019, 176, 435–447.e15. [Google Scholar] [CrossRef] [PubMed]
- Arulkumaran, N.; Pollen, S.; Greco, E.; Courtneidge, H.; Hall, A.M.; Duchen, M.R.; Tam, F.W.K.; Unwin, R.J.; Singer, M. Renal Tubular Cell Mitochondrial Dysfunction Occurs Despite Preserved Renal Oxygen Delivery in Experimental Septic Acute Kidney Injury. Crit. Care Med. 2018, 46, e318–e325. [Google Scholar] [CrossRef] [PubMed]
- Asfar, P.; Schortgen, F.; Boisramé-Helms, J.; Charpentier, J.; Guérot, E.; Megarbane, B.; Grimaldi, D.; Grelon, F.; Anguel, N.; Lasocki, S.; et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): A two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir. Med. 2017, 5, 180–190. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Hough, R.F.; Islam, M.N.; Gusarova, G.A.; Jin, G.; Das, S.; Bhattacharya, J. Endothelial mitochondria determine rapid barrier failure in chemical lung injury. JCI Insight 2019, 4, e124329. [Google Scholar] [CrossRef]
- Powers, S.K.; Hudson, M.B.; Nelson, W.B.; Talbert, E.E.; Min, K.; Szeto, H.H.; Kavazis, A.N.; Smuder, A.J. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 2011, 39, 1749–1759. [Google Scholar] [CrossRef]
- Supinski, G.S.; Schroder, E.A.; Callahan, L.A. Mitochondria and Critical Illness. Chest 2020, 157, 310–322. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Molkentin, J.D. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Huo, J.; Bround, M.J.; Boyer, J.G.; Schwanekamp, J.A.; Ghazal, N.; Maxwell, J.T.; Jang, Y.C.; Khuchua, Z.; Shi, K.; et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 2018, 3, e121689. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Deutschman, C.S.; Pinsky, M.R.; Zuckerbraun, B.; Schumacker, P.T.; Gomez, H.; Gomez, A.; Murray, P.; Kellum, J.A. Mitochondrial Function in Sepsis. Shock 2016, 45, 271–281. [Google Scholar] [CrossRef]
- Layec, G.; Haseler, L.J.; Trinity, J.D.; Hart, C.R.; Liu, X.; Le Fur, Y.; Jeong, E.-K.; Richardson, R.S. Mitochondrial function and increased convective O2 transport: Implications for the assessment of mitochondrial respiration in vivo. J. Appl. Physiol. 2013, 115, 803–811. [Google Scholar] [CrossRef]
- Zubieta-Calleja, G.R.; Zubieta-DeUrioste, N. High Altitude Pulmonary Edema, High Altitude Cerebral Edema, and Acute Mountain Sickness: An enhanced opinion from the High Andes—La Paz, Bolivia 3500 m. Rev. Environ. Health 2023, 38, 327–338. [Google Scholar] [CrossRef]
- Balestra, G.M.; Legrand, M.; Ince, C. Microcirculation and mitochondria in sepsis: Getting out of breath. Curr. Opin. Anaesthesiol. 2009, 22, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, K.; Hammarqvist, F.; Strigård, K.; Hultenby, K.; Ljungqvist, O.; Wernerman, J.; Rooyackers, O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1044–E1050. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P. Bench-to-bedside review: Cytopathic hypoxia. Crit. Care 2002, 6, 491–499. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and endothelial function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef]
- Heine, K.B.; Hood, W.R. Mitochondrial behaviour, morphology, and animal performance. Biol. Rev. Camb. Philos. Soc. 2020, 95, 730–737. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
- Khwaja, B.; Thankam, F.G.; Agrawal, D.K. Mitochondrial DAMPs and altered mitochondrial dynamics in OxLDL burden in atherosclerosis. Mol. Cell. Biochem. 2021, 476, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Hong, Z.; Wu, W.; Xiong, S.; Zhong, M.; Gao, X.; Rehman, J.; Malik, A.B. mtDNA Activates cGAS Signaling and Suppresses the YAP-Mediated Endothelial Cell Proliferation Program to Promote Inflammatory Injury. Immunity 2020, 52, 475–486.e5. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Almendros, I.; Díaz-García, E.; Toledano, V.; Casitas, R.; Galera, R.; López-Collazo, E.; Farre, R.; Gozal, D.; García-Rio, F. Differential effect of intermittent hypoxia and sleep fragmentation on PD-1/PD-L1 upregulation. Sleep 2020, 43, zsz285. [Google Scholar] [CrossRef]
- Wang, L.T.; He, P.C.; Li, A.Q.; Cao, K.X.; Yan, J.W.; Guo, S.; Jiang, L.; Yao, L.; Dai, X.Y.; Feng, D.; et al. Caffeine promotes angiogenesis through modulating endothelial mitochondrial dynamics. Acta Pharmacol. Sin. 2021, 42, 2033–2045. [Google Scholar] [CrossRef]
- Yan, Y.R.; Zhang, L.; Lin, Y.N.; Sun, X.W.; Ding, Y.J.; Li, N.; Li, H.P.; Li, S.Q.; Zhou, J.P.; Li, Q.Y. Chronic intermittent hypoxia-induced mitochondrial dysfunction mediates endothelial injury via the TXNIP/NLRP3/IL-1beta signaling pathway. Free Radic. Biol. Med. 2021, 165, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hilderink, B.N.; Crane, R.F.; Baysan, M.; Arbous, S.M.; Bogaard, B.v.D.; Mik, E.G.; Ince, C.; Pillay, J.; Juffermans, N.P. A simulation of skin mitochondrial Po(2) in circulatory shock. J. Appl. Physiol. 2023, 134, 1165–1176. [Google Scholar] [CrossRef]
- Bettink, M.A.W.; Harms, F.A.; Dollee, N.; Specht, P.A.; Raat, N.J.; Schoonderwoerd, G.C.; Mik, E.G. Non-invasive versus ex vivo measurement of mitochondrial function in an endotoxemia model in rat: Toward monitoring of mitochondrial therapy. Mitochondrion 2020, 50, 149–157. [Google Scholar] [CrossRef]
- Mik, E.G.; Balestra, G.M.; Harms, F.A. Monitoring mitochondrial PO2: The next step. Curr. Opin. Crit. Care 2020, 26, 289–295. [Google Scholar] [CrossRef]
- Harms, F.A.; Stolker, R.J.; Mik, E.G. Cutaneous Respirometry as Novel Technique to Monitor Mitochondrial Function: A Feasibility Study in Healthy Volunteers. PLoS ONE 2016, 11, e0159544. [Google Scholar]
- Baumbach, P.; Neu, C.; Derlien, S.; Bauer, M.; Nisser, M.; Buder, A.; Coldewey, S.M. A pilot study of exercise-induced changes in mitochondrial oxygen metabolism measured by a cellular oxygen metabolism monitor (PICOMET). Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 749–758. [Google Scholar] [CrossRef]
- Ubbink, R.; Bettink, M.A.W.; Janse, R.; Harms, F.A.; Johannes, T.; Münker, F.M.; Mik, E.G. A monitor for Cellular Oxygen METabolism (COMET): Monitoring tissue oxygenation at the mitochondrial level. J. Clin. Monit. Comput. 2017, 31, 1143–1150. [Google Scholar] [CrossRef]
- Katarzynska, J.; Cholewinski, T.; Sieron, L.; Marcinek, A.; Gebicki, J. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): A Tool for Characterization of Microcirculatory Status. Front. Physiol. 2020, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-Invasive Assessment of Vascular Circulation Based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Katarzynska, J.; Borkowska, A.; Czajkowski, P.; Los, A.; Szczerbinski, L.; Milewska-Kranc, A.; Marcinek, A.; Kretowski, A.; Cypryk, K.; Gebicki, J. Flow Mediated Skin Fluorescence technique reveals remarkable effect of age on microcirculation and metabolic regulation in type 1 diabetes. Microvasc. Res. 2019, 124, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mayevsky, A.; Walden, R.; Pewzner, E.; Deutsch, A.; Heldenberg, E.; Lavee, J.; Tager, S.; Kachel, E.; Raanani, E.; Preisman, S.; et al. Mitochondrial function and tissue vitality: Bench-to-bedside real-time optical monitoring system. J. Biomed. Opt. 2011, 16, 067004. [Google Scholar] [CrossRef]
- Ubbink, R.; Wefers Bettink, M.A.; van Weteringen, W.; Mik, E.G. Mitochondrial oxygen monitoring with COMET: Verification of calibration in man and comparison with vascular occlusion tests in healthy volunteers. J. Clin. Monit. Comput. 2021, 35, 1357–1366. [Google Scholar] [CrossRef]
- van Dijk, L.J.D.; Ubbink, R.; Terlouw, L.G.; van Noord, D.; Mik, E.G.; Bruno, M.J. Oxygen-dependent delayed fluorescence of protoporphyrin IX measured in the stomach and duodenum during upper gastrointestinal endoscopy. J. Biophotonics 2019, 12, e201900025. [Google Scholar] [CrossRef]
- Harms, F.A.; Ubbink, R.; de Wijs, C.J.; Ligtenberg, M.P.; Ter Horst, M.; Mik, E.G. Mitochondrial Oxygenation during Cardiopulmonary Bypass: A Pilot Study. Front. Med. 2022, 9, 785734. [Google Scholar] [CrossRef]
- Raia, L.; Zafrani, L. Endothelial Activation and Microcirculatory Disorders in Sepsis. Front. Med. 2022, 9, 907992. [Google Scholar] [CrossRef]
- Boyd, J.H.; Forbes, J.; Nakada, T.A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Tseng, C.H.; Chen, T.T.; Wu, M.Y.; Chan, M.C.; Shih, M.C.; Tu, Y.K. Resuscitation fluid types in sepsis, surgical, and trauma patients: A systematic review and sequential network meta-analyses. Crit. Care 2020, 24, 693. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, H.; Liu, D.; Wang, X. Resuscitation fluids as drugs: Targeting the endothelial glycocalyx. Chin. Med. J. 2022, 135, 137–144. [Google Scholar] [CrossRef]
- Smart, L.; Boyd, C.J.; Claus, M.A.; Bosio, E.; Hosgood, G.; Raisis, A. Large-Volume Crystalloid Fluid Is Associated with Increased Hyaluronan Shedding and Inflammation in a Canine Hemorrhagic Shock Model. Inflammation 2018, 41, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- da Luz, L.T.; Shah, P.S.; Strauss, R.; Mohammed, A.A.; D’Empaire, P.P.; Tien, H.; Nathens, A.B.; Nascimento, B. Does the evidence support the importance of high transfusion ratios of plasma and platelets to red blood cells in improving outcomes in severely injured patients: A systematic review and meta-analyses. Transfusion 2019, 59, 3337–3349. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Koyama, K. Open randomized trial of the effects of 6% hydroxyethyl starch 130/0.4/9 and 5% albumin on safety profile, volume efficacy, and glycocalyx degradation in hepatic and pancreatic surgery. J. Anesth. 2020, 34, 912–923. [Google Scholar] [CrossRef]
- Straat, M.; Müller, M.C.; Meijers, J.C.; Arbous, M.S.; Spoelstra-de Man, A.M.; Beurskens, C.J.; Vroom, M.B.; Juffermans, N.P. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: A prospective substudy of a randomized trial. Crit. Care 2015, 19, 163. [Google Scholar] [CrossRef]
- Spronk, P.E.; Ince, C.; Gardien, M.J.; Mathura, K.R.; Oudemans-van Straaten, H.M.; Zandstra, D.F. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002, 360, 1395–1396. [Google Scholar] [CrossRef]
- Boerma, E.C.; Koopmans, M.; Konijn, A.; Kaiferova, K.; Bakker, A.J.; van Roon, E.N.; Buter, H.; Bruins, N.; Egbers, P.H.; Gerritsen, R.T.; et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: A double-blind randomized placebo controlled trial. Crit. Care Med. 2010, 38, 93–100. [Google Scholar] [CrossRef]
- Chen, J.L.; Hsu, Y.C.; Huang, G.S.; Lin, C.Y.; Ke, H.Y.; Hsu, P.S.; Chung, C.H.; Tsai, C.S.; Lin, T.C. Cerebral Oximetry-Monitored Nitroglycerin Infusion and Tissue Perfusion during Rewarming of Cardiopulmonary Bypass in Cardiac Surgery: A Prospective Randomized Trial. J. Clin. Med. 2022, 11, 712. [Google Scholar] [CrossRef]
- Greenwood, J.C.; Talebi, F.M.; Jang, D.H.; Spelde, A.E.; Tonna, J.E.; Gutsche, J.T.; Horak, J.; Acker, M.A.; Kilbaugh, T.J.; Shofer, F.S.; et al. Topical nitroglycerin to detect reversible microcirculatory dysfunction in patients with circulatory shock after cardiovascular surgery: An observational study. Sci. Rep. 2022, 12, 15257. [Google Scholar] [CrossRef]
- Bomberg, H.; Bierbach, B.; Flache, S.; Novak, M.; Schafers, H.J.; Menger, M.D. Dobutamine Versus Vasopressin After Mesenteric Ischemia. J. Surg. Res. 2019, 235, 410–423. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Creteur, J.; Dubois, M.J.; Sakr, Y.; Koch, M.; Verdant, C.; Vincent, J.L. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit. Care Med. 2006, 34, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Bruhn, A.; Luengo, C.; Regueira, T.; Kattan, E.; Fuentealba, A.; Florez, J.; Castro, R.; Aquevedo, A.; Pairumani, R.; et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: A randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Ospina-Tascón, G.A.; García Marin, A.F.; Echeverri, G.J.; Bermudez, W.F.; Madriñán-Navia, H.; Valencia, J.D.; Quiñones, E.; Rodríguez, F.; Marulanda, A.; Arango-Dávila, C.A.; et al. Effects of dobutamine on intestinal microvascular blood flow heterogeneity and O(2) extraction during septic shock. J. Appl. Physiol. 2017, 122, 1406–1417. [Google Scholar] [CrossRef]
- Chommeloux, J.; Montero, S.; Franchineau, G.; Lebreton, G.; Bréchot, N.; Barhoum, P.; Lefèvre, L.; de Chambrun, M.P.; Hékimian, G.; Luyt, C.E.; et al. Venoarterial extracorporeal membrane oxygenation flow or dobutamine to improve microcirculation during ECMO for refractory cardiogenic shock. J. Crit. Care 2022, 71, 154090. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Su, F.; Velissaris, D.; Salgado, D.R.; Barros, D.d.S.; Lorent, S.; Taccone, F.S.; Vincent, J.-L.; De Backer, D. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit. Care Med. 2012, 40, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Glaspey, L.J.; Dellinger, R.P.; Durflinger, P.; Anderson, K.; Dezfulian, C.; Roberts, B.W.; Chansky, M.E.; Parrillo, J.E.; Hollenberg, S.M. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis. Crit. Care Med. 2014, 42, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Johannes, T.; Ince, C.; Klingel, K.; Unertl, K.E.; Mik, E.G. Iloprost preserves renal oxygenation and restores kidney function in endotoxemia-related acute renal failure in the rat. Crit. Care Med. 2009, 37, 1423–1432. [Google Scholar] [CrossRef]
- Dépret, F.; Sitbon, A.; Soussi, S.; De Tymowski, C.; Blet, A.; Fratani, A.; Legrand, M. Intravenous iloprost to recruit the microcirculation in septic shock patients? Intensive Care Med. 2018, 44, 121–122. [Google Scholar] [CrossRef]
- Legrand, M.; Oufella, H.A.; De Backer, D.; Leone, M.; Levy, B.; Rossignol, P.; Vicaut, E.; Depret, F.; Constantin, J.-M.; Dureanteau, J.; et al. The I-MICRO trial, Ilomedin for treatment of septic shock with persistent microperfusion defects: A double-blind, randomized controlled trial-study protocol for a randomized controlled trial. Trials 2020, 21, 601. [Google Scholar] [CrossRef]
- Murray, K.O.; Ludwig, K.R.; Darvish, S.; Coppock, M.E.; Seals, D.R.; Rossman, M.J. Chronic mitochondria antioxidant treatment in older adults alters the circulating milieu to improve endothelial cell function and mitochondrial oxidative stress. Am. J.Physiol. Heart Circ. Physiol. 2023, 325, H187–H194. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Guigas, B.; Chauvin, C.; Batandier, C.; Fontaine, E.; Wiernsperger, N.; Leverve, X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 2005, 54, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Pukenas, B.; Chawla, S.; Ehinger, J.K.; Plyler, R.; Stolow, M.; Gabello, M.; Hugerth, M.; Elmér, E.; Hansson, M.J.; et al. Neuroprotective Effects of Cyclosporine in a Porcine Pre-Clinical Trial of Focal Traumatic Brain Injury. J. Neurotrauma 2018, 36, 14–24. [Google Scholar] [CrossRef]
- Borrelli, E.; Roux-Lombard, P.; Grau, G.E.; Girardin, E.; Ricou, B.; Dayer, J.; Suter, P.M. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996, 24, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; García, J.A.; Acuña-Castroviejo, D.; Doerrier, C.; López, A.; Venegas, C.; Volt, H.; Luna-Sánchez, M.; López, L.C.; Escames, G. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: Inhibition of iNOS and preservation of nNOS. J. Pineal. Res. 2014, 56, 71–81. [Google Scholar] [CrossRef]
- Truse, R.; Nolten, I.; Schulz, J.; Herminghaus, A.; Holtmanns, T.; Gördes, L.; Raupach, A.; Bauer, I.; Picker, O.; Vollmer, C. Topical Melatonin Improves Gastric Microcirculatory Oxygenation during Hemorrhagic Shock in Dogs but Does Not Alter Barrier Integrity of Caco-2 Monolayers. Front. Med. 2020, 7, 510. [Google Scholar] [CrossRef]
- Herminghaus, A.; Buitenhuis, A.J.; Schulz, J.; Truse, R.; Vollmer, C.; Relja, B.; Bauer, I.; Picker, O. Indomethacin Increases the Efficacy of Oxygen Utilization of Colonic Mitochondria and Uncouples Hepatic Mitochondria in Tissue Homogenates From Healthy Rats. Front. Med. 2020, 7, 463. [Google Scholar] [CrossRef]
- McCully, J.D.; Cowan, D.B.; Emani, S.M.; Del Nido, P.J. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion 2017, 34, 127–134. [Google Scholar] [CrossRef]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D. Transit and integration of extracellular mitochondria in human heart cells. Sci. Rep. 2017, 7, 17450. [Google Scholar] [CrossRef]
- Carrara, M.; Ferrario, M.; Bollen Pinto, B.; Herpain, A. The autonomic nervous system in septic shock and its role as a future therapeutic target: A narrative review. Ann. Intensive Care 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Badke, C.M.; Marsillio, L.E.; Weese-Mayer, D.E.; Sanchez-Pinto, L.N. Autonomic Nervous System Dysfunction in Pediatric Sepsis. Front. Pediatr. 2018, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W. Autonomic cardiac innervation: Development and adult plasticity. Organogenesis 2013, 9, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Geloen, A.; Chapelier, K.; Cividjian, A.; Dantony, E.; Rabilloud, M.; May, C.N.; Quintin, L. Clonidine and dexmedetomidine increase the pressor response to norepinephrine in experimental sepsis: A pilot study. Crit. Care Med. 2013, 41, e431–e438. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Ertmer, C.; Westphal, M.; Rehberg, S.; Kampmeier, T.; Ligges, S.; Orecchioni, A.; D’Egidio, A.; D’Ippoliti, F.; Raffone, C.; et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial. JAMA 2013, 310, 1683–1691. [Google Scholar] [CrossRef]
- Pan, P.; Su, L.; Liu, D.; Wang, X. Microcirculation-guided protection strategy in hemodynamic therapy. Clin. Hemorheol. Microcirc. 2020, 75, 243–253. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Dries, D.J. The contemporary role of blood products and components used in trauma resuscitation. Scand. J. Trauma. Resusc. Emerg. Med. 2010, 18, 63. [Google Scholar] [CrossRef]
- Clifford, K.M.; Dy-Boarman, E.A.; Haase, K.K.; Maxvill, K.; Pass, S.E.; Alvarez, C.A. Challenges with Diagnosing and Managing Sepsis in Older Adults. Expert. Rev. Anti Infect. Ther. 2016, 14, 231–241. [Google Scholar] [CrossRef]
- Andreis, D.T.; Singer, M. Catecholamines for inflammatory shock: A Jekyll-and-Hyde conundrum. Intensive Care Med. 2016, 42, 1387–1397. [Google Scholar] [CrossRef]
- Hartmann, C.; Radermacher, P.; Wepler, M.; Nussbaum, B. Non-Hemodynamic Effects of Catecholamines. Shock 2017, 48, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef] [PubMed]
| Technical Term | Monitoring Parameter | Author (Year) Ref. |
|---|---|---|
| PpIX-TSLT | mitoPO2 | Hilderink et al. (2023) [46] Wefers Bettink et al. (2020) [47] Mik et al. (2020) [48] |
| COMET | mitoPO2 | Harms et al. (2016) [49] Baumbach et al. (2019) [50] Ubbink et al. (2017) [51] |
| FMSF-PORH | NADH fluorescence, RHR, HS, NOI | Katarzynska et al. (2020) [52] Marcinek et al. (2023) [53] Katarzynska et al. (2019) [54] |
| CritiView | NADH, microcirculatory blood flow, blood volume, hemoglobin saturation | Mayevsky et al. (2011) [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Lian, H.; Zhang, H.; Wang, X. Microcirculation and Mitochondria: The Critical Unit. J. Clin. Med. 2023, 12, 6453. https://doi.org/10.3390/jcm12206453
Wang G, Lian H, Zhang H, Wang X. Microcirculation and Mitochondria: The Critical Unit. Journal of Clinical Medicine. 2023; 12(20):6453. https://doi.org/10.3390/jcm12206453
Chicago/Turabian StyleWang, Guangjian, Hui Lian, Hongmin Zhang, and Xiaoting Wang. 2023. "Microcirculation and Mitochondria: The Critical Unit" Journal of Clinical Medicine 12, no. 20: 6453. https://doi.org/10.3390/jcm12206453
APA StyleWang, G., Lian, H., Zhang, H., & Wang, X. (2023). Microcirculation and Mitochondria: The Critical Unit. Journal of Clinical Medicine, 12(20), 6453. https://doi.org/10.3390/jcm12206453






