Renal Arteriovenous (AV) Fistula after High-Grade Blunt Renal Trauma Caused by Traffic Accidents
Abstract
1. Introduction
2. Materials and Methods
3. Results
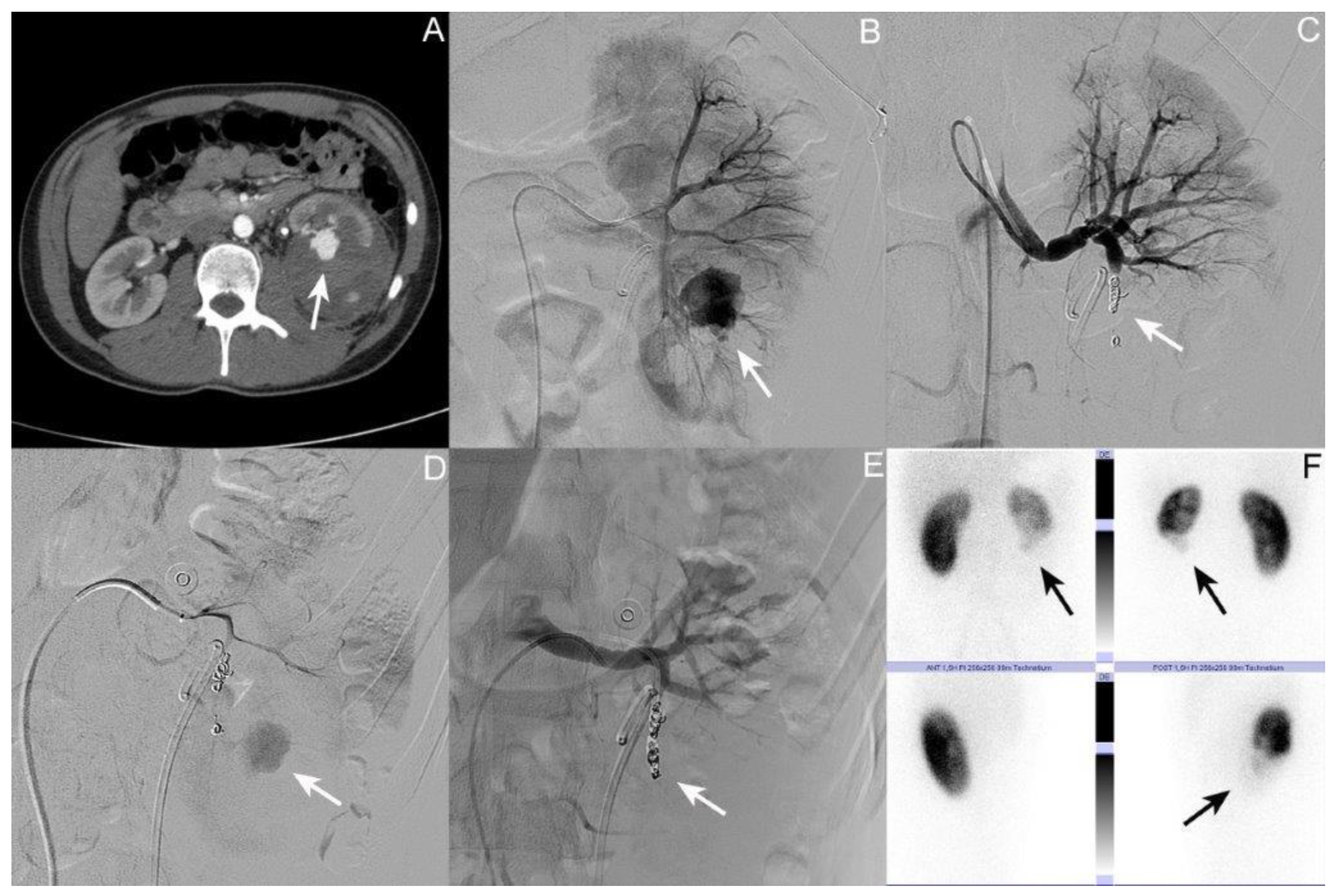
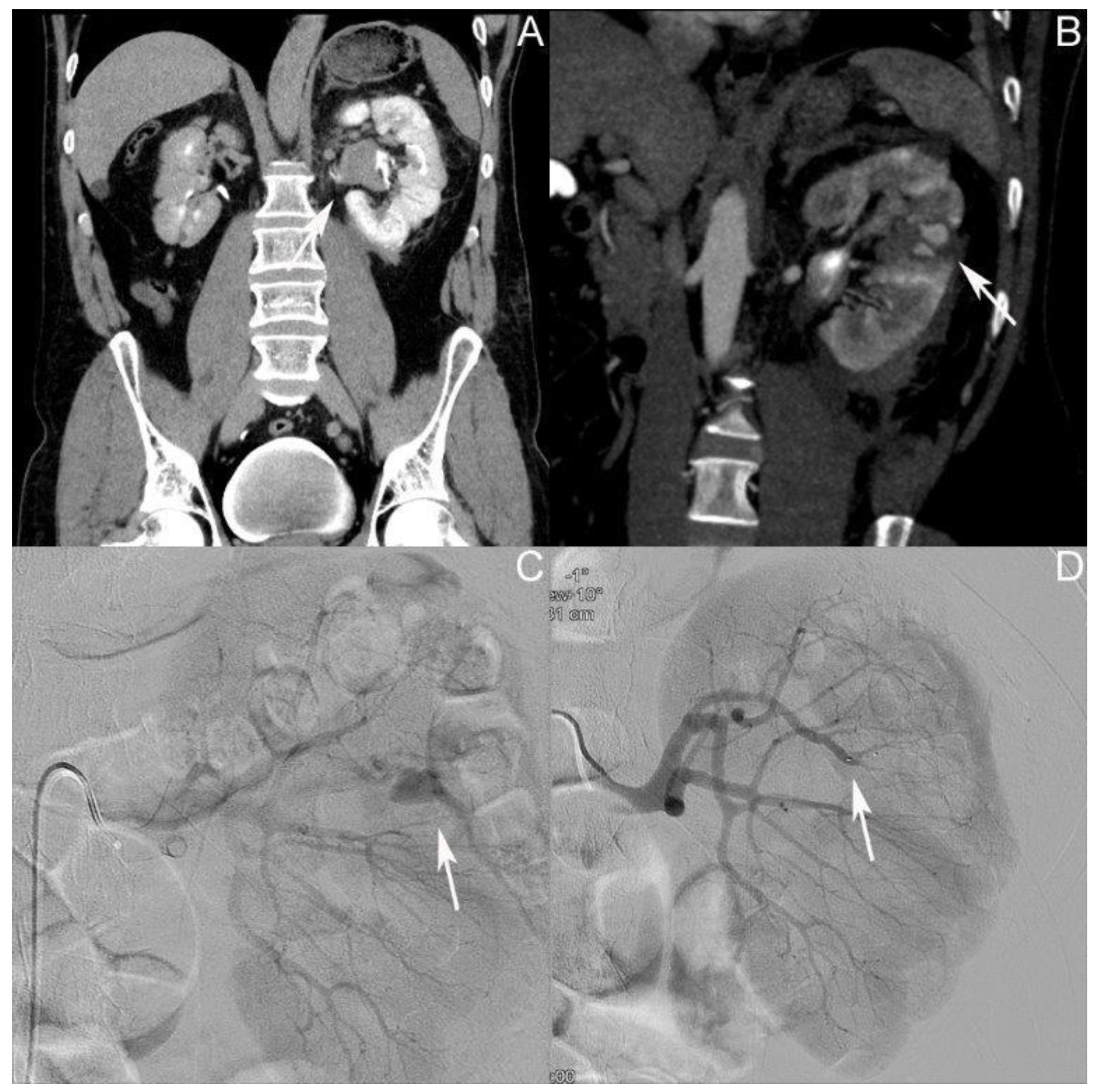
3.1. Patient I: Male Patient, Aged 17 Years
3.2. Patient II: Male Patient, Aged 44 Years
3.3. Patient III: Female Patient, Aged 56 Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heuer, M.; Taeger, G.; Kaiser, G.M.; Nast-Kolb, D.; Kuhne, C.A.; Ruchholtz, S.; Lefering, R.; Paul, A.; Lendemans, S.; Trauma Registry of The DGU. No further incidence of sepsis after splenectomy for severe trauma: A multi-institutional experience of The trauma registry of the DGU with 1,630 patients. Eur. J. Med. Res. 2010, 15, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sookraj, K. Kidney Trauma. In StatPearls; StatPearls Publishing LLC: Orlando, FL, USA, 2023. [Google Scholar]
- Ehrlich, T.; Kitrey, N.D. Renal trauma: The current best practice. Ther. Adv. Urol. 2018, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Goin, G.; Massalou, D.; Bege, T.; Contargyris, C.; Avaro, J.P.; Pauleau, G.; Balandraud, P. Feasibility of selective non-operative management for penetrating abdominal trauma in France. J. Visc. Surg. 2017, 154, 167–174. [Google Scholar] [CrossRef]
- Miller, K.S.; McAninch, J.W. Radiographic assessment of renal trauma: Our 15-year experience. J. Urol. 1995, 154, 352–355. [Google Scholar] [CrossRef]
- Voelzke, B.B.; Leddy, L. The epidemiology of renal trauma. Transl. Androl. Urol. 2014, 3, 143–149. [Google Scholar]
- Hurtuk, M.; Reed, R.L., II; Esposito, T.J.; Davis, K.A.; Luchette, F.A. Trauma surgeons practice what they preach: The NTDB story on solid organ injury management. J. Trauma 2006, 61, 243–254, discussion 254–245. [Google Scholar] [CrossRef]
- European Association of Urology. EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan 2023; EAU Guidelines Office: Arnhem, The Netherlands, 2023. [Google Scholar]
- Mingoli, A.; La Torre, M.; Migliori, E.; Cirillo, B.; Zambon, M.; Sapienza, P.; Brachini, G. Operative and nonoperative management for renal trauma: Comparison of outcomes. A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2017, 13, 1127–1138. [Google Scholar] [CrossRef]
- Starnes, M.; Demetriades, D.; Hadjizacharia, P.; Inaba, K.; Best, C.; Chan, L. Complications following renal trauma. Arch. Surg. 2010, 145, 377–381, discussion 381–372. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Stanley, J.C. Non-neoplastic congenital and acquired renal arteriovenous malformations and fistulas. Radiology 1978, 129, 333–343. [Google Scholar] [CrossRef]
- Van den Broecke, M.; Vereecke, E.; De Visschere, P. Renal Arteriovenous Fistula. J. Belg. Soc. Radiol. 2020, 104, 10. [Google Scholar] [CrossRef]
- Hyams, E.S.; Pierorazio, P.; Proteek, O.; Sukumar, S.; Wagner, A.A.; Mechaber, J.L.; Rogers, C.; Kavoussi, L.; Allaf, M. Iatrogenic vascular lesions after minimally invasive partial nephrectomy: A multi-institutional study of clinical and renal functional outcomes. Urology 2011, 78, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Albani, J.M.; Novick, A.C. Renal artery pseudoaneurysm after partial nephrectomy: Three case reports and a literature review. Urology 2003, 62, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Asero, V.; Proietti, F.; Flammia, R.S.; Franco, G.; Leonardo, C. Arteriovenous fistula after robotic partial nephrectomy: Case report and narrative review. Radiol. Case Rep. 2022, 17, 2550–2553. [Google Scholar] [CrossRef] [PubMed]
- Omae, K.; Kondo, T.; Takagi, T.; Morita, S.; Hashimoto, Y.; Kobayashi, H.; Iizuka, J.; Nozaki, T.; Yoshida, K.; Tanabe, K. Renal sinus exposure as an independent factor predicting asymptomatic unruptured pseudoaneurysm formation detected in the early postoperative period after minimally invasive partial nephrectomy. Int. J. Urol. 2015, 22, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Abdulla, A.N.; Kim, S.; Hoogenes, J.; Matsumoto, E.D. A novel endoscopic treatment for renal arteriopelvic fistula post-percutaneous nephrolithotomy (PCNL). Int. Braz. J. Urol. 2014, 40, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Manhas, A. Post PCNL Renal Pseudoaneurysm and AV Fistula: Single Institution Analysis. Arch. Urol. Complic. 2020, 6, 74. [Google Scholar]
- Arora, A.M.; Pawar, P.W.; Tamhankar, A.S.; Sawant, A.S.; Mundhe, S.T.; Patil, S.R. Predictors for severe hemorrhage requiring angioembolization post percutaneous nephrolithotomy: A single-center experience over 3 years. Urol. Ann. 2019, 11, 180–186. [Google Scholar] [CrossRef]
- Sato, O.; Tada, Y.; Sudo, K.; Ueno, A.; Nobori, M.; Idezuki, Y. Arteriovenous fistula following central venous catheterization. Arch. Surg. 1986, 121, 729–731. [Google Scholar] [CrossRef]
- Stern, A.B.; Klemmer, P.J. High-output heart failure secondary to arteriovenous fistula. Hemodial. Int. 2011, 15, 104–107. [Google Scholar] [CrossRef]
- Cohen, S.D.; Goins, J.L.; Butler, S.G.; Morris, P.P.; Browne, J.D. Dural arteriovenous fistula: Diagnosis, treatment, and outcomes. Laryngoscope 2009, 119, 293–297. [Google Scholar] [CrossRef]
- Pick, A.; Deschamps, C.; Stanson, A.W. Pulmonary arteriovenous fistula: Presentation, diagnosis, and treatment. World J. Surg. 1999, 23, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Kollmeyer, K.R.; Hunt, J.L.; Ellman, B.A.; Fry, W.J. Acute and chronic traumatic arteriovenous fistulae in civilians. Epidemiology and treatment. Arch. Surg. 1981, 116, 697–702. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Kang, T.G.; Yun, H.J.; Kim, M.J.; Jung, J.A.; Yoo, J.H.; Lee, Y.S. Hypertension caused by renal arteriovenous fistula. Korean Circ. J. 2009, 39, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Dayal, M.; Gamanagatti, S.; Kumar, A. Imaging in renal trauma. World J. Radiol. 2013, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Szmigielski, W.; Kumar, R.; Al Hilli, S.; Ismail, M. Renal trauma imaging: Diagnosis and management. A pictorial review. Pol. J. Radiol. 2013, 78, 27–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez, T.V.; Bookwalter, C.A.; Foley, T.A.; Rajiah, P.S. Multimodality imaging evaluation of arteriovenous fistulas and grafts: A clinical practice review. Cardiovasc. Diagn. Ther. 2023, 13, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Deane, C.; Cowan, N.; Giles, J.; Walters, H.; Rifkin, I.; Severn, A.; Parsons, V. Arteriovenous fistulas in renal transplants: Color Doppler ultrasound observations. Urol. Radiol. 1992, 13, 211–217. [Google Scholar] [CrossRef]
- Araújo, N.; Mendes, R. Postbiopsy Arteriovenous Fistula in Renal Transplant: Two Cases of Spontaneous Resolution. J. Diagn. Med. Sonogr. 2010, 26, 290–295. [Google Scholar] [CrossRef]
- Zullo, M.A.; Wallerson, D.C.; Lang, S. Formation and spontaneous closure of an arteriovenous fistula after transvenous pacemaker placement. Chest 1991, 100, 572–574. [Google Scholar] [CrossRef]
- Stocks, L.O.; Halpern, M.; Turner, A.F. Spontaneous closure of an arteriovenous fistula. Report of a case in hereditary hemorrhagic telangiectasia. Radiology 1969, 92, 1499–1500. [Google Scholar] [CrossRef]
- Maruno, M.; Kiyosue, H.; Tanoue, S.; Hongo, N.; Matsumoto, S.; Mori, H.; Sagara, Y.; Kashiwagi, J. Renal Arteriovenous Shunts: Clinical Features, Imaging Appearance, and Transcatheter Embolization Based on Angioarchitecture. Radiographics 2016, 36, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, M.; Song, L.; Tong, X.; Zou, Y. Endovascular treatment of renal artery aneurysms and renal arteriovenous fistulas. J. Vasc. Surg. 2013, 57, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, M.; Tong, X.; Wang, J.; Guan, H.; Niu, G.; Yan, Z.; Zhang, B.; Zou, Y. Transarterial embolization for renal angiomyolipomas: A single centre experience in 79 patients. J. Int. Med. Res. 2017, 45, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Brountzos, E.N.; Ptohis, N.; Grammenou-Pomoni, M.; Panagiotou, I.; Kelekis, D.; Gouliamos, A.; Kelekis, N. High-flow renal arteriovenous fistula treated with the Amplatzer vascular plug: Implementation of an arterial and venous approach. Cardiovasc. Intervent. Radiol. 2009, 32, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Tarmiz, A.; Jerbi, S.; Jaidane, M.; Ben Sorba, N.; Mlika, S.; Romdhani, N.; Limayem, F.; Ennabli, K. An 18-cm-large renal arteriovenous fistula treated by nephrectomy. Ann. Vasc. Surg. 2010, 24, 551.e9–551.e11. [Google Scholar] [CrossRef] [PubMed]

| Case I | Case II | Case III | Mean if Applicable | |
|---|---|---|---|---|
| Age at trauma | 17 | 44 | 56 | 39 |
| Gender | Male | Male | Female | |
| Trauma mechanism | Motorcycle accident | Bike accident | Bike accident | |
| Concomitant injuries | None | None | None | |
| AAST grade of renal trauma | IV | IV | IV | |
| Side of renal trauma | Left | Left | Right | |
| Primary treatment | Placement of US | Conservative | Placement of US | |
| Time of first diagnosis of AV fistula after the initial trauma in days | 13 | 1 | 7 | 7 |
| Size of AV fistula in mm | 24 | 12 | 20 | 18.7 |
| Time between trauma and onset of symptoms in days | 13 | 9 | NA | 11 |
| Symptoms | Flank pain, HU |
| None | |
| Number of coil embolizations performed | 2 | 1 | 2 | 1.7 |
| Time of coil embolization(s) in days after the trauma |
|
|
|
|
| Time of intensive care treatment in days | 1 | 3 | 3 | 2.3 |
| Time of inpatient treatment in days | 16 | 12 | 12 | 13.3 |
| Hb loss in g/dL | 4 | 5.4 | 1.4 | 3.6 |
| Number of erythrocyte concentrates given | 0 | 1 | 0 | 0.3 |
| Creatinine change in mg/dL | +0.3 | −0.2 | −0.27 | |
| Complications | HU, UTI | HU, need for transfusion, IP | ||
| Time of DMSA renal scintigraphy after the trauma in days | 248 | NA | 106 | 177 |
| Result of DMSA renal scintigraphy | -No TA in the lower kidney pole on the left side -Contribution: right/left kidney 65%:35% | NA | -No TA in the lower kidney pole on the right side -Contribution: left/right kidney 61%:39% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deininger, S.; Törzsök, P.; Lusuardi, L.; Deininger, S.H.M.; Freude, T.; Wichlas, F.; Deininger, C. Renal Arteriovenous (AV) Fistula after High-Grade Blunt Renal Trauma Caused by Traffic Accidents. J. Clin. Med. 2023, 12, 6362. https://doi.org/10.3390/jcm12196362
Deininger S, Törzsök P, Lusuardi L, Deininger SHM, Freude T, Wichlas F, Deininger C. Renal Arteriovenous (AV) Fistula after High-Grade Blunt Renal Trauma Caused by Traffic Accidents. Journal of Clinical Medicine. 2023; 12(19):6362. https://doi.org/10.3390/jcm12196362
Chicago/Turabian StyleDeininger, Susanne, Peter Törzsök, Lukas Lusuardi, Sebastian Hubertus Markus Deininger, Thomas Freude, Florian Wichlas, and Christian Deininger. 2023. "Renal Arteriovenous (AV) Fistula after High-Grade Blunt Renal Trauma Caused by Traffic Accidents" Journal of Clinical Medicine 12, no. 19: 6362. https://doi.org/10.3390/jcm12196362
APA StyleDeininger, S., Törzsök, P., Lusuardi, L., Deininger, S. H. M., Freude, T., Wichlas, F., & Deininger, C. (2023). Renal Arteriovenous (AV) Fistula after High-Grade Blunt Renal Trauma Caused by Traffic Accidents. Journal of Clinical Medicine, 12(19), 6362. https://doi.org/10.3390/jcm12196362






