The Burden of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome: A Case–Control Study
Abstract
1. Introduction
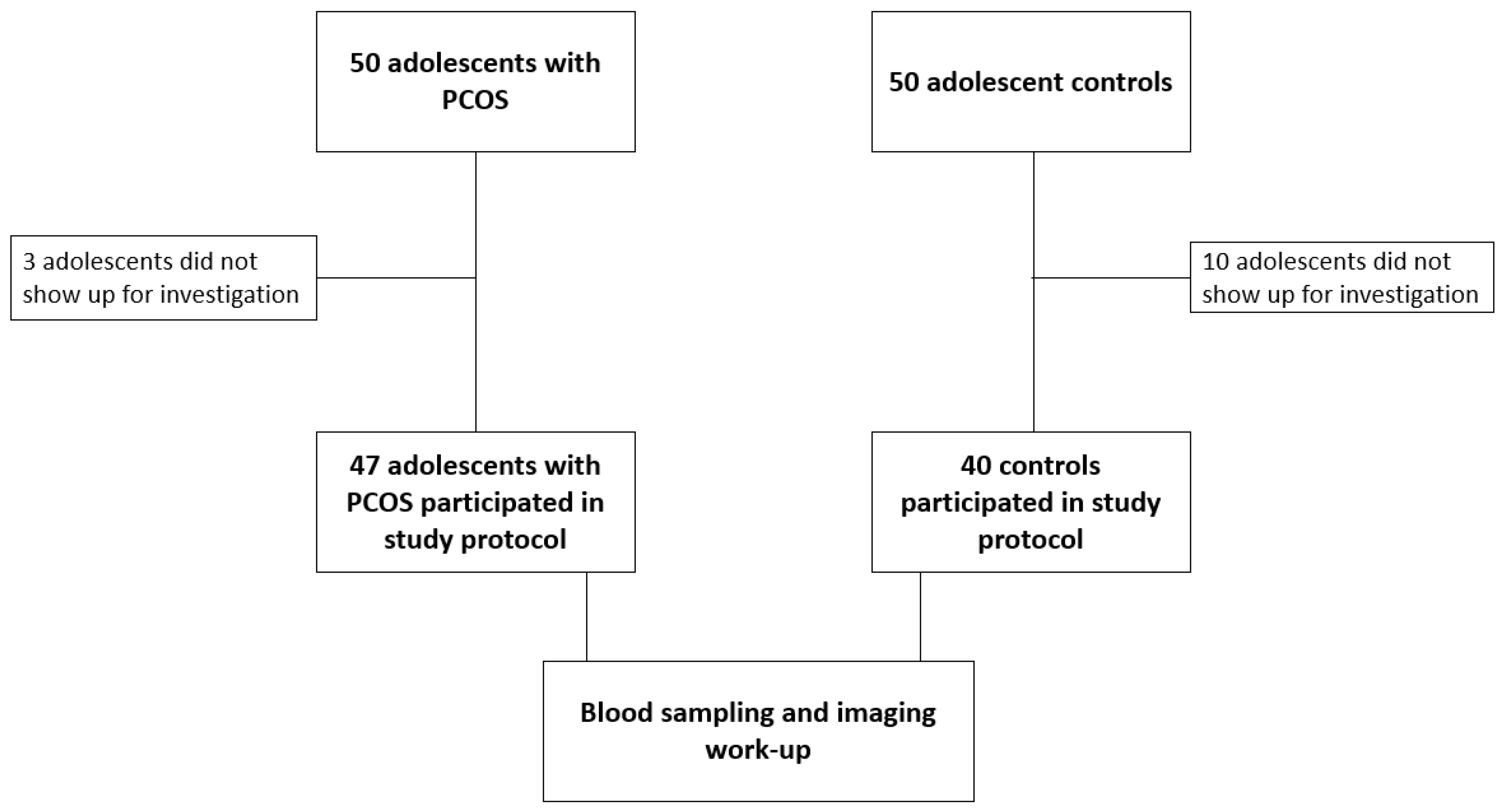
2. Materials and Methods
3. Results
3.1. PCOS Adolescents vs. Controls
3.2. PCOS vs. Controls: BMI Stratification
3.3. PCOS Adolescents: Low vs. High FAI
3.4. PCOS Adolescents: Presence vs. Absence of Hepatic Steatosis
3.5. PCOS Adolescents: Multiple Regression for NAFLD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witchel, S.F.; Oberfield, S.; Rosenfield, R.L.; Codner, E.; Bonny, A.; Ibáñez, L.; Pena, A.; Horikawa, R.; Gomez-Lobo, V.; Joel, D.; et al. The Diagnosis of Polycystic Ovary Syndrome during Adolescence. Horm. Res. Paediatr. 2015, 83, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L. The Diagnosis of Polycystic Ovary Syndrome in Adolescents. Pediatrics 2015, 136, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef]
- Zawadski, J.K.; Dunaif, A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. In Polycystic Ovary Syndrome; Blackwell Scientific: Boston, MA, USA, 1992; pp. 377–384. [Google Scholar]
- Eshre, R.; ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E. PCOS in Adolescents. Best Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 173–183. [Google Scholar] [CrossRef]
- Anagnostis, P.; Tarlatzis, B.C.; Kauffman, R.P. Polycystic Ovarian Syndrome (PCOS): Long-Term Metabolic Consequences. Metabolism 2018, 86, 33–43. [Google Scholar] [CrossRef]
- Labrecque, D.; Abbas, Z.; Frank, P.; Usa, A.; Ferenci, P.; Aamir, A.; Khan, G.; Goh, P.K.-L.; Saeed, M.; Hamid, S.; et al. World Gastroenterology Organisation Global Guidelines Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. J. Clin. Gastroenterol. 2012, 48, 467–473. [Google Scholar] [CrossRef]
- Monelli, F.; Venturelli, F.; Bonilauri, L.; Manicardi, E.; Manicardi, V.; Rossi, P.G.; Massari, M.; Ligabue, G.; Riva, N.; Schianchi, S.; et al. Systematic Review of Existing Guidelines for NAFLD Assessment. Hepatoma Res. 2021, 7, 25. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Le Garf, S.; Nègre, V.; Anty, R.; Gual, P. Metabolic Fatty Liver Disease in Children: A Growing Public Health Problem. Biomedicines 2021, 9, 1915. [Google Scholar] [CrossRef]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.T.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319. [Google Scholar] [CrossRef] [PubMed]
- Ayonrinde, O.T.; Adams, L.A.; Doherty, D.A.; Mori, T.A.; Beilin, L.J.; Oddy, W.H.; Hickey, M.; Sloboda, D.M.; Olynyk, J.K.; Hart, R. Adverse Metabolic Phenotype of Adolescent Girls with Non-Alcoholic Fatty Liver Disease plus Polycystic Ovary Syndrome Compared with Other Girls and Boys. J. Gastroenterol. Hepatol. 2016, 31, 980–987. [Google Scholar] [CrossRef]
- Carreau, A.M.; Pyle, L.; Garcia-Reyes, Y.; Rahat, H.; Vigers, T.; Jensen, T.; Scherzinger, A.; Nadeau, K.J.; Cree-Green, M. Clinical Prediction Score of Nonalcoholic Fatty Liver Disease in Adolescent Girls with Polycystic Ovary Syndrome (PCOS-HS Index). Clin. Endocrinol. 2019, 91, 544–552. [Google Scholar] [CrossRef]
- Andrisse, S.; Garcia-Reyes, Y.; Pyle, L.; Kelsey, M.M.; Nadeau, K.J.; Cree-Green, M. Racial and Ethnic Differences in Metabolic Disease in Adolescents With Obesity and Polycystic Ovary Syndrome. J. Endocr. Soc. 2021, 5, bvab008. [Google Scholar] [CrossRef] [PubMed]
- Cree-Green, M.; Bergman, B.C.; Coe, G.V.; Newnes, L.; Baumgartner, A.D.; Bacon, S.; Sherzinger, A.; Pyle, L.; Nadeau, K.J. Hepatic Steatosis Is Common in Adolescents with Obesity and PCOS and Relates to De Novo Lipogenesis but Not Insulin Resistance. Obesity 2016, 24, 2399–2406. [Google Scholar] [CrossRef]
- Barfield, E.; Liu, Y.H.; Kessler, M.; Pawelczak, M.; David, R.; Shah, B. The Prevalence of Abnormal Liver Enzymes and Metabolic Syndrome in Obese Adolescent Females with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2009, 22, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Michaliszyn, S.F.; Lee, S.; Tfayli, H.; Arslanian, S. Polycystic Ovary Syndrome and Nonalcoholic Fatty Liver in Obese Adolescents: Association with Metabolic Risk Profile. Fertil. Steril. 2013, 100, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Garoufi, A.; Pagoni, A.; Papadaki, M.; Marmarinos, A.; Karapostolakis, G.; Michala, L.; Soldatou, A. Cardiovascular Risk Factors and Subclinical Atherosclerosis in Greek Adolescents with Polycystic Ovary Syndrome: Its Relationship with Body Mass Index. Children 2021, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.E.; Brown, A.J.; Diehl, A.M.; Setji, T.L. Review of Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. World J. Gastroenterol. 2014, 20, 14172. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F.D.; Zafar, S.; Panthangi, V.; Cyril Kurupp, A.R.; Raju, A.; Luthra, G.; Khan, S. A Systematic Review of the Risk of Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. Cureus 2022, 14, 1279–1288. [Google Scholar] [CrossRef]
- Won, Y.B.; Seo, S.K.; Yun, B.H.; Cho, S.H.; Choi, Y.S.; Lee, B.S. Non-Alcoholic Fatty Liver Disease in Polycystic Ovary Syndrome Women. Sci. Rep. 2021, 11, 7085. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Goulis, D.G.; Kountouras, J.; Mintziori, G.; Chatzis, P.; Papadakis, E.; Katsikis, I.; Panidis, D. Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome: Assessment of Non-Invasive Indices Predicting Hepatic Steatosis and Fibrosis. Hormones 2014, 13, 519–531. [Google Scholar] [CrossRef]
- Vassilatou, E.; Lafoyianni, S.; Vryonidou, A.; Ioannidis, D.; Kosma, L.; Katsoulis, K.; Papavassiliou, E.; Tzavara, I. Increased Androgen Bioavailability Is Associated with Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. Hum. Reprod. 2010, 25, 212–220. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Legro, R.S.; Finegood, D.; Dunaif, A. A Fasting Glucose to Insulin Ratio Is a Useful Measure of Insulin Sensitivity in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 2694–2698. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin Sensitivity Indices Obtained from Oral Glucose Tolerance Testing: Comparison with the Euglycemic Insulin Clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- de Barros Caxiano Chissini, R.; Kuschnir, M.C.; de Oliveira, C.L.; Giannini, D.T.; Santos, B. Cutoff Values for HOMA-IR Associated with Metabolic Syndrome in the Study of Cardiovascular Risk in Adolescents (ERICA Study). Nutrition 2020, 71, 110608. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Celestre, M.; Anania, C.; Paolantonio, P.; Chiesa, C.; Laghi, A. MRI and Ultrasound for Hepatic Fat Quantification: Relationships to Clinical and Metabolic Characteristics of Pediatric Nonalcoholic Fatty Liver Disease. Acta Pædiatrica 2007, 96, 542–547. [Google Scholar] [CrossRef]
- Tokuhara, D.; Cho, Y.; Shintaku, H. Transient Elastography-Based Liver Stiffness Age-Dependently Increases in Children. PLoS ONE 2016, 11, e0166683. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A Simple Index of Lipid Overaccumulation Is a Good Marker of Liver Steatosis. BMC Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of Non-Alcoholic Fatty Liver Disease and Liver Fat Using Metabolic and Genetic Factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Tomizawa, M.; Kawanabe, Y.; Shinozaki, F.; Sato, S.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Elevated Levels of Alanine Transaminase and Triglycerides within Normal Limits Are Associated with Fatty Liver. Exp. Ther. Med. 2014, 8, 759–762. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD Fibrosis Score: A Noninvasive System That Identifies Liver Fibrosis in Patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kruger, F.C.; Daniels, C.R.; Kidd, M.; Swart, G.; Brundyn, K.; van Rensburg, C.; Kotze, M. APRI: A Simple Bedside Marker for Advanced Fibrosis That Can Avoid Liver Biopsy in Patients with NAFLD/NASH. S. Afr. Med. J. 2011, 101, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and Validation of a Simple NAFLD Clinical Scoring System for Identifying Patients without Advanced Disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Giral, P.; Charlotte, F.; Bruckert, E.; Thibault, V.; Theodorou, I.; Khalil, L.; Turpin, G.; Opolon, P.; Poynard, T. Liver Fibrosis in Overweight Patients. Gastroenterology 2000, 118, 1117–1123. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, G.K.M.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The Metabolic Syndrome in Children and Adolescents—An IDF Consensus Report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Bacopoulou, F.; Efthymiou, V.; Landis, G.; Rentoumis, A.; Chrousos, G.P. Waist Circumference, Waist-to-Hip Ratio and Waist-to-Height Ratio Reference Percentiles for Abdominal Obesity among Greek Adolescents. BMC Pediatr. 2015, 15, 50. [Google Scholar] [CrossRef]
- Brown, A.J.; Tendler, D.A.; McMurray, R.G.; Setji, T.L. Polycystic Ovary Syndrome and Severe Nonalcoholic Steatohepatitis: Beneficial Effect of Modest Weight Loss and Exercise on Liver Biopsy Findings. Endocr. Pract. 2005, 11, 319–324. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Newton, K.P.; Awai, H.I.; Choi, L.J.; Garcia, M.A.; Ellis, L.L.; Vanderwall, K.; Fontanesi, J. Paediatric Gastroenterology Evaluation of Overweight and Obese Children Referred from Primary Care for Suspected Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2013, 38, 1267. [Google Scholar] [CrossRef]
- Awai, H.I.; Newton, K.P.; Sirlin, C.B.; Behling, C.; Schwimmer, J.B. Evidence and Recommendations for Imaging Liver Fat in Children, Based upon Systematic Review. Clin. Gastroenterol. Hepatol. 2014, 12, 765. [Google Scholar] [CrossRef]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent Predictors of Liver Fibrosis in Patients with Nonalcoholic Steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Hernández, H.; Rodríguez-Morán, M.; Guerrero-Romero, F. The Alanine Aminotransferase to Triglycerides Ratio as a Marker to Identify Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Tran, T.P.; Birerdinc, A.; Younossi, Z.M. Systematic Review: Association of Polycystic Ovary Syndrome with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2011, 33, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.L.; Faria, L.C.; Guimarães, T.C.M.; Moreira, G.V.; Cândido, A.L.; Couto, C.A.; Reis, F.M. Non-Alcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2017, 40, 1279–1288. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Palmert, M.R.; Gordon, C.M.; Kartashov, A.I.; Legro, R.S.; Emans, J.J.; Dunaif, A. Screening for Abnormal Glucose Tolerance in Adolescents with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 1017–1023. [Google Scholar] [CrossRef]
- Peppa, M.; Koliaki, C.; Nikolopoulos, P.; Raptis, S.A. Skeletal Muscle Insulin Resistance in Endocrine Disease. J. Biomed. Biotechnol. 2010, 2010, 13. [Google Scholar] [CrossRef]
| Homeostatic model assessment for insulin resistance (HOMA-IR) | (HOMA-IR = fasting glucose (mg/dL) × insulin (μU/mL)/405) | |
| Quantitative insulin sensitivity check index (QUICKI) | QUICKI = 1/[log(fasting insulin μU/mL) + log(fasting glucose mg/dL)] | |
| Matsuda index (M-ISI) | M-ISI = 10,000/(glucose0 mmol/dL × insulin0 mU/L × glucose mean mmol/dL × insulin-mean mU/L)1/2 | |
| Glucose to insulin ratio (G/I ratio) | G/I ratio = fasting glucose/fasting insulin | |
| Fatty liver index (FLI) | FLI = (e0.953 × loge(TG) + 0.139 × BMI + 0.718 ∗ loge(GGT) + 0.053 × WC − 15.745)/(1 + e0.953 × loge(TG) + 0.319 × BMI + 0.718 × loge(GGT) + 0.053 × WC − 15.745) × 100 | |
| Liver fat score (LFS) | LFS = −2.89 + 1.18 × MetS (yes = 1, no = 0) + 0.45 × T2DM (yes = 2, no = 0) + 0.15 × fS-insulin (mU/L) + 0.04 × fS-AST (U/L) − 0.94 × AST/ALT | |
| Lipid accumulation product (LAP) | LAP = (WC [cm] − 58) × (TG [mmol/L]) | |
| Hepatic steatosis index (HSI) | HIS = 8 × ALT/AST ratio +BMI(+2 if T2DM, +2 if female) | |
| ALT/TG ratio | ALT/TG | |
| Tomizawa index | positive if ALT > 19 IU/L and TG > 101 mg/dL | |
| PCOS hepatic steatosis index for obese individuals (PCOS-HS) | PCOS-HS = 1/1 + [exp (−(26.01 + (−0.3761 × BMI percentile + 0.05781 × WC (cm) + 0.0448 × HOMA IR + 0.00095519 × HDLc (mmol/L) + 0.00005892 × TG (mmol/L) + 0.0964 × ALT (IU/L) + 0.001548 × free testosterone (nmol/L) − 0.06806 × SHBG (nmol/L))] | |
| Visceral adiposity index (VAI) | VAI = [WC/(36.58 + 1.88 × BMI)] × TG/0.81 × 1.52/HDLc | |
| FIB-4 index | FIB-4 = age (years) × AST(U/L)/[PLT(109/L) × ALT1/2 (U/L)] | |
| NAFLD fibrosis score (NFS) | NFS = −1.6750 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/T2DM (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × PLT (109/L) − 0.66 × albumin (g/dL) | |
| AST to platelet ratio index (APRI) | APRI = (AST/upper limit of AST × 100)/PLT (×109/L) | |
| BARD score | BARD = sum of points, BMI ≥ 28 = 1 point, AST/ALT ≥ 0.8 = 2 points, T2DM = 1 point | |
| BAAT score | BAAT = sum of points, BMI ≥ 28 = 1 point, <28 = 0, age ≥ 50 = 1 point, <50 = 0, ALT ≥ 2 × upper normal = 1 point, <2 upper normal = 0, TG ≥ 1.7 mmol/L = 1, <1.7 = 0 | |
| Variables | PCOS Group (n = 47) | Control Group (n = 40) | p-Value |
|---|---|---|---|
| Age (years) § | 15.66 (2.49) | 14.81 (2.00) | 0.082 |
| Menarche (years) | 11.96 ± 1.41 | 11.68 ± 0.91 | 0.281 |
| Physical activity (h/w) § | 1.00 (5.00) | 2.00 (4.75) | 0.797 |
| BMI (kg/m2) | 25.56 ± 5.28 | 23.14 ± 4.07 | 0.021 |
| SBP (mmHg) | 116.35 ± 10.44 | 113.00 ± 7.75 | 0.116 |
| DBP (mmHg) | 67.92 ± 10.35 | 66.30 ± 7.45 | 0.442 |
| Waist circumference (cm) | 76.36 ± 10.23 | 72.71 ± 7.90 | 0.071 |
| Hip Circumference (cm) | 102.54 ± 10.21 | 97.20 ± 8.57 | 0.011 |
| Waist to height ratio | 0.47 ± 0.07 | 0.46 ± 0.05 | 0.196 |
| Waist to hip ratio | 0.744 ± 0.058 | 0.749 ± 0.068 | 0.704 |
| AST (U/L) § | 17.50 (6.00) | 16.00 (5.00) | 0.315 |
| ALT (U/L) § | 14.00 (10.00) | 13.00 (6.00) | 0.011 |
| GGT (U/L) § | 11.90 (8.00) | 10.00 (5.00) | 0.009 |
| ALP (U/L) § | 74.00 (27.00) | 82.00 (40.00) | 0.388 |
| TC (mg/dL) § | 148.50(28.00) | 146.50 (43.15) | 0.657 |
| LDLc (mg/dL) § | 84.00 (32.50) | 82.10 (34.70) | 0.984 |
| HDLc (mg/dL) | 53.38 ± 12.54 | 59.19 ± 11.76 | 0.039 |
| TG (mg/dL) § | 66.65 (41.00) | 59.00 (32.30) | 0.699 |
| Lpa (nmol/L) § | 14.50 (40.70) | 12.10 (15.25) | 0.380 |
| ApoB (mg/dL) § | 74.60 (21.00) | 55.00 (32.00) | 0.308 |
| ApoA1 (mg/dL) | 135.12 ± 19.11 | 141.83 ± 30.29 | 0.533 |
| VitD (ng/mL) | 26.13 ± 8.66 | 26.70 ± 7.76 | 0.790 |
| Glu (mg/dL) | 87.88 ± 7.48 | 83.37 ± 5.92 | 0.004 |
| Insulin (μU/mL) § | 11.80 (7.80) | 9.60 (6.50) | 0.066 |
| Ovarian volume (cm3) | 11.45 ± 3.71 | 5.52 ± 2.22 | <0.001 |
| G/I ratio § | 7.37 (5.15) | 8.58 (6.95) | 0.090 |
| QUICKI | 0.33 ± 0.02 | 0.35 ± 0.03 | 0.006 |
| HOMA-IR § | 2.45 (1.63) | 1.88 (1.24) | 0.015 |
| LFS § | −1.56 (1.32) | −2.07 (1.21) | 0.019 |
| FLI § | 2.78 (22.82) | 0.44 (2.82) | 0.007 |
| HIS § | 35.46 (10.73) | 30.98 (6.33) | 0.004 |
| ALT/TG ratio § | 3.73 (2.49) | 3.12 (1.67) | 0.009 |
| Tomizawa index | 8.5% | 0% | 0.05 |
| VAI § | 0.92 (0.55) | 0.75 (0.65) | 0.239 |
| LAP | 16.62 ± 12.00 | 12.76 ± 8.05 | 0.078 |
| PCOS-HS | 0.48 ± 0.44 | 0.48 ± 0.46 | 0.998 |
| FIB-4 § | 0.24 (0.08) | 0.24 (0.11) | 0.178 |
| NFS | −4.53 ± 1.03 | −4.16 ± 1.31 | 0.411 |
| APRI § | 0.20 (0.09) | 0.18 (0.10) | 0.957 |
| BAAT | 2.1% | 0% | 0.353 |
| BARD | 92.5% | 97.2% | 0.357 |
| Fibroscan® stiffness (kPa) | 6.25 ± 1.55 | 6.87 ± 3.61 | 0.570 |
| Variables | Control Group BMI < 25 kg/m2 (n = 28) | Control Group BMI ≥ 25 kg/m2 (n = 12) | p-Value | PCOS Group BMI < 25 kg/m2 (n = 24) | PCOS Group BMI ≥ 25 kg/m2 (n = 23) | p-Value |
|---|---|---|---|---|---|---|
| SBP (mmHg) | 111.20 ± 6.72 | 116.75 ± 8.66 | 0.067 | 111.28 ± 8.64 | 119.48 ± 9.09 | 0.007 |
| WC (cm) | 67.41 ± 8.19 | 69.04 ± 7.17 | <0.001 | 68.61 ± 4.43 | 83.22 ± 8.89 | <0.001 |
| HC (cm) § | 94.35 ± 6.55 | 103.83 ± 9.30 | <0.001 | 96.00 (7.00) | 109.00 (11.00) | <0.001 |
| WHtR | 0.43 ± 0.04 | 0.51 ± 0.02 | <0.001 | 0.42 ± 0.03 | 0.52 ± 0.05 | <0.001 |
| GGT (U/L) § | 9 (5) | 10 (6.5) | 0.602 | 10.00 (4.50) | 15.00 (9.00) | 0.014 |
| FAI § | 1.89 (2.13) | 2.4 (1.3) | 0.384 | 2.76 (2.14) | 4.92 (4.68) | 0.009 |
| QUICKI | 0.35 ± 0.02 | 0.33 ± 0.02 | 0.002 | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.012 |
| HOMA-IR § | 1.58 (0.94) | 2.56 (2.09) | 0.006 | 2.01 (1.04) | 2.98 (2.48) | 0.012 |
| G/I ratio | 11.67 ± 5.66 | 6.94 ± 2.42 | 0.002 | 9.69 ± 3.41 | 6.56 ± 2.52 | 0.002 |
| ALT (U/L) § | 13 (6) | 11 (5.25) | 0.439 | 13.00 (7.00) | 20.00 (11.00) | 0.040 |
| LFS § | −2.38 (1.32) | −1.56 (1.20) | 0.011 | −1.97 (1.03) | −1.20 (1.67) | 0.007 |
| FLI § | 0.36 (0.37) | 4.76 (3.62) | <0.001 | 0.37 (0.63) | 19.71 (22.92) | <0.001 |
| LAP | 8.98 ± 5.43 | 21.57 ± 2.42 | <0.001 | 8.07 ± 4.38 | 24.55 ± 11.41 | <0.001 |
| HSI § | 29.11 (4.47) | 35.86 (3.62) | <0.001 | 29.24 (2.33) | 40.10 (6.40) | <0.001 |
| HSI ≤ 36 (n = 26) | HSI > 36 (n = 18) | p-Value | |
|---|---|---|---|
| BMI (kg/m2) | 22.33 ± 3.09 | 30.69 ± 3.98 | <0.001 |
| Waist circumference (cm) | 70.80 ± 5.53 | 85.31 ± 9.22 | <0.001 |
| Hip circumference (cm) | 96.82 ± 7.06 | 111.03 ± 8.86 | <0.001 |
| Waist to height ratio | 0.44 ± 0.04 | 0.53 ± 0.06 | <0.001 |
| Waist to hip ratio | 0.73 ± 0.45 | 0.77 ± 0.06 | 0.030 |
| SBP (mmHg) | 113.57 ± 8.93 | 121.06 ± 11.05 | 0.025 |
| FAI § | 3.05 (3.48) | 5.31 (5.69) | 0.034 |
| Insulin (μU/mL) § | 9.40 (3.75) | 15.00 (6.40) | 0.001 |
| QUICKI | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.002 |
| HOMA-IR § | 2.02 (0.89) | 3.16 (2.03) | 0.002 |
| G/I ratio | 9.34 ± 3.33 | 5.88 ± 2.42 | 0.001 |
| Total cholesterol (mg/dL) | 143.12 ± 18.44 | 159.27 ± 29.49 | 0.031 |
| LDLc (mg/dL) | 77.56 ± 20.21 | 94.07 ± 29.08 | 0.031 |
| Triglycerides § (mg/dL) | 51.50 (30.90) | 75.00 (31.00) | 0.019 |
| Fibroscan® stiffness (kPa) | 5.30 ± 1.30 | 7.37 ± 0.99 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannouli, A.; Efthymiou, V.; Konidari, M.; Mani, I.; Aravantinos, L.; Dourakis, S.P.; Antoniou, A.; Deligeoroglou, E.; Bacopoulou, F. The Burden of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome: A Case–Control Study. J. Clin. Med. 2023, 12, 557. https://doi.org/10.3390/jcm12020557
Giannouli A, Efthymiou V, Konidari M, Mani I, Aravantinos L, Dourakis SP, Antoniou A, Deligeoroglou E, Bacopoulou F. The Burden of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome: A Case–Control Study. Journal of Clinical Medicine. 2023; 12(2):557. https://doi.org/10.3390/jcm12020557
Chicago/Turabian StyleGiannouli, Aikaterini, Vasiliki Efthymiou, Marianna Konidari, Iliana Mani, Leon Aravantinos, Spyridon P. Dourakis, Aristeidis Antoniou, Efthymios Deligeoroglou, and Flora Bacopoulou. 2023. "The Burden of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome: A Case–Control Study" Journal of Clinical Medicine 12, no. 2: 557. https://doi.org/10.3390/jcm12020557
APA StyleGiannouli, A., Efthymiou, V., Konidari, M., Mani, I., Aravantinos, L., Dourakis, S. P., Antoniou, A., Deligeoroglou, E., & Bacopoulou, F. (2023). The Burden of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome: A Case–Control Study. Journal of Clinical Medicine, 12(2), 557. https://doi.org/10.3390/jcm12020557







