Putative Bidirectionality of Chronic Obstructive Pulmonary Disease and Periodontal Disease: A Review of the Literature
Abstract
1. Introduction
2. The Relationship between Periodontal Disease and COPD
2.1. The Association between Periodontal Disease and the Development of COPD
2.2. Does Periodontal Disease Promote a Decline in Pulmonary Function?
| Author (Year) | Location | Study Design | Study Population | n | Measured Outcome | Main Findings |
|---|---|---|---|---|---|---|
| Pérez Barrionuevo et al., 2018 [26] | Norway | Cross-sectional | Norwegian participants in the Respiratory Health in northern Europe, Spain, and Australia (RHINESSA) generation study and the third wave of the European Community Respiratory Health Survey (ECRHS) study | 656 | Periodontal parameter: CPI Pulmonary function measurement: spirometry | Participants with CPI 3–4 (worse periodontal health) had significantly lower FEV1/FVC ratios compared to participants with CPI 0 (healthy periodontal status): regression coefficients β (95% CI) = −0.032 (−0.055, −0.009), p-trend = 0.004. |
| Holtfreter et al., 2013 [27] | Germany | Cross-sectional | Participants in the Study of Health in Pomerania | 1463 | Periodontal parameters: CAL, PD, and number of missing teeth Pulmonary function measurements: spirometry, body plethysmography, and diffusing capacity of the lung for carbon monoxide | Mean CAL was significantly associated with reduced FVC (p < 0.05), FEV1 (p < 0.001), functional residual capacity (p < 0.001), FEV1/FVC ratio (p < 0.01), maximal expiratory flow at 25% of FVC (p < 0.05), and residual volume/total lung capacity ratio (p < 0.001). |
| Winning et al., 2020 [28] | Sweden | Cross-sectional | Individuals selected from the Swedish civil registration database representing the aging population in Karlskrona, Sweden | 826 | Periodontal parameter: periodontal bone loss Pulmonary function measurement: spirometry | The percentage of participants in the airflow limitation (FEV1/FVC < 0.7) group who presented with periodontitis was 65.1%, compared to 41.5% in the group with normal pulmonary function (p < 0.001). Periodontitis was independently associated with airflow limitation (OR = 2.31). |
| Katancik et al., 2005 [29] | USA | Cross-sectional | Community-dwelling, well-functioning older adults selected from participants enrolled in the Health, Aging, and Body Composition Study (Health ABC) | 860 | Periodontal parameters: PI, GI, PD, and LOA Pulmonary function measurement: spirometry | Participants with airflow limitation (defined as a reduced FEV1/FVC as determined by age-, gender-, and race-normalized values) had significantly worse GI (p = 0.022) and LOA (p = 0.009) than those with normal pulmonary function. |
| Moeintaghavi et al., 2018 [31] | Iran | Cross-sectional | Patients with COPD who had been referred to the specialty clinic | 50 | Periodontal parameters: PD, LOA, GI, and PI Pulmonary function measurements: spirometry and SpO2 | The FEV1 and FVC indices showed significant negative correlations with PI (FEV1: r = −0.481, p < 0.001; FVC: r = −0.296, p = 0.037) and LOA (FEV1: r = −0.370, p = 0.008; FVC: r = −0.370, p = 0.008). The SpO2 index showed a significant negative correlation with GI (r = −0.339, p = 0.016) and attachment loss (r = −0.319, p = 0.024) variables. |
| Winning et al., 2019 [35] | Northern Ireland | Cross-sectional | Participants in the Prospective Epidemiological Study of Myocardial Infarction (PRIME), which is a longitudinal cohort study of cardiovascular disease in Northern Ireland | 1380 (male only) | Periodontal parameters: CAL, PD, and number of teeth Pulmonary function measurement: spirometry | A 2-fold increase in mean CAL corresponded to a predicted FEV1 value of −3.33% (95% CI = −4.80–1.86), with a p < 0.001. |
| Lee et al., 2020 [37] | USA | Cross-sectional | Participants in the third National Health and Nutrition Examination Survey (NHANES III; 1988–1994), | 10,645 | Periodontal parameters: PD and LOA Pulmonary function measurement: spirometry | There was a significant inverse correlation between pulmonary function (predicted FEV1%, predicted FVC%, and FEV1/FVC) and the severity of periodontitis (p < 0.001). |
| Chen et al., 2022 [38] | USA | Cross-sectional | Participants in the National Health and Nutrition Examination Survey (NHANES 2009–2012) | 6313 | Periodontal parameters: PD and LOA Pulmonary function measurement: spirometry | The ORs for airflow obstruction (FEV1/FVC < 0.70) in moderate and severe periodontitis were 1.38 (95% CI = 1.01–1.75) and 1.47 (95% CI = 1.06–2.01). |
| Lee et al., 2019 [40] | Korea | Cross-sectional | Participants in the sixth Korea National Health and Nutrition Examination Survey (KNHANES; 2014) | 4004 | Periodontal parameter: CPI Pulmonary function measurement: spirometry | No statistically significant association was found (adjusted OR = 1.140, 95% CI 0.849–1.530) between periodontitis and obstructive pulmonary function impairment (FEV1/FVC < 0.7). |
| Henke et al., 2016 [41] | Cross-sectional | Patients consulting a general dental practice | 206 | Periodontal parameter: periodontal screening index Pulmonary function measurement: spirometry | After adjustment for covariates, periodontitis was not significantly associated with spirometric measurements (FEV1, FVC, FEV1/FVC, and peak expiratory flow). | |
| Hämäläinen et al., 2004 [39] | Finland | Cross-sectional and prospective cohort | Participants in the population-based prospective epidemiological cohort study on health and functional capacity (the Evergreen project) | 203 | Periodontal parameters: BOP, calculus and deepened periodontal pockets Pulmonary function measurement: spirometry (standing position) | At baseline, edentulous male participants had the lowest FEV1. After five years of follow-up, the decline in FEV1 was greatest in participants with periodontitis or edentulism (−9.4%), whereas no decline was observed in those with healthy periodontal tissue (+1.0%, p < 0.006). |
| Si et al., 2012 [30] | China | Case-control | Patients being treated in the respiratory and dental departments of eight hospitals in Beijing | 1019 (case, n = 581; control, n = 438) | Periodontal parameters: PD, LOA, BI, PI, and ABL Pulmonary function measurements: spirometry and 6-minute walk test | PD, LOA, PI, ABL, and the number of teeth were significantly associated with all stages of COPD (all p < 0.001). Patients with higher BODE scores had significantly higher LOA (p < 0.001), BI (p = 0.027), PI (p < 0.001), ABL (p < 0.001), and fewer number of teeth (p < 0.001). |
| Peter et al., 2013 [32] | India | Case-control | The case group included well-functioning and ambulatory patients having COPD. The control group consisted of systemically healthy individuals enrolled from the outpatient clinic of the periodontics department. | 501 (case, n = 102; control, n = 399) | Periodontal parameters: OHI, PI, GI, PD, and CAL Pulmonary function measurement: spirometry | Patients with COPD had significantly higher CAL, PD, and OHI than healthy individuals (p < 0.0001). A significant negative relationship was found between three periodontal indices (CAL, PD, and GI) and FEV1 (p < 0.0001). |
| Tan et al., 2019 [34] | China | Case-control | Participants in a hospital-based study of consecutive cases of COPD at 4 hospitals in Shenyang | 160 (case, n = 80; control, n = 80) | Periodontal parameters: OHI-S, SBI, PD, and CAL Pulmonary function measurement: spirometry | Significant negative correlations of OHI-S (r = −0.748, p < 0.01) and CAL (r = −0.571, p < 0.01) with FEV1 were observed in the COPD group. Significant negative correlations of OHI-S (r = −0.422, p < 0.01), SBI (r = −0.239, p = 0.03), and CAL (r = −0.465, p < 0.01) with FEV1 were also noted in the control group. |
| Javaheri et al., 2020 [36] | Iran | Case-control | Participants selected from patients with stable COPD with a history of smoking (case) and no pulmonary symptoms with normal spirometry (control) in the same hospital, matched for age and number of teeth | 71 (male only: case, n = 35; control, n = 36) | Periodontal parameters: PD, BOP, and LOA Pulmonary function measurement: spirometry | The PD, BOP, and LOA were negatively correlated with the predicted FEV1% (r = −0.53, p = 0.001), (r = −0.62, p = 0.001), and (r = −0.72, p = 0.001) as well as FEV1/FVC ratio (r = −0.45, p = 0.007), (r = −0.47, p = 0.004) and (r = −0.61, p = 0.001), respectively. |
| Hayes et al., 1998 [33] | USA | Nested case-control | Participants in the Veterans Affairs dental longitudinal study and normative aging study | 1118 (male only) | Periodontal parameter: radiographic ABL Pulmonary function measurement: spirometry (those whose FEV1 was less than 65% of the predicted value were defined as having COPD) | Whole-mouth bone loss was a risk factor for developing COPD (RR = 1.6, 95%CI = 1.2–2.0). |
| Takeuchi et al., 2019 [42] | Japan | Prospective cohort | Community-dwelling adults without COPD (participants from the Hisayama study) | 900 | Periodontal parameters: PD, CAL, and the number of teeth Pulmonary function measurement: spirometry | The risk of developing COPD was positively correlated with the severity of periodontitis (p for trend = 0.043) after adjusting for smoking intensity and other covariates. The adjusted RR for developing COPD was significantly higher in patients with severe periodontitis than in those with mild periodontitis (RR = 3.51, 95% CI = 1.15–10.74). |
2.3. The Influence of Periodontal Disease on QOL Deterioration
2.4. Periodontal Disease and COPD Exacerbations
| Author (Year) | Location | Study Design | Study Population | n | Measured Outcome | Main Findings |
|---|---|---|---|---|---|---|
| Shen et al., 2009 [50] | Taiwan | Retrospective cohort study (1:1 propensity score matching) | The National Health Insurance claims data from the National Health Research Institutes of Taiwan | Patients with COPD receiving periodontal treatment (n = 5562) vs. COPD patients without periodontal disease (n = 5562) The periodontal treatment included a basic form of subgingival curettage (scaling, root planing) and an invasive form of periodontal flap surgery. | Definition of adverse respiratory event: emergency room visit or hospitalization due to exacerbation of COPD, pneumonia, and acute respiratory failure |
|
| Liu et al., 2012 [51] | China | Cross-sectional | Ambulatory patients with COPD, treated at eight hospitals in Beijing (frequent exacerbator vs. infrequent exacerbator) | 392 (frequent exacerbator, n = 183; infrequent exacerbator, n = 209) | Periodontal parameters: PD, CAL, BOP and PI Definition of COPD exacerbation: the presence of two or more of the following symptoms and a change in medication; increased dyspnoea, cough, sputum volume, or sputum purulence compared with their baseline status Definition of frequent exacerbator: those who experienced two or more exacerbations in the last 12 months | Fewer remaining teeth (p = 0.02), higher PI scores (p = 0.02), and less frequent tooth brushing (p = 0.008) were statistically significantly associated with COPD exacerbations. When stratified by smoking, higher PI scores (OR = 3.43, 95% CI = 1.19–9.94) were significantly associated with COPD exacerbations in never-smokers. |
| Baldomero et al., 2019 [44] | USA | Case-control (exacerbators vs. non-exacerbators) | Individuals from the Minneapolis Veterans Affairs health care system | 136 (patients with COPD: exacerbator, n = 70; non-exacerbator, n = 66) | Periodontal parameters: OHIP-5; PD, CAL, BOP, GI, PI, and caries risk assessment (subset of patients) Definition of exacerbator: at least one COPD exacerbation in the previous 12 months Definition of COPD exacerbation: taking antibiotics and/or oral corticosteroids for respiratory symptoms or hospitalization for respiratory symptoms or emergency room visit for respiratory illness | Unadjusted odds ratios for severe exacerbations to mild exacerbations tended to be higher for those with worse measures of periodontitis severity, PD, CAL, BOP, PI, and GI, and caries risk assessment, but the difference was not statistically significant. Due to the small sample size, adjustment for covariates was not performed. |
| Barros et al., 2013 [52] | USA | Prospective cohort | Participants in the Dental Atherosclerosis Risk in Communities study | 1635 (patients with COPD: individuals with COPD-related events at 5-year follow-up, n = 399; individuals without events at 5-year follow-up, n = 1236) | Periodontal parameters: PD, CAL, and the number of teeth Definition of COPD-related event: hospitalization due to COPD exacerbation or COPD-related death | There was a statistically significant association between oral health status and COPD-related events (p < 0.0001). The event rates showed a gradient associated with worse oral health status, ranging from 10.5% in those with teeth and healthy periodontium to 23.8% in those with severe periodontal disease, with the highest event rate in the edentulous (43.9%). |
2.5. Impact of Therapeutic Interventions for Periodontal Disease on the Health Outcome of COPD
| Author (Year) | Location | Study Design | Study Population | n | Periodontal Intervention | Measured Outcome of COPD | Main Findings |
|---|---|---|---|---|---|---|---|
| Madalli et al., 2016 [57] | India | Prospective cohort | Patients diagnosed with COPD and chronic periodontitis | 30 | Supragingival scaling and oral hygiene instructions to all patients | Spirometric data (FEV1 and FVC) |
|
| Kucukcoskun et al., 2013 [53] | Turkey | Prospective case–control | Patients with COPD attending the outpatient clinics of the three chest clinics with a history of at least one infectious exacerbation in the past year and with moderate to severe chronic periodontitis Treatment group: patients who were able to visit the authors’ department regularly for treatment and subsequent follow-up Control group: patients who came from hospitals distant from the authors’ periodontology department and had transportation problems | 40 | Oral hygiene instructions, full-mouth scaling, and root planing using hand instruments and ultrasonic devices under local anaesthesia, n = 20; no periodontal treatment, n = 20 | Rate of exacerbation (sustained worsening of baseline respiratory symptoms for ≥2 days that required oral corticosteroids and antibiotics/hospitalization) over the 12 months |
|
| Sharma et al., 2021 [54] | India | Prospective case–control | Case group: patients with COPD having chronic periodontal disease and a history of exacerbation within the last month Control group: systemically healthy outpatients with periodontitis | 75 | Non-surgical periodontal therapy: oral hygiene instructions and professional full mouth SRP using an Ultrasonic scaler and periodontal hand instruments without local anaesthesia, n = 37; no periodontal treatment, n = 38 | Spirometric data (FEV1 and FVC) |
|
| Das et al., 2017 [55] | India | Randomized controlled trial | Patients with COPD | 35 | Full-mouth scaling and root planing using hand instruments, and oral hygiene instructions, n = 18; no periodontal treatment, n = 17 | SGRQ |
|
| Zhou et al., 2014 [56] | China | Randomized controlled trial | Symptomatic patients with COPD attending a hospital in Beijing | 60 | SRP treatment, n = 20; supragingival scaling treatment, n = 20; no periodontal treatment, n = 20 | Pulmonary function (FEV1 % predicted, FEV1/ FVC) and the frequencies of COPD exacerbation |
|
| Agado et al., 2012 [46] | USA | Randomized controlled trial | Patients diagnosed with COPD and chronic periodontitis | 30 | Magnetostrictive ultrasonic instrument, n = 10; hand instrument, n = 10; control, n = 10 | SGRQ-A and Illness Questionnaire (developed by the principal investigator) |
|
| Sundh et al., 2021 [58] | Sweden | Randomized controlled trial | Patients with COPD recruited at hospitals and primary healthcare centers | 101 | Advanced dental cleaning (modification of the full-mouth disinfection protocol), n = 45; control (dental examination and supra-gingival cleaning using toothpaste, corresponding to tooth brushing), n = 56 | Exacerbation frequency, pulmonary function (FEV1 % predicted), and CAT score |
|
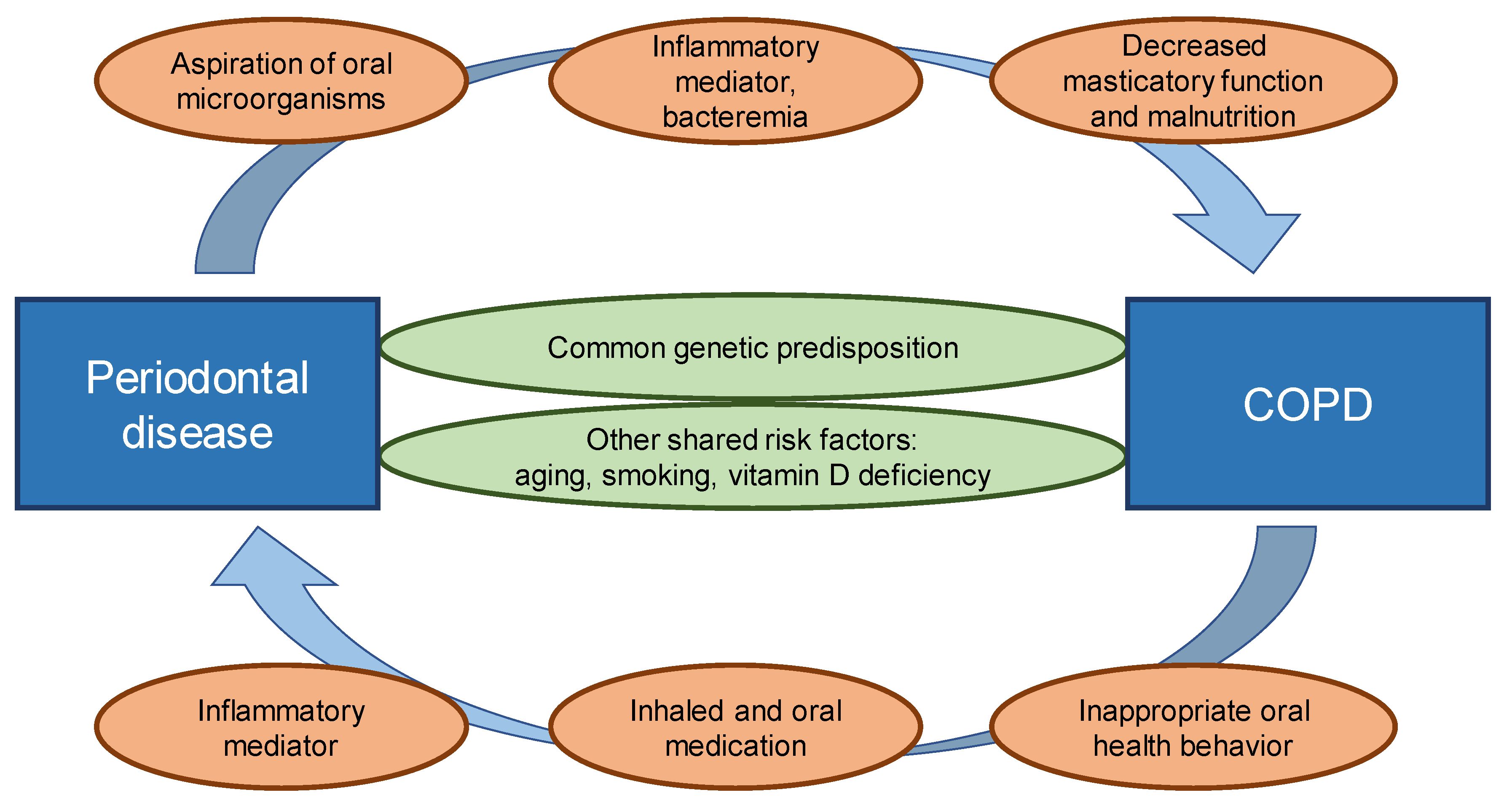
3. Biological Mechanisms Underlying This Relationship
3.1. Common Genetic Predisposition
3.2. Other Shared Risk Factors
3.3. The Role of Microorganisms
3.4. Therapeutic Agents
3.5. Inflammatory Mediators
3.6. Sarcopenia, Masticatory Function and Brushing Behavior
4. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 2015, 1, 15076. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Kouanda, B.; Sattar, Z.; Geraghty, P. Periodontal Diseases: Major Exacerbators of Pulmonary Diseases? Pulm. Med. 2021, 2021, 4712406. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontol. 2000 2020, 82, 257–267. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.O.D.; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Graves, C.L.; Gonzalez, O.A.; Dawson, D., 3rd; Morford, L.A.; Huja, P.E.; Hartsfield, J.K., Jr.; Huja, S.S.; Pandruvada, S.; Wallet, S.M. Aging, inflammation, immunity and periodontal disease. Periodontol. 2000 2016, 72, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, M.P.; Ford, P.J.; Seymour, G.J. Periodontal disease and systemic health: Current status. Aust. Dent. J. 2009, 54 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hobbins, S.; Chapple, I.L.; Sapey, E.; Stockley, R.A. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.; Swann, A.; Morris, A.J.; Martins, S.M.; Adams, R.; Jordan, R.E. Knowledge, attitudes and practices of patients and healthcare professionals regarding oral health and COPD in Sao Paulo, Brazil: A qualitative study. NPJ Prim. Care Respir. Med. 2021, 31, 20. [Google Scholar] [CrossRef]
- Kelly, N.; Winning, L.; Irwin, C.; Lundy, F.T.; Linden, D.; McGarvey, L.; Linden, G.J.; El Karim, I.A. Periodontal status and chronic obstructive pulmonary disease (COPD) exacerbations: A systematic review. BMC Oral Health 2021, 21, 425. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Leake, J.L. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 2006, 77, 1465–1482. [Google Scholar] [CrossRef]
- Garcia, R.I.; Nunn, M.E.; Vokonas, P.S. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann. Periodontol. 2001, 6, 71–77. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, B.; Xing, H.; Yang, S.; Xu, J.; Liu, H. Patients with Chronic Obstructive Pulmonary Disease Suffer from Worse Periodontal Health-Evidence from a Meta-Analysis. Front. Physiol. 2018, 9, 33. [Google Scholar] [CrossRef]
- Zeng, X.T.; Tu, M.L.; Liu, D.Y.; Zheng, D.; Zhang, J.; Leng, W. Periodontal disease and risk of chronic obstructive pulmonary disease: A meta-analysis of observational studies. PLoS ONE 2012, 7, e46508. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Cruz, S.S.D.; Trindade, S.C.; Passos-Soares, J.S.; Carvalho-Filho, P.C.; Figueiredo, A.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xiao, C.; Chen, F.; Wang, Y.; Guo, Z. Pulmonary disease and periodontal health: A meta-analysis. Sleep Breath. 2022, 26, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Huck, O.; Herrera, D.; Montero, E. The association between respiratory diseases and periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 842–887. [Google Scholar] [CrossRef] [PubMed]
- Perez Barrionuevo, A.M.; Gomez Real, F.; Igland, J.; Johannessen, A.; Omenaas, E.; Franklin, K.A.; Perez Barrionuevo, L.; Astrom, A.N.; Svanes, C.; Bertelsen, R.J. Periodontal health status and lung function in two Norwegian cohorts. PLoS ONE 2018, 13, e0191410. [Google Scholar] [CrossRef]
- Holtfreter, B.; Richter, S.; Kocher, T.; Dorr, M.; Volzke, H.; Ittermann, T.; Obst, A.; Schaper, C.; John, U.; Meisel, P.; et al. Periodontitis is related to lung volumes and airflow limitation: A cross-sectional study. Eur. Respir. J. 2013, 42, 1524–1535. [Google Scholar] [CrossRef]
- Winning, L.; Polyzois, I.; Sanmartin Berglund, J.; Renvert, S. Periodontitis and airflow limitation in older Swedish individuals. J. Clin. Periodontol. 2020, 47, 715–725. [Google Scholar] [CrossRef]
- Katancik, J.A.; Kritchevsky, S.; Weyant, R.J.; Corby, P.; Bretz, W.; Crapo, R.O.; Jensen, R.; Waterer, G.; Rubin, S.M.; Newman, A.B. Periodontitis and airway obstruction. J. Periodontol. 2005, 76, 2161–2167. [Google Scholar] [CrossRef]
- Si, Y.; Fan, H.; Song, Y.; Zhou, X.; Zhang, J.; Wang, Z. Association between periodontitis and chronic obstructive pulmonary disease in a Chinese population. J. Periodontol. 2012, 83, 1288–1296. [Google Scholar] [CrossRef]
- Moeintaghavi, A.; Mohammadzadeh Lari, S.; Shiezadeh, F.; Mohammadian, Z.; Tajik, S.; Nasrabadi, N. Relationship between periodontal variables and disease severity in patients with chronic obstructive pulmonary disease. J. Adv. Periodontol. Implant. Dent. 2018, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.P.; Mute, B.R.; Doiphode, S.S.; Bardapurkar, S.J.; Borkar, M.S.; Raje, D.V. Association between periodontal disease and chronic obstructive pulmonary disease: A reality or just a dogma? J. Periodontol. 2013, 84, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.; Sparrow, D.; Cohen, M.; Vokonas, P.S.; Garcia, R.I. The association between alveolar bone loss and pulmonary function: The VA Dental Longitudinal Study. Ann. Periodontol. 1998, 3, 257–261. [Google Scholar] [CrossRef]
- Tan, L.; Tang, X.; Pan, C.; Wang, H.; Pan, Y. Relationship among clinical periodontal, microbiologic parameters and lung function in participants with chronic obstructive pulmonary disease. J. Periodontol. 2019, 90, 134–140. [Google Scholar] [CrossRef]
- Winning, L.; Patterson, C.C.; Cullen, K.M.; Kee, F.; Linden, G.J. Chronic periodontitis and reduced respiratory function. J. Clin. Periodontol. 2019, 46, 266–275. [Google Scholar] [CrossRef]
- Javaheri, N.; Matin, S.; Naghizadeh-Baghi, A.; Bagheri, A.; Andreasian, A.; Ghobadi, H. Periodontal Status, Its Treatment Needs, and Its Relationship with Airflow Limitation and Quality of Life in COPD Patients. Eurasian J. Med. 2020, 52, 259–264. [Google Scholar] [CrossRef]
- Lee, W.C.; Fu, E.; Li, C.H.; Huang, R.Y.; Chiu, H.C.; Cheng, W.C.; Chen, W.L. Association between periodontitis and pulmonary function based on the Third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Periodontol. 2020, 47, 788–795. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Luo, J.; Dong, X.; Jiang, X. The association between periodontitis and lung function: Results from the National Health and Nutrition Examination Survey 2009 to 2012. J. Periodontol. 2022, 93, 901–910. [Google Scholar] [CrossRef]
- Hamalainen, P.; Suominen, H.; Keskinen, M.; Meurman, J.H. Oral health and reduction in respiratory capacity in a cohort of community-dwelling elderly people: A population-based 5-year follow-up study. Gerodontology 2004, 21, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, S.W. Prevalence of Periodontitis and its Association with Reduced Pulmonary Function: Results from the Korean National Health and Nutrition Examination Survey. Medicina 2019, 55, 581. [Google Scholar] [CrossRef] [PubMed]
- Henke, C.; Budweiser, S.; Jorres, R.A. Lung function and associations with multiple dimensions of dental health: A prospective observational cross-sectional study. BMC Res. Notes 2016, 9, 274. [Google Scholar] [CrossRef]
- Takeuchi, K.; Matsumoto, K.; Furuta, M.; Fukuyama, S.; Takeshita, T.; Ogata, H.; Suma, S.; Shibata, Y.; Shimazaki, Y.; Hata, J.; et al. Periodontitis Is Associated with Chronic Obstructive Pulmonary Disease. J. Dent. Res. 2019, 98, 534–540. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Song, Y.; Zhang, J.; Wang, C. Periodontal health and quality of life in patients with chronic obstructive pulmonary disease. Respir. Med. 2011, 105, 67–73. [Google Scholar] [CrossRef]
- Baldomero, A.K.; Siddiqui, M.; Lo, C.Y.; Petersen, A.; Pragman, A.A.; Connett, J.E.; Kunisaki, K.M.; Wendt, C.H. The relationship between oral health and COPD exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 881–892. [Google Scholar] [CrossRef]
- Gaeckle, N.T.; Heyman, B.; Criner, A.J.; Criner, G.J. Markers of Dental Health Correlate with Daily Respiratory Symptoms in COPD. Chronic Obstr. Pulm. Dis. 2018, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Agado, B.E.; Crawford, B.; DeLaRosa, J.; Bowen, D.M.; Peterson, T.; Neill, K.; Paarmann, C. Effects of periodontal instrumentation on quality of life and illness in patients with chronic obstructive pulmonary disease: A pilot study. J. Dent. Hyg. 2012, 86, 204–214. [Google Scholar] [PubMed]
- Soler-Cataluna, J.J.; Martinez-Garcia, M.A.; Roman Sanchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.; Miravitlles, M.; Metzdorf, N.; Celli, B. Impact and prevention of severe exacerbations of COPD: A review of the evidence. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Viniol, C.; Vogelmeier, C.F. Exacerbations of COPD. Eur. Respir. Rev. 2018, 27, 170103. [Google Scholar] [CrossRef]
- Shen, T.C.; Chang, P.Y.; Lin, C.L.; Chen, C.H.; Tu, C.Y.; Hsia, T.C.; Shih, C.M.; Hsu, W.H.; Sung, F.C.; Kao, C.H. Periodontal Treatment Reduces Risk of Adverse Respiratory Events in Patients with Chronic Obstructive Pulmonary Disease: A Propensity-Matched Cohort Study. Medicine 2016, 95, e3735. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhang, J.; Zhou, X.; Zhang, L.; Song, Y.; Wang, Z. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J. Clin. Periodontol. 2012, 39, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Suruki, R.; Loewy, Z.G.; Beck, J.D.; Offenbacher, S. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: Modulatory role of systemic biomarkers of inflammation. PLoS ONE 2013, 8, e68592. [Google Scholar] [CrossRef]
- Kucukcoskun, M.; Baser, U.; Oztekin, G.; Kiyan, E.; Yalcin, F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J. Periodontol. 2013, 84, 863–870. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A.; Verma, A.K.; Pathak, A.; Verma, S.; Chaudhary, S.C.; Kaushal, S.; Lal, N.; Kant, S.; Verma, U.P. Impact of Non-surgical Periodontal Therapy on Pulmonary functions, Periodontal Health and Salivary Matrix Metalloproteinase-8 of COPD Patients with Chronic Periodontitis: A Clinico-biochemical Study. Turk. Thorac. J. 2021, 22, 324–332. [Google Scholar] [CrossRef]
- Das, K.; Das, S.J.; Sarma, J. Effects of Phase I Periodontal Therapy on the Quality of Life in COPD Patients. J. Med. Sci. Clin. Res. 2017, 5, 28724–28732. [Google Scholar] [CrossRef]
- Zhou, X.; Han, J.; Liu, Z.; Song, Y.; Wang, Z.; Sun, Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: A 2-year pilot randomized controlled trial. J. Clin. Periodontol. 2014, 41, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Madalli, R.; Kheur, S.; Reddy, M.G.S.; Kheur, M.; Mahalle, A. Assessment of role of Porphyromonas gingivalis as an aggravating factor for chronic obstructive pulmonary disease patients with periodontitis. Dent. Hypotheses 2016, 7, 100–106. [Google Scholar]
- Sundh, J.; Tanash, H.; Arian, R.; Neves-Guimaraes, A.; Broberg, K.; Lindved, G.; Kern, T.; Zych, K.; Nielsen, H.B.; Halling, A.; et al. Advanced Dental Cleaning is Associated with Reduced Risk of COPD Exacerbations-A Randomized Controlled Trial. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 3203–3215. [Google Scholar] [CrossRef]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13 (Suppl. 4), 3–10. [Google Scholar] [CrossRef]
- Yu, H.; Lin, M.; Wang, X.; Wang, S.; Wang, Z. Toll-like receptor 4 polymorphism is associated with increased susceptibility to chronic obstructive pulmonary disease in Han Chinese patients with chronic periodontitis. J. Oral Sci. 2016, 58, 555–560. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Fu, Y.; Ziebolz, D.; Li, S.; Schmalz, G.; Li, F. Transcriptomic analysis reveals pathophysiological relationship between chronic obstructive pulmonary disease (COPD) and periodontitis. BMC Med. Genom. 2022, 15, 130. [Google Scholar] [CrossRef]
- Tadjoedin, F.M.; Fitri, A.H.; Kuswandani, S.O.; Sulijaya, B.; Soeroso, Y. The correlation between age and periodontal diseases. J. Int. Dent. Med. Res. 2017, 10, 327–332. [Google Scholar]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J. Tobacco smoking and chronic destructive periodontal disease. Odontology 2004, 92, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.S.; Dolmage, T.E.; Stephenson, A.; Jackson, B. Chronic obstructive pulmonary disease and socioeconomic status: A systematic review. COPD 2012, 9, 216–226. [Google Scholar] [CrossRef]
- Prasanna, S.J. Causal relationship between periodontitis and chronic obstructive pulmonary disease. J. Indian Soc. Periodontol. 2011, 15, 359–365. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, J.C.; Rim, T.T.; Jung, U.W.; Kim, C.S.; Donos, N.; Cha, I.H.; Choi, S.H. Socioeconomic disparities of periodontitis in Koreans based on the KNHANES IV. Oral Dis. 2014, 20, 551–559. [Google Scholar] [CrossRef]
- Lenk, M.; Noack, B.; Weidner, K.; Lorenz, K. Psychopathologies and socioeconomic status as risk indicators for periodontitis: A survey-based investigation in German dental practices. Clin. Oral Investig. 2022, 26, 2853–2862. [Google Scholar] [CrossRef]
- Zhou, X.; Han, J.; Song, Y.; Zhang, J.; Wang, Z. Serum levels of 25-hydroxyvitamin D, oral health and chronic obstructive pulmonary disease. J. Clin. Periodontol. 2012, 39, 350–356. [Google Scholar] [CrossRef]
- Krall, E.A.; Wehler, C.; Garcia, R.I.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 111, 452–456. [Google Scholar] [CrossRef]
- Dietrich, T.; Nunn, M.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am. J. Clin. Nutr. 2005, 82, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Black, P.N.; Scragg, R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef]
- Chalmers, J.D.; McHugh, B.J.; Docherty, C.; Govan, J.R.; Hill, A.T. Vitamin-D deficiency is associated with chronic bacterial colonisation and disease severity in bronchiectasis. Thorax 2013, 68, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Laaksi, I. Vitamin D and respiratory infection in adults. Proc. Nutr. Soc. 2012, 71, 90–97. [Google Scholar] [CrossRef]
- Sandford, A.J.; Weir, T.D.; Pare, P.D. Genetic risk factors for chronic obstructive pulmonary disease. Eur. Respir. J. 1997, 10, 1380–1391. [Google Scholar] [CrossRef]
- Boyan, B.D.; Wong, K.L.; Fang, M.; Schwartz, Z. 1alpha,25(OH)2D3 is an autocrine regulator of extracellular matrix turnover and growth factor release via ERp60 activated matrix vesicle metalloproteinases. J. Steroid Biochem. Mol. Biol. 2007, 103, 467–472. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Nelson, A.; Wang, X.; Farid, M.; Gunji, Y.; Ikari, J.; Iwasawa, S.; Basma, H.; Feghali-Bostwick, C.; Rennard, S.I. Vitamin D modulates prostaglandin E2 synthesis and degradation in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2014, 50, 40–50. [Google Scholar] [CrossRef]
- Togo, S.; Holz, O.; Liu, X.; Sugiura, H.; Kamio, K.; Wang, X.; Kawasaki, S.; Ahn, Y.; Fredriksson, K.; Skold, C.M.; et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am. J. Respir. Crit. Care Med. 2008, 178, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Proenca, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- Han, J.; Cheng, C.; Zhu, Z.; Lin, M.; Zhang, D.X.; Wang, Z.M.; Wang, S. Vitamin D reduces the serum levels of inflammatory cytokines in rat models of periodontitis and chronic obstructive pulmonary disease. J. Oral Sci. 2019, 61, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Iinuma, T.; Sato, S. Relationship between the oral cavity and respiratory diseases: Aspiration of oral bacteria possibly contributes to the progression of lower airway inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Yokoe, S.; Ogata, Y.; Sato, S.; Imai, K. Exposure to Porphyromonas gingivalis Induces Production of Proinflammatory Cytokine via TLR2 from Human Respiratory Epithelial Cells. J. Clin. Med. 2020, 9, 3433. [Google Scholar] [CrossRef] [PubMed]
- Hayata, M.; Watanabe, N.; Tamura, M.; Kamio, N.; Tanaka, H.; Nodomi, K.; Miya, C.; Nakayama, E.; Ueda, K.; Ogata, Y.; et al. The Periodontopathic Bacterium Fusobacterium nucleatum Induced Proinflammatory Cytokine Production by Human Respiratory Epithelial Cell Lines and in the Lower Respiratory Organs in Mice. Cell Physiol. Biochem. 2019, 53, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, Z.; Wang, X.; Liu, W.; Wang, Z. Role of experimental periodontitis in inducing pulmonary inflammation in mice. Oral Dis. 2022, 28, 2294–2303. [Google Scholar] [CrossRef]
- Miya, C.; Cueno, M.E.; Suzuki, R.; Maruoka, S.; Gon, Y.; Kaneko, T.; Yonehara, Y.; Imai, K. Porphyromonas gingivalis gingipains potentially affect MUC5AC gene expression and protein levels in respiratory epithelial cells. FEBS Open Bio 2021, 11, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Yanagihara, K.; Harada, Y.; Yamada, K.; Migiyama, Y.; Morinaga, Y.; Hasegawa, H.; Izumikawa, K.; Kakeya, H.; Nishimura, M.; et al. Macrolides inhibit Fusobacterium nucleatum-induced MUC5AC production in human airway epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 1844–1849. [Google Scholar] [CrossRef]
- Suzuki, R.; Kamio, N.; Kaneko, T.; Yonehara, Y.; Imai, K. Fusobacterium nucleatum exacerbates chronic obstructive pulmonary disease in elastase-induced emphysematous mice. FEBS Open Bio 2022, 12, 638–648. [Google Scholar] [CrossRef]
- Hendrix, A.Y.; Kheradmand, F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog. Mol. Biol. Transl. Sci. 2017, 148, 1–29. [Google Scholar] [CrossRef]
- Suzuki, R.; Kamio, N.; Sugimoto, K.; Maruoka, S.; Gon, Y.; Kaneko, T.; Yonehara, Y.; Imai, K. Periodontopathic Bacterium Fusobacterium nucleatum Affects Matrix Metalloproteinase-9 Expression in Human Alveolar Epithelial Cells and Mouse Lung. Vivo 2022, 36, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yoshida, K.; Fujiwara, N.; Seyama, M.; Ono, K.; Kawai, H.; Guo, J.; Wang, Z.; Weng, Y.; Yu, Y.; et al. Extracellular vesicles of P. gingivalis-infected macrophages induce lung injury. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166236. [Google Scholar] [CrossRef] [PubMed]
- Rigauts, C.; Aizawa, J.; Taylor, S.L.; Rogers, G.B.; Govaerts, M.; Cos, P.; Ostyn, L.; Sims, S.; Vandeplassche, E.; Sze, M.; et al. R othia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur. Respir. J. 2022, 59, 3433. [Google Scholar] [CrossRef]
- Wang, Z.; Bafadhel, M.; Haldar, K.; Spivak, A.; Mayhew, D.; Miller, B.E.; Tal-Singer, R.; Johnston, S.L.; Ramsheh, M.Y.; Barer, M.R.; et al. Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 2016, 47, 1082–1092. [Google Scholar] [CrossRef]
- Tan, L.; Wang, H.; Li, C.; Pan, Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J. Periodontal. Res. 2014, 49, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, J.; Xu, M.; Zhu, D.; Wang, X.; Chen, Y.; Wu, J.; Cui, C.; Zhang, W.; Yu, L. 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J. Oral Microbiol. 2017, 9, 1324725. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, X.; Wang, J.; Cheng, C.; Zhang, T.; Han, X.; Song, Y.; Wang, Z.; Wang, S. Saliva Microbiome Changes in Patients with Periodontitis with and without Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol. 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Khijmatgar, S.; Belur, G.; Venkataram, R.; Karobari, M.I.; Marya, A.; Shetty, V.; Chowdhury, A.; Gootveld, M.; Lynch, E.; Shetty, S.; et al. Oral Candidal Load and Oral Health Status in Chronic Obstructive Pulmonary Disease (COPD) Patients: A Case-Cohort Study. Biomed. Res. Int. 2021, 2021, 5548746. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Liu, W.; Huang, X.; Song, Y.; Wang, Z.; Jia, X. Periodontal Status and Microbiologic Pathogens in Patients with Chronic Obstructive Pulmonary Disease and Periodontitis: A Case-Control Study. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Muro, S.; Tanabe, N.; Terada, K.; Kiyokawa, H.; Sato, S.; Hoshino, Y.; Ogawa, E.; Uno, K.; Naruishi, K.; et al. Relationship between periodontitis-related antibody and frequent exacerbations in chronic obstructive pulmonary disease. PLoS ONE 2012, 7, e40570. [Google Scholar] [CrossRef][Green Version]
- Thomas, M.S.; Parolia, A.; Kundabala, M.; Vikram, M. Asthma and oral health: A review. Aust. Dent. J. 2010, 55, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gani, F.; Caminati, M.; Bellavia, F.; Baroso, A.; Faccioni, P.; Pancera, P.; Batani, V.; Senna, G. Oral health in asthmatic patients: A review: Asthma and its therapy may impact on oral health. Clin. Mol. Allergy 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Khassawneh, B.; Alhabashneh, R.; Ibrahim, F. The association between bronchial asthma and periodontitis: A case-control study in Jordan. J. Asthma 2019, 56, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J.; Wong, C.A.; Oborne, J.; Cooper, S.; Lewis, S.A.; Pringle, M.; Hubbard, R.; Tattersfield, A.E. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax 2001, 56, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, M.; Moller, C.; Ericson, T. Effect of beta 2-adrenoceptor agonists on saliva proteins and dental caries in asthmatic children. J. Dent. Res. 1987, 66, 1404–1406. [Google Scholar] [CrossRef]
- Ryberg, M.; Moller, C.; Ericson, T. Saliva composition and caries development in asthmatic patients treated with beta 2-adrenoceptor agonists: A 4-year follow-up study. Scand. J. Dent. Res. 1991, 99, 212–218. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Stellini, E.; Cavaleri, E.; Angelova Volponi, A.; Ferro, R.; Fochesato Colombani, S. Dental caries in children with asthma undergoing treatment with short-acting beta2-agonists. Eur. J. Paediatr. Dent. 2008, 9, 132–138. [Google Scholar]
- Kargul, B.; Tanboga, I.; Ergeneli, S.; Karakoc, F.; Dagli, E. Inhaler medicament effects on saliva and plaque pH in asthmatic children. J. Clin. Pediatr. Dent. 1998, 22, 137–140. [Google Scholar]
- Dubus, J.C.; Marguet, C.; Deschildre, A.; Mely, L.; Le Roux, P.; Brouard, J.; Huiart, L.; Réseau de Recherche Clinique en Pneumonologie Pédiatrique. Local side-effects of inhaled corticosteroids in asthmatic children: Influence of drug, dose, age, and device. Allergy 2001, 56, 944–948. [Google Scholar] [CrossRef]
- Knight, L.; Fletcher, J. Growth of Candida albicans in saliva: Stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J. Infect. Dis. 1971, 123, 371–377. [Google Scholar] [CrossRef]
- Chellaih, P.; Sivadas, G.; Chintu, S.; Vaishnavi Vedam, V.K.; Arunachalam, R.; Sarsu, M. Effect of anti-asthmatic drugs on dental health: A comparative study. J. Pharm. Bioallied. Sci. 2016, 8, S77–S80. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, C.; Matsuse, H.; Saeki, S.; Kawano, T.; Machida, I.; Kondo, Y.; Kohno, S. Salivary IgA and oral candidiasis in asthmatic patients treated with inhaled corticosteroid. J. Asthma 2005, 42, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Tiisanoja, A.; Syrjala, A.M.; Anttonen, V.; Ylostalo, P. Anticholinergic burden, oral hygiene practices, and oral hygiene status-cross-sectional findings from the Northern Finland Birth Cohort 1966. Clin. Oral Investig. 2021, 25, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Bozejac, B.V.; Stojsin, I.; Ethuric, M.; Zvezdin, B.; Brkanic, T.; Budisin, E.; Vukoje, K.; Secen, N. Impact of inhalation therapy on the incidence of carious lesions in patients with asthma and COPD. J. Appl. Oral Sci. 2017, 25, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.C.; Chang, P.Y.; Lin, C.L.; Chen, C.H.; Tu, C.Y.; Hsia, T.C.; Shih, C.M.; Hsu, W.H.; Sung, F.C.; Kao, C.H. Risk of Periodontal Diseases in Patients with Chronic Obstructive Pulmonary Disease: A Nationwide Population-based Cohort Study. Medicine 2015, 94, e2047. [Google Scholar] [CrossRef]
- Raj, R.; Manu, M.K.; Prakash, P.Y.; Singhal, D.K.; Acharya, S. The effect of 6 months or longer duration of chronic obstructive respiratory disease medication on the oral health parameters of adults. Spec. Care Dent. 2018, 38, 133–138. [Google Scholar] [CrossRef]
- Oztekin, G.; Baser, U.; Kucukcoskun, M.; Tanrikulu-Kucuk, S.; Ademoglu, E.; Isik, G.; Ozkan, G.; Yalcin, F.; Kiyan, E. The association between periodontal disease and chronic obstructive pulmonary disease: A case control study. COPD 2014, 11, 424–430. [Google Scholar] [CrossRef]
- Winslow, S.; Odqvist, L.; Diver, S.; Riise, R.; Abdillahi, S.; Wingren, C.; Lindmark, H.; Wellner, A.; Lundin, S.; Yrlid, L.; et al. Multi-omics links IL-6 trans-signalling with neutrophil extracellular trap formation and Haemophilus infection in COPD. Eur. Respir. J. 2021, 58, 2003312. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Zeng, K.; Deng, F.; Lin, C.; Huang, W. Interleukin-6 is a Strong Predictor of the Frequency of COPD Exacerbation within 1 Year. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2945–2951. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Zhang, D.W.; Ye, J.J.; Lan, Q.X.; Ji, S.; Sun, L.; Li, F.; Fei, G.H. Interleukin-6 neutralizing antibody attenuates the hypersecretion of airway mucus via inducing the nuclear translocation of Nrf2 in chronic obstructive pulmonary disease. Biomed. Pharmacother. 2022, 152, 113244. [Google Scholar] [CrossRef]
- Fung, K.Y.; Louis, C.; Metcalfe, R.D.; Kosasih, C.C.; Wicks, I.P.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for IL-11 in inflammatory diseases. Cytokine 2022, 149, 155750. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.; Rohde, G.; Arinir, U.; Hagedorn, M.; Durig, N.; Schultze-Werninghaus, G.; Epplen, J.T. A promotor polymorphism in the Interleukin 11 gene is associated with chronic obstructive pulmonary disease. Electrophoresis 2004, 25, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.B.; Wood, N.; Serio, F.G. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J. Periodontol. 2004, 75, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Usher, A.K.; Stockley, R.A. The link between chronic periodontitis and COPD: A common role for the neutrophil? BMC Med. 2013, 11, 241. [Google Scholar] [CrossRef]
- Sapey, E.; Yonel, Z.; Edgar, R.; Parmar, S.; Hobbins, S.; Newby, P.; Crossley, D.; Usher, A.; Johnson, S.; Walton, G.M.; et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J. Clin. Periodontol. 2020, 47, 1040–1052. [Google Scholar] [CrossRef]
- Vernooy, J.H.; Lindeman, J.H.; Jacobs, J.A.; Hanemaaijer, R.; Wouters, E.F. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest 2004, 126, 1802–1810. [Google Scholar] [CrossRef]
- Vignola, A.M.; Riccobono, L.; Mirabella, A.; Profita, M.; Chanez, P.; Bellia, V.; Mautino, G.; D’Accardi, P.; Bousquet, J.; Bonsignore, G. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1998, 158, 1945–1950. [Google Scholar] [CrossRef]
- Culpitt, S.V.; Rogers, D.F.; Traves, S.L.; Barnes, P.J.; Donnelly, L.E. Sputum matrix metalloproteases: Comparison between chronic obstructive pulmonary disease and asthma. Respir. Med. 2005, 99, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Umeizudike, K.; Raisanen, I.; Gupta, S.; Nwhator, S.; Grigoriadis, A.; Sakellari, D.; Sorsa, T. Active matrix metalloproteinase-8: A potential biomarker of oral systemic link. Clin. Exp. Dent. Res. 2022, 8, 359–365. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e9642. [Google Scholar] [CrossRef]
- Ghosh, P.; Muthuraj, T.S.; Bandyopadhyay, P.; Swarnakar, S.; Sarkar, P.; Varatharajan, A. Expression of matrix metalloproteinase-9 in gingival tissue biopsy in patients with slowly/ moderately and rapidly progressing periodontitis: An observational study. J. Indian Soc. Periodontol. 2021, 25, 386–392. [Google Scholar] [CrossRef]
- Yang, S.; Gu, B.; Zhao, L.; Shi, Q.; Xu, J.; Wen, N. Meta-analysis of the association between serum and gingival crevicular fluid matrix metalloproteinase-9 and periodontitis. J. Am. Dent. Assoc. 2019, 150, 34–41. [Google Scholar] [CrossRef]
- Ji, J.; von Scheele, I.; Bergstrom, J.; Billing, B.; Dahlen, B.; Lantz, A.S.; Larsson, K.; Palmberg, L. Compartment differences of inflammatory activity in chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 104. [Google Scholar] [CrossRef]
- Yildirim, E.; Kormi, I.; Basoglu, O.K.; Gurgun, A.; Kaval, B.; Sorsa, T.; Buduneli, N. Periodontal health and serum, saliva matrix metalloproteinases in patients with mild chronic obstructive pulmonary disease. J. Periodontal. Res. 2013, 48, 269–275. [Google Scholar] [CrossRef]
- Rosa, E.P.; Murakami-Malaquias-da-Silva, F.; Palma-Cruz, M.; de Carvalho Garcia, G.; Brito, A.A.; Andreo, L.; Kamei, S.K.; Negreiros, R.M.; Rodrigues, M.; Mesquita-Ferrari, R.A.; et al. The impact of periodontitis in the course of chronic obstructive pulmonary disease: Pulmonary and systemic effects. Life Sci. 2020, 261, 118257. [Google Scholar] [CrossRef]
- Marengoni, A.; Vetrano, D.L.; Manes-Gravina, E.; Bernabei, R.; Onder, G.; Palmer, K. The Relationship between COPD and Frailty: A Systematic Review and Meta-Analysis of Observational Studies. Chest 2018, 154, 21–40. [Google Scholar] [CrossRef]
- Demircioglu, H.; Cihan, F.G.; Kutlu, R.; Yosunkaya, S.; Zamani, A. Frequency of sarcopenia and associated outcomes in patients with chronic obstructive pulmonary disease. Turk. J. Med. Sci. 2020, 50, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Nakajima, T.; Matsuzaki, T.; Iwami, E.; Shibui, T.; Nomura, T.; Katakura, A. Chewing ability and desaturation during chewing in patients with COPD. Monaldi Arch. Chest Dis. 2019, 89, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ogawa, H.; Yoshihara, A.; Yamaga, T.; Takiguchi, T.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Fukutomi, E.; Chen, W.; et al. Evaluation of chewing ability and its relationship with activities of daily living, depression, cognitive status and food intake in the community-dwelling elderly. Geriatr. Gerontol. Int. 2013, 13, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Chubachi, S.; Matsuzaki, T.; Nakajima, T.; Satoh, M.; Iwami, E.; Yoshida, K.; Katakura, A.; Betsuyaku, T. The association between dental health and nutritional status in chronic obstructive pulmonary disease. Chronic Respir. Dis. 2017, 14, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kaluzniak-Szymanowska, A.; Krzyminska-Siemaszko, R.; Deskur-Smielecka, E.; Lewandowicz, M.; Kaczmarek, B.; Wieczorowska-Tobis, K. Malnutrition, Sarcopenia, and Malnutrition-Sarcopenia Syndrome in Older Adults with COPD. Nutrients 2021, 14, 44. [Google Scholar] [CrossRef]
- Han, C.H.; Chung, J.H. Association between Sarcopenia and Tooth Loss. Ann. Geriatr. Med. Res. 2018, 22, 145–150. [Google Scholar] [CrossRef]
- Chung, J.H.; Hwang, H.J.; Kim, S.H.; Kim, T.H. Associations between Periodontitis and Chronic Obstructive Pulmonary Disease: The 2010 to 2012 Korean National Health and Nutrition Examination Survey. J. Periodontol. 2016, 87, 864–871. [Google Scholar] [CrossRef]
- Bhavsar, N.V.; Dave, B.D.; Brahmbhatt, N.A.; Parekh, R. Periodontal status and oral health behavior in hospitalized patients with chronic obstructive pulmonary disease. J. Nat. Sci. Biol. Med. 2015, 6, S93–S97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Zhang, J.; Zhang, L.; Song, Y.; Hu, F.B.; Wang, C. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J. Clin. Periodontol. 2009, 36, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.; Pavord, I.D.; Landis, S.; Pascoe, S.; Lettis, S.; Morjaria, N.; Barnes, N.; Hilton, E. Blood eosinophil levels as a biomarker in COPD. Respir. Med. 2018, 138, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Singh, D.; Hurst, J.R.; Martinez, F.J.; Rabe, K.F.; Bafadhel, M.; Jenkins, M.; Salazar, D.; Dorinsky, P.; Darken, P. Predictive modeling of COPD exacerbation rates using baseline risk factors. Ther. Adv. Respir. Dis. 2022, 16, 1–15. [Google Scholar] [CrossRef]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R.; Environmental and Occupational Health Assembly Committee on Nonsmoking COPD; et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef]
- Nicolaou, L.; Checkley, W. Differences between cigarette smoking and biomass smoke exposure: An in silico comparative assessment of particulate deposition in the lungs. Environ. Res. 2021, 197, 111116. [Google Scholar] [CrossRef]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Pare, P.D.; Muller, N.L.; Silva, C.I.; Rojas, C.E.; et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef]
- Agarwal, D.M.; Dhotre, D.P.; Kumbhare, S.V.; Gaike, A.H.; Brashier, B.B.; Shouche, Y.S.; Juvekar, S.K.; Salvi, S.S. Disruptions in oral and nasal microbiota in biomass and tobacco smoke associated chronic obstructive pulmonary disease. Arch. Microbiol. 2021, 203, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J. Patients with chronic obstructive pulmonary disease: Management considerations for the dental team. Br. Dent. J. 2014, 217, 235–237. [Google Scholar] [CrossRef][Green Version]
- Hayton, C.; Clark, A.; Olive, S.; Browne, P.; Galey, P.; Knights, E.; Staunton, L.; Jones, A.; Coombes, E.; Wilson, A.M. Barriers to pulmonary rehabilitation: Characteristics that predict patient attendance and adherence. Respir. Med. 2013, 107, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Goncalves, N.; Scully, B.; Heredia, R.; Hegde, S. A Teledentistry Pilot Study on Patient-Initiated Care. Int. J. Environ. Res. Public Health 2022, 19, 9403. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Borgnakke, W.S. Self-Reported Periodontal Disease: Validation in an Epidemiological Survey. J. Periodontol. 2007, 78 (Suppl. 7S), 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulos, G.S.; Tsalikis, L.; Konstantinidis, A.; Kotsakis, G.A. A Two-Domain Self-Report Measure of Periodontal Disease Has Good Accuracy for Periodontitis Screening in Dental School Outpatients. J. Periodontol. 2016, 87, 1165–1173. [Google Scholar] [CrossRef]
- Skou, S.T.; Mair, F.S.; Fortin, M.; Guthrie, B.; Nunes, B.P.; Miranda, J.J.; Boyd, C.M.; Pati, S.; Mtenga, S.; Smith, S.M. Multimorbidity. Nat. Rev. Dis. Prim. 2022, 8, 48. [Google Scholar] [CrossRef]
- Chen, B.K.; Jalal, H.; Hashimoto, H.; Suen, S.C.; Eggleston, K.; Hurley, M.; Schoemaker, L.; Bhattacharya, J. Forecasting Trends in Disability in a Super-Aging Society: Adapting the Future Elderly Model to Japan. J. Econ. Ageing 2016, 8, 42–51. [Google Scholar] [CrossRef]
- Aida, J.; Takeuchi, K.; Furuta, M.; Ito, K.; Kabasawa, Y.; Tsakos, G. Burden of Oral Diseases and Access to Oral Care in an Ageing Society. Int. Dent. J. 2022, 72, S5–S11. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Murthy, M.; Balch, W.E.; Chandel, N.S.; Meiners, S.; Eickelberg, O.; Selman, M.; Pardo, A.; White, E.S.; Levy, B.D.; et al. Blue journal conference. Aging and susceptibility to lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 261–269. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Location | Study Design | Study Population | n | Measured Outcome | Main Findings |
|---|---|---|---|---|---|---|
| Zhou et al., 2011 [43] | China | Cross-sectional | Patients with COPD being treated at eight hospitals in Beijing | 306 | Periodontal parameters: PD, CAL, BOP, PI, and the number of missing teeth QOL measurement: SGRQ | The missing teeth were significantly associated with symptom score (p = 0.030) and activity score (p = 0.033); PI was also significantly associated with symptom score (p = 0.007). |
| Baldomero et al., 2019 [44] | USA | Case-control (exacerbators vs. non-exacerbators) | Individuals from the Minneapolis Veterans Affairs health care system | 136 (patients with COPD: exacerbator, n = 70; non-exacerbator, n = 66) | Periodontal parameters: OHIP-5; PD, CAL, BOP, GI, PI, and caries risk assessment (subset of patients) QOL measurement: SGRQ | Worse OHRQoL as measured by OHIP-5 was associated with worse respiratory health scores (SGRQ total score): difficulty chewing (regression coefficient, 2.57; p = 0.023), painful ache in the mouth (regression coefficient, 5.43; p < 0.001), uncomfortable about appearance (regression coefficient, 3.17; p = 0.003), less flavor (regression coefficient, 3.53; p = 0.005), and difficulty performing jobs (regression coefficient, 7.31; p < 0.001). |
| Gaeckle et al., 2018 [45] | USA | Prospective cohort | Healthy individuals without lung disease and patients with severe COPD, recruited at a single medical center | 30 (case, n = 20; control, n = 10) | Periodontal parameters: PI and OHIP-14 QOL measurement: electronic COPD daily diary | In patients with COPD, the number of teeth showed a significant positive correlation with the percentage of days with cough (β = 2.70, p = 0.04) and wheezing (β = 2.65, p = 0.01), whereas PI showed no significant correlation with daily respiratory symptoms. |
| Agado et al., 2012 [46] | USA | Randomized controlled trial | Patients diagnosed with COPD and chronic periodontitis | 30 (magnetostrictive ultrasonic instrument, n = 10; hand instrument, n = 10; control, n = 10) | Periodontal parameters: PI and CAL QOL measurements: SGRQ-A and illness questionnaire (developed by the principal investigator) | SGRQ-A (symptom, p = 0.124; activity, p = 0.702; impact, p = 0.926) and illness questionnaire scores did not demonstrate significant differences in QOL or illness after periodontal debridement between groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamiya, H.; Mitani, A.; Abe, M.; Nagase, T. Putative Bidirectionality of Chronic Obstructive Pulmonary Disease and Periodontal Disease: A Review of the Literature. J. Clin. Med. 2023, 12, 5935. https://doi.org/10.3390/jcm12185935
Tamiya H, Mitani A, Abe M, Nagase T. Putative Bidirectionality of Chronic Obstructive Pulmonary Disease and Periodontal Disease: A Review of the Literature. Journal of Clinical Medicine. 2023; 12(18):5935. https://doi.org/10.3390/jcm12185935
Chicago/Turabian StyleTamiya, Hiroyuki, Akihisa Mitani, Masanobu Abe, and Takahide Nagase. 2023. "Putative Bidirectionality of Chronic Obstructive Pulmonary Disease and Periodontal Disease: A Review of the Literature" Journal of Clinical Medicine 12, no. 18: 5935. https://doi.org/10.3390/jcm12185935
APA StyleTamiya, H., Mitani, A., Abe, M., & Nagase, T. (2023). Putative Bidirectionality of Chronic Obstructive Pulmonary Disease and Periodontal Disease: A Review of the Literature. Journal of Clinical Medicine, 12(18), 5935. https://doi.org/10.3390/jcm12185935






