Subtypes of Patients with Mild to Moderate Airflow Limitation as Predictors of Chronic Obstructive Pulmonary Disease Exacerbation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comorbidities and Medical History
3.3. Occurrence of Acute Exacerbation and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 3 October 2023).
- GOLD. Available online: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf (accessed on 3 October 2023).
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Edwards, L.D.; Lomas, D.A.; MacNee, W.; Miller, B.E.; Rennard, S.; Silverman, E.K.; et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 2010, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D. The Importance of Early Chronic Obstructive Pulmonary Disease: A Lecture from 2022 Asian Pacific Society of Respirology. Tuberc. Respir. Dis. 2023, 86, 71–81. [Google Scholar] [CrossRef]
- Choi, J.Y.; Rhee, C.K. Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD). J. Clin. Med. 2020, 9, 3426. [Google Scholar] [CrossRef]
- Rennard, S.I.; Drummond, M.B. Early chronic obstructive pulmonary disease: Definition, assessment, and prevention. Lancet 2015, 385, 1778–1788. [Google Scholar] [CrossRef]
- Colak, Y.; Afzal, S.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Prevalence, Characteristics, and Prognosis of Early Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2020, 201, 671–680. [Google Scholar] [CrossRef]
- MacQueen, J. Some Methods for Classification and Analysis of Multivariate Observations. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, 21–18 July 1965 and 27 December 1965–7 January 1966. [Google Scholar]
- McLachlan, G.J. Cluster analysis and related techniques in medical research. Stat. Methods Med. Res. 1992, 1, 27–48. [Google Scholar] [CrossRef]
- Elbattah, M.; Molloy, O. Data-driven patient segmentation using K-means clustering: The case of hip fracture care in Ireland. In Proceedings of the Australasian Computer Science Week Multiconference, Geelong, Australia, 30 January–3 February 2017; p. 60. [Google Scholar]
- Lee, J.Y.; Chon, G.R.; Rhee, C.K.; Kim, D.K.; Yoon, H.K.; Lee, J.H.; Yoo, K.H.; Lee, S.H.; Lee, S.Y.; Kim, T.E.; et al. Characteristics of Patients with Chronic Obstructive Pulmonary Disease at the First Visit to a Pulmonary Medical Center in Korea: The KOrea COpd Subgroup Study Team Cohort. J. Korean Med. Sci. 2016, 31, 553–560. [Google Scholar] [CrossRef]
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [Google Scholar] [CrossRef]
- ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef] [PubMed]
- Burney, P.G.; Luczynska, C.; Chinn, S.; Jarvis, D. The European Community Respiratory Health Survey. Eur. Respir. J. 1994, 7, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.; Wedzicha, J.A. COPD exacerbations: Definitions and classifications. Eur. Respir. J. 2003, 21, 46s–53s. [Google Scholar] [CrossRef] [PubMed]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Kim, V.; Davey, A.; Comellas, A.P.; Han, M.K.; Washko, G.; Martinez, C.H.; Lynch, D.; Lee, J.H.; Silverman, E.K.; Crapo, J.D.; et al. Clinical and computed tomographic predictors of chronic bronchitis in COPD: A cross sectional analysis of the COPDGene study. Respir. Res. 2014, 15, 52. [Google Scholar] [CrossRef]
- Kim, V.; Han, M.K.; Vance, G.B.; Make, B.J.; Newell, J.D.; Hokanson, J.E.; Hersh, C.P.; Stinson, D.; Silverman, E.K.; Criner, G.J. The chronic bronchitic phenotype of COPD: An analysis of the COPDGene Study. Chest 2011, 140, 626–633. [Google Scholar] [CrossRef]
- Saure, E.W.; Eagan, T.M.; Jensen, R.L.; Bakke, P.S.; Johannessen, A.; Aanerud, M.; Nilsen, R.M.; Thorsen, E.; Hardie, J.A. Predictors for PaO2 and hypoxemic respiratory failure in COPD-A three-year follow-up. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 531–538. [Google Scholar] [CrossRef]
- Mannino, D.M.; Tal-Singer, R.; Lomas, D.A.; Vestbo, J.; Graham Barr, R.; Tetzlaff, K.; Lowings, M.; Rennard, S.I.; Snyder, J.; Goldman, M.; et al. Plasma Fibrinogen as a Biomarker for Mortality and Hospitalized Exacerbations in People with COPD. Chronic Obstr. Pulm. Dis. 2015, 2, 23–34. [Google Scholar] [CrossRef]
- Singh, D.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Lange, P.; Lettis, S.; Lipson, D.A.; Mannino, D.; Martin, N.; et al. InforMing the PAthway of COPD Treatment (IMPACT) trial: Fibrinogen levels predict risk of moderate or severe exacerbations. Respir. Res. 2021, 22, 130. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; Macnee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.H.; Kim, Y.; Kim, K.; Oh, Y.-M.; Yoo, K.H.; Rhee, C.K.; Yoon, H.K.; Kim, Y.S.; Park, Y.B.; et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: A national cross-sectional cohort study. BMC Pulm. Med. 2013, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, R.; Lee, S.W. Effects of gastroesophageal reflux disease treatment with proton pump inhibitors on the risk of acute exacerbation and pneumonia in patients with COPD. Respir. Res. 2023, 24, 75. [Google Scholar] [CrossRef]
- Halpin, D.M.G.; Decramer, M.; Celli, B.; Kesten, S.; Leimer, I.; Tashkin, D.P. Risk of Nonlower Respiratory Serious Adverse Events Following COPD Exacerbations in the 4-year UPLIFT® Trial. Lung 2011, 189, 261–268. [Google Scholar] [CrossRef]
- Husebø, G.R.; Bakke, P.S.; Aanerud, M.; Hardie, J.A.; Ueland, T.; Grønseth, R.; Persson, L.J.P.; Aukrust, P.; Eagan, T.M. Predictors of Exacerbations in Chronic Obstructive Pulmonary Disease—Results from the Bergen COPD Cohort Study. PLoS ONE 2014, 9, e109721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hillas, G.; Perlikos, F.; Tzanakis, N. Acute exacerbation of COPD: Is it the “stroke of the lungs”? Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Kunisaki, K.M.; Strand, M.J.; Anzueto, A.; Bhatt, S.P.; Bowler, R.P.; Criner, G.J.; Curtis, J.L.; Hanania, N.A.; Nath, H.; et al. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 195, 324–330. [Google Scholar] [CrossRef]
- Wan, E.S.; Hokanson, J.E.; Murphy, J.R.; Regan, E.A.; Make, B.J.; Lynch, D.A.; Crapo, J.D.; Silverman, E.K. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am. J. Respir. Crit. Care Med. 2011, 184, 57–63. [Google Scholar] [CrossRef]
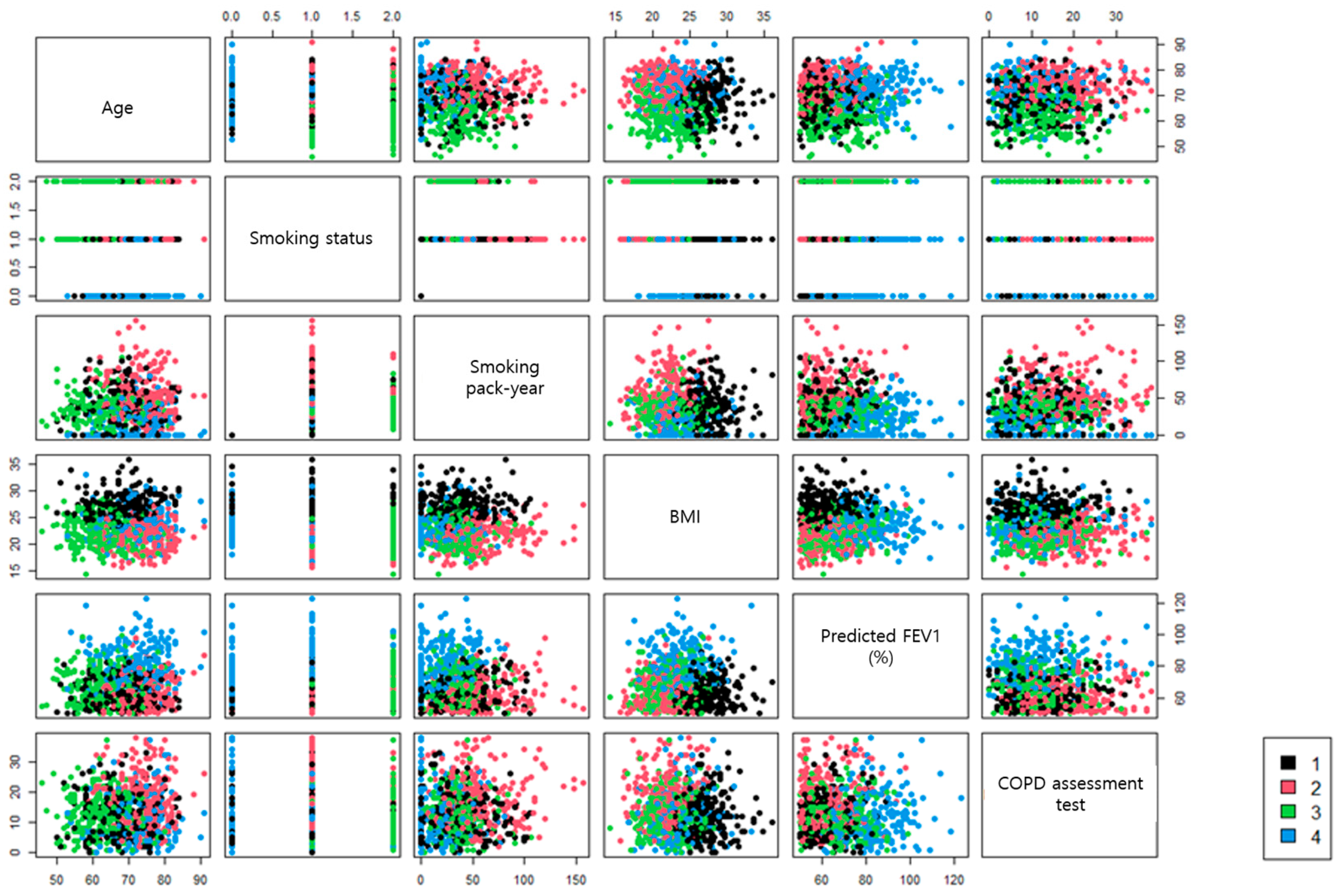

| Chronic Bronchitis (N = 224) | Emphysema (N = 235) | Young Smokers (N = 248) | Near Normal (N = 217) | p-Value | |
|---|---|---|---|---|---|
| Age, yr, | 68.6 ± 6.7 | 74.2 ± 5.4 | 63.3 ± 6.6 | 71.9 ± 6.6 | <0.001 |
| Male | 210 (93.8%) | 230 (97.9%) | 236 (95.2%) | 177 (81.6%) | <0.001 |
| Height, cm | 165.1 ± 7.1 | 164.3 ± 6.2 | 165.5 ± 6.3 | 163.1 ± 7.9 | 0.004 |
| Weight, kg | 73.9 ± 8.8 | 58.6 ± 7.8 | 61.5 ± 8.5 | 62.9 ± 8.9 | <0.001 |
| BMI, kg/m2 | 27.1 ± 2.4 | 21.7 ± 2.3 | 22.4 ± 2.6 | 23.6 ± 2.7 | <0.001 |
| Smoking status | <0.001 | ||||
| Never smoker | 13 (5.8%) | 1 (0.4%) | 0 (0.0%) | 62 (28.6%) | |
| Former smoker | 196 (87.5%) | 199 (84.7%) | 66 (26.6%) | 152 (70.0%) | |
| Current smoker | 15 (6.7%) | 35 (14.9%) | 182 (73.4%) | 3 (1.4%) | |
| Smoking age, yr | 22.6 ± 9.0 | 23.3 ± 12.1 | 22.0 ± 8.4 | 25.7 ± 11.9 | <0.001 |
| Smoking, pack-yrs | 42.5 ± 21.8 | 55.3 ± 27.7 | 37.1 ± 16.0 | 29.8 ± 16.4 | <0.001 |
| Pulmonary function test | |||||
| FEV1, % predicted | 62.3 ± 8.5 | 62.5 ± 9.3 | 66.9 ± 10.3 | 80.8 ± 12.9 | <0.001 |
| FVC, % predicted | 79.4 ± 13.9 | 82.7 ± 13.0 | 88.9 ± 12.2 | 92.4 ± 13.6 | <0.001 |
| DLCO, % predicted | 72.7 ± 19.7 | 58.7 ± 19.1 | 70.0 ± 19.6 | 73.8 ± 19.6 | <0.001 |
| FRC, % predicted | 106.6 ± 26.9 | 115.1 ± 28.2 | 117.1 ± 24.1 | 102.4 ± 24.8 | <0.001 |
| RV, % predicted | 95.8 ± 33.2 | 104.4 ± 39.1 | 98.8 ± 33.2 | 83.3 ± 33.5 | <0.001 |
| TLC, L | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | <0.001 |
| Symptom scores | |||||
| CAT score | 12.1 ± 6.7 | 17.5 ± 8.1 | 12.2 ± 6.5 | 11.4 ± 7.4 | <0.001 |
| SGRQ score | 27.8 ±15.1 | 36.6 ± 18.9 | 24.8 ± 12.8 | 11.4 ± 7.4 | <0.001 |
| 6 min walk distance, m | 396.8 ± 115.9 | 351.7 ± 119.3 | 427.4 ± 99.4 | 388.2 ± 113.9 | <0.001 |
| Dyspnea score after 6MWT | 1.7 ± 1.6 | 1.9 ± 1.8 | 1.3 ± 1.3 | 1.2 ± 1.3 | <0.001 |
| Laboratory findings | |||||
| WBCs, ×103/mm3 | 7.4 ± 2.5 | 7.4 ± 2.2 | 7.6 ± 2.2 | 6.9 ± 2.1 | 0.002 |
| Hb, g/dL | 14..3 ± 1.7 | 13.8 ± 1.6 | 14.5 ± 1.4 | 14.1 ± 1.4 | <0.001 |
| ESR, mm/h | 15.9 ± 17.1 | 20.1 ± 19.3 | 15.7 ± 16.4 | 14.6 ± 16.5 | <0.001 |
| Neutrophil, % | 56.5 ± 11.7 | 60.7 ± 11.6 | 56.1 ± 10.9 | 57.2 ± 10.3 | <0.001 |
| Lymphocyte, % | 30.3 ± 9.5 | 26.6 ± 9.5 | 31.6 ± 9.1 | 30.1 ± 8.4 | <0.001 |
| Monocyte, % | 7.8 ± 2.4 | 7.6 ± 2.3 | 7.7 ± 4.5 | 8.1 ± 2.9 | <0.001 |
| Albumin, g/dL | 4.4 ± 0.4 | 4.3 ± 0.4 | 4.4 ± 0.4 | 4.4 ± 0.4 | <0.001 |
| NT Pro-BNP, pg/mL | 216.4 ± 551.5 | 325.5 ± 847.9 | 91.8 ± 167.5 | 118.5 ± 256.7 | <0.001 |
| D-dimer, ug/mL | 0.7 ± 0.9 | 0.6 ± 0.5 | 0.5 ± 1.3 | 0.6 ± 0.7 | 0.001 |
| Fibrinogen, mg/dL | 332.9 ± 108.8 | 341.6 ± 101.5 | 324.4 ± 98.2 | 305.6 ± 73.8 | <0.001 |
| Chronic Bronchitis (N = 224) | Emphysema (N = 235) | Young Smokers (N = 248) | Near Normal (N = 217) | p-Value | |
|---|---|---|---|---|---|
| Myocardial infarction | 12 (5.4%) | 16 (6.8%) | 5 (2.0%) | 11 (5.1%) | 0.089 |
| Heart failure | 9 (4.0%) | 8 (3.4%) | 5 (2.0%) | 6 (2.8%) | 0.621 |
| Peripheral vascular disease | 5 (2.2%) | 9 (3.8%) | 4 (1.6%) | 1 (0.5%) | 0.086 |
| Diabetes mellitus | 69 (30.8%) | 41 (17.5%) | 41 (16.7%) | 29 (13.4%) | 0.000 |
| Hypertension | 124 (55.9%) | 94 (40.2%) | 93 (37.7%) | 91 (42.1%) | 0.000 |
| Osteoporosis | 10 (4.5%) | 8 (3.4%) | 9 (3.7%) | 11 (5.1%) | 0.797 |
| GERD | 38 (17.0%) | 30 (12.8%) | 33 (13.4%) | 44 (20.3%) | 0.103 |
| Hyperlipidemia | 42 (18.9%) | 32 (13.7%) | 29 (11.7%) | 30 (14.0%) | 0.159 |
| Thyroid | 8 (3.6%) | 4 (1.7%) | 4 (1.6%) | 12 (5.6%) | 0.046 |
| Inflammatory bowel disease | 1 (0.4%) | 3 (1.3%) | 1 (0.4%) | 1 (0.5%) | 0.586 |
| Asthma | 82 (39.8%) | 83 (38.6%) | 74 (32.6%) | 46 (23.4%) | 0.002 |
| Chronic Bronchitis (N = 224) | Emphysema (N = 235) | Young Smokers (N = 248) | Near Normal (N = 217) | p-Value | |
|---|---|---|---|---|---|
| Drug | 191 (88.8%) | 200 (90.5%) | 212 (91.8%) | 173 (84.0%) | 0.056 |
| ICSs | 4 (1.9%) | 2 (0.9%) | 1 (0.4%) | 0 (0.0%) | 0.163 |
| LABAs | 27 (12.6%) | 43 (19.5%) | 29 (12.6%) | 25 (12.1%) | 0.081 |
| LAMAs | 113 (52.6%) | 109 (49.3%) | 129 (55.8%) | 83 (40.3%) | 0.009 |
| LABAs + LAMAs | 24 (11.2%) | 29 (13.1%) | 36 (15.6%) | 32 (15.5%) | 0.481 |
| ICSs + LABAs | 73 (34.0%) | 75 (33.9%) | 64 (27.7%) | 42 (20.4%) | 0.005 |
| PDE4 inhibitor | 13 (6.0%) | 8 (3.6%) | 6 (2.6%) | 6 (2.9%) | 0.225 |
| Methylxanthine | 57 (26.5%) | 76 (34.4%) | 58 (25.1%) | 47 (22.8%) | 0.040 |
| Chronic Bronchitis (N = 224) | Emphysema (N = 235) | Young Smokers (N = 248) | Near Normal (N = 217) | p-Value | |
|---|---|---|---|---|---|
| Moderate exacerbation | 68 (30.4%) | 86 (36.6%) | 72 (29.0%) | 52 (24.0%) | 0.033 |
| Severe exacerbation | 17 (7.6%) | 14 (6.0%) | 10 (4.0%) | 7 (3.2%) | 0.153 |
| Moderate exacerbation (frequency) | 0.7 ± 1.4 | 0.8 ± 1.6 | 0.5 ± 1.1 | 0.5 ± 1.3 | 0.019 |
| Severe exacerbation (frequency) | 0.1 ± 0.3 | 0.1 ± 0.6 | 0.0 ± 0.2 | 0.0 ± 0.2 | 0.146 |
| Death | 2 (0.9%) | 9 (3.8%) | 1 (0.4%) | 2 (0.9%) | 0.009 |
| Odds Ratio | 95% Confidence Intervals | p | |
|---|---|---|---|
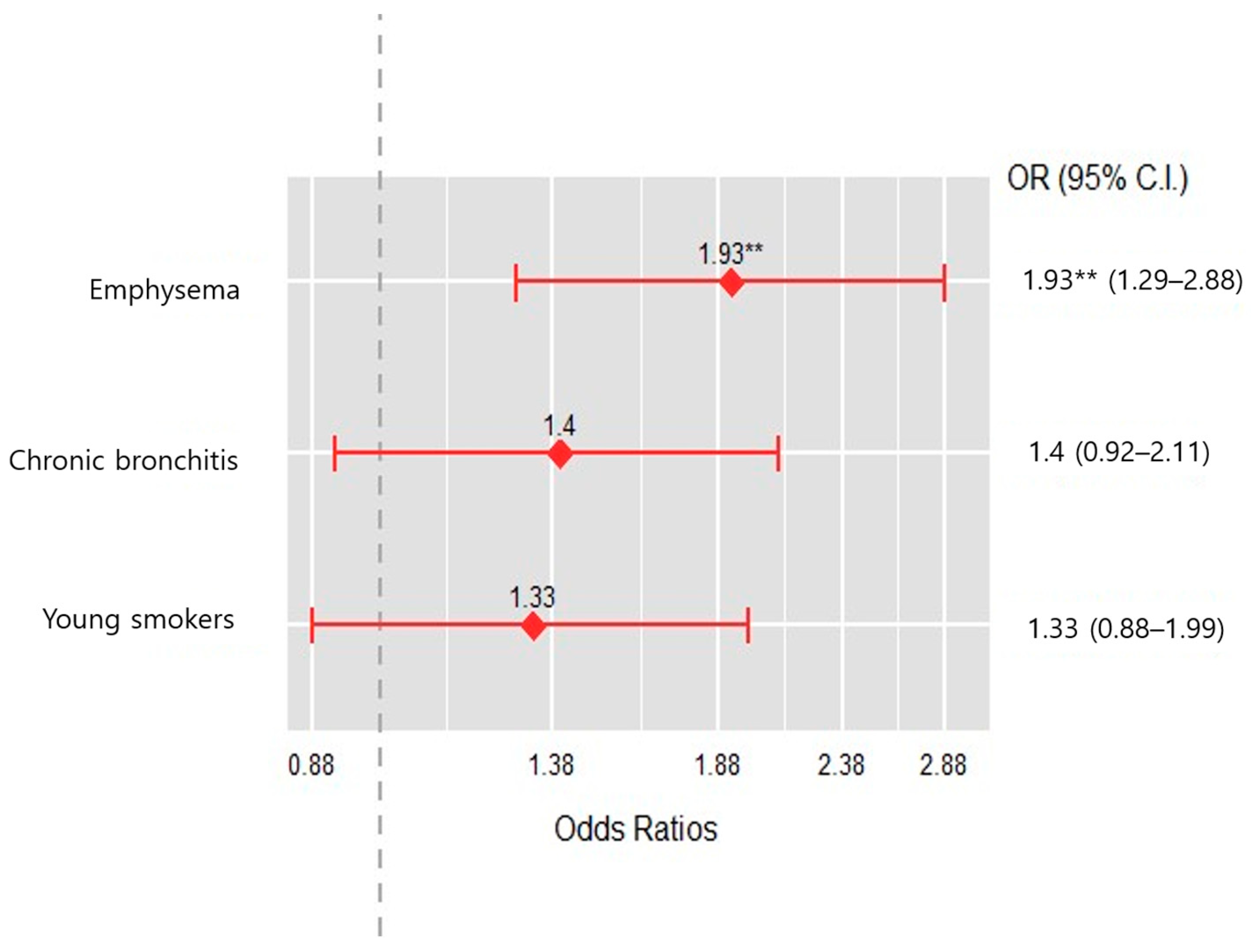
| Young smokers cluster | 1.704 | 0.638–4.688 | 0.292 |
| Emphysema cluster | 2.834 | 0.893–9.237 | 0.078 |
| Chronic bronchitis cluster | 2.887 | 1.065–8.192 | 0.040 |
| Post-bronchodilator FVC, % predicted | 1.007 | 0.978–1.036 | 0.661 |
| Functional residual capacity, % predicted | 1.023 | 1.007–1.040 | 0.007 |
| Past history of asthma diagnosis | 1.527 | 0.549–4.045 | 0.402 |
| 6 min walk distance, m | 0.999 | 0.996–1.003 | 0.693 |
| White blood cells, ×103/mm3 | 1.133 | 0.953–1.351 | 0.156 |
| Monocyte, % | 0.922 | 0.776–1.084 | 0.336 |
| Albumin, g/dL | 0.470 | 0.149–1.440 | 0.190 |
| Fibrinogen, mg/dL | 1.004 | 1.001–1.008 | 0.015 |
| Osteoporosis | 0.471 | 0.045–3.120 | 0.470 |
| Gastroesophageal reflux diseases | 2.646 | 1.142–6.181 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.E.; Kang, E.-H.; Jung, J.Y.; Lee, C.Y.; Lee, W.Y.; Lim, S.Y.; Park, D.I.; Yoo, K.H.; Jung, K.-S.; Lee, J.H. Subtypes of Patients with Mild to Moderate Airflow Limitation as Predictors of Chronic Obstructive Pulmonary Disease Exacerbation. J. Clin. Med. 2023, 12, 6643. https://doi.org/10.3390/jcm12206643
Kim NE, Kang E-H, Jung JY, Lee CY, Lee WY, Lim SY, Park DI, Yoo KH, Jung K-S, Lee JH. Subtypes of Patients with Mild to Moderate Airflow Limitation as Predictors of Chronic Obstructive Pulmonary Disease Exacerbation. Journal of Clinical Medicine. 2023; 12(20):6643. https://doi.org/10.3390/jcm12206643
Chicago/Turabian StyleKim, Nam Eun, Eun-Hwa Kang, Ji Ye Jung, Chang Youl Lee, Won Yeon Lee, Seong Yong Lim, Dong Il Park, Kwang Ha Yoo, Ki-Suck Jung, and Jin Hwa Lee. 2023. "Subtypes of Patients with Mild to Moderate Airflow Limitation as Predictors of Chronic Obstructive Pulmonary Disease Exacerbation" Journal of Clinical Medicine 12, no. 20: 6643. https://doi.org/10.3390/jcm12206643
APA StyleKim, N. E., Kang, E.-H., Jung, J. Y., Lee, C. Y., Lee, W. Y., Lim, S. Y., Park, D. I., Yoo, K. H., Jung, K.-S., & Lee, J. H. (2023). Subtypes of Patients with Mild to Moderate Airflow Limitation as Predictors of Chronic Obstructive Pulmonary Disease Exacerbation. Journal of Clinical Medicine, 12(20), 6643. https://doi.org/10.3390/jcm12206643






