Exploring Current Concepts and Challenges in the Identification and Management of Early-Stage COPD
Abstract
1. Introduction
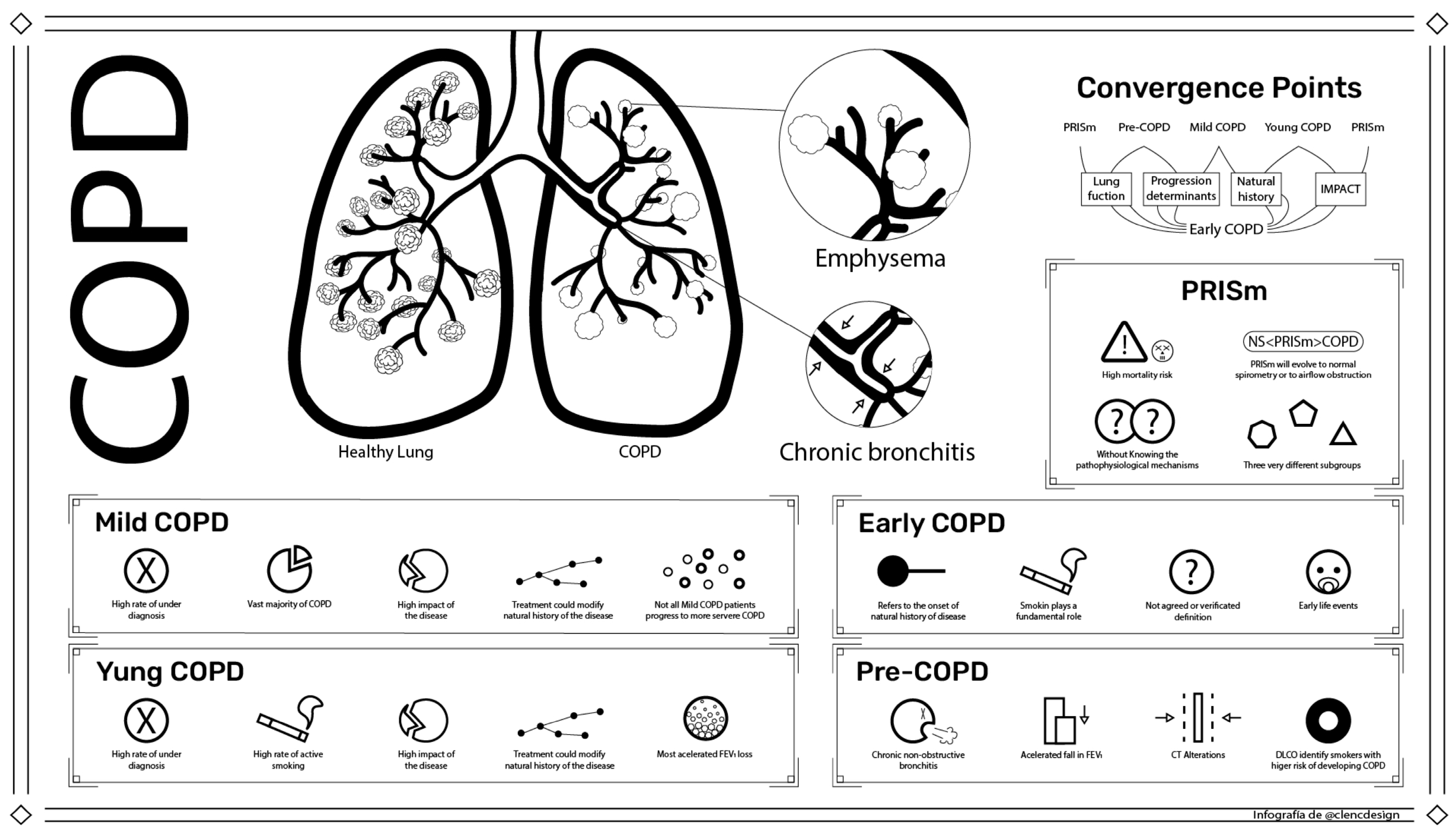
2. Mild COPD
2.1. Concept and Importance
2.2. Limitations
2.3. Summary
3. Young COPD
3.1. Concept and Importance
3.2. Limitations
3.3. Summary
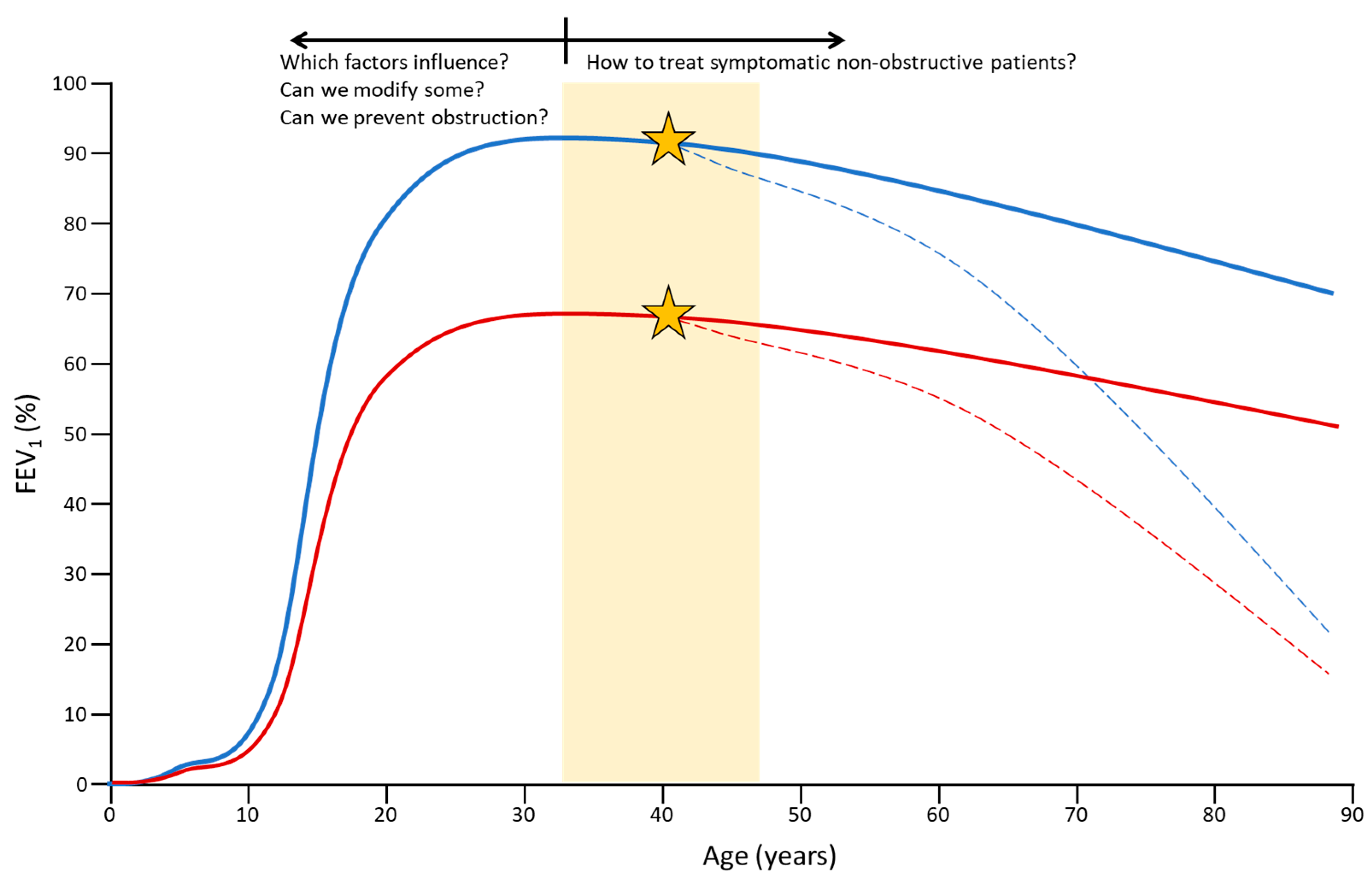
4. Early COPD
4.1. Concept and Importance
4.2. Limitations
4.3. Summary
5. Pre-COPD
5.1. Concept and Importance
5.2. Limitations
5.3. Summary
6. PRISm
6.1. Concept and Importance
6.2. Limitations
- Secondly, this restrictive pattern could also be due to different comorbidities, such as associated pulmonary fibrosis or heart failure, and the presence of bronchiectasis or mutual or many other respiratory and non-respiratory comorbidities [72].
- Thirdly, the situation could arise where the spirometry shows a normal FEV1/FVC ratio and a normal FVC, but decreased FEV1. This latter circumstance is a spirometric pattern with uncertain consequences that should be further explored in future research.
6.3. Summary
7. Bronchodilators in Non-Obstructive Lung Disease
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipson, D.A.; Crim, C.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; Kilbride, S.; Lange, P.; et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2020, 201, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Rabe, K.F.; Ferguson, G.T.; Wedzicha, J.A.; Singh, D.; Wang, C.; Rossman, K.; St Rose, E.; Trivedi, R.; Ballal, S.; et al. Reduced all-cause mortality in the ethos trial of budesonide/glycopyrrolate/formoterol for copd: A randomized, double-blind, multi-center parallel-group study. Am. J. Respir. Crit. Care Med. 2020, 203, 553–564. [Google Scholar] [CrossRef]
- Lindenauer, P.K.; Stefan, M.S.; Pekow, P.S.; Mazor, K.M.; Priya, A.; Spitzer, K.A.; Lagu, T.C.; Pack, Q.R.; Pinto-Plata, V.M.; ZuWallack, R. Association between initiation of pulmonary rehabilitation after hospitalization for copd and 1-year survival among medicare beneficiaries. JAMA 2020, 323, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Cosio, B.G.; Hernandez, C.; Chiner, E.; Gimeno-Santos, E.; Pleguezuelos, E.; Seijas, N.; Rigau, D.; Lopez-Campos, J.L.; Soler-Cataluna, J.J.; Calle, M.; et al. Spanish copd guidelines (gesepoc 2021): Non-pharmacological treatment update. Arch. Bronconeumol. 2022, 58, 345–351. [Google Scholar] [CrossRef]
- Ancochea, J.; Soriano, J.B. Copd in spain at the start of a new decade. Arch. Bronconeumol. 2021, 57, 1–2. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax 2012, 67, 957–963. [Google Scholar] [CrossRef]
- Ezponda, A.; Casanova, C.; Cabrera, C.; Martin-Palmero, Á.; Marin-Oto, M.; Marín, J.M.; Pinto-Plata, V.; Divo, M.; Celli, B.R.; Zulueta, J.J.; et al. Psoas muscle density evaluated by chest ct and long-term mortality in copd patients. Arch. Bronconeumol. 2021, 57, 533–539. [Google Scholar] [CrossRef]
- Rodrigo-Troyano, A.; Giner, J.; Perea, L.; Merino, J.L.; Albacar, N.; Solarat, B.; Castillo, D.; Faner, R.; Agustí, A.; Sibila, O. Predicting early hospital readmissions in copd patients using an electronic nose. Arch. Bronconeumol. 2022, 58, 663–665. [Google Scholar] [CrossRef]
- Agusti, A.; Alcazar, B.; Cosio, B.; Echave, J.M.; Faner, R.; Izquierdo, J.L.; Marin, J.M.; Soler-Cataluña, J.J.; Celli, B. Time for a change: Anticipating the diagnosis and treatment of copd. Eur. Respir. J. 2020, 56, 2002104. [Google Scholar] [CrossRef]
- Agustí, A.; Alcázar, B.; Ancochea, J.; Casanova, C.; Celli, B.; Cosio, B.; Echave-Sustaeta, J.M.; Fernandez Villar, A.; Garcia Rivero, J.L.; González, C.; et al. The antes program in copd: First year. Arch. Bronconeumol. 2022, 58, 291–294. [Google Scholar] [CrossRef]
- Morice, A.H.; Celli, B.; Kesten, S.; Lystig, T.; Tashkin, D.; Decramer, M. Copd in young patients: A pre-specified analysis of the four-year trial of tiotropium (uplift). Respir. Med. 2010, 104, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Celli, B.R.; Decramer, M.; Lystig, T.; Liu, D.; Kesten, S. Efficacy of tiotropium in copd patients with fev1 >/= 60% participating in the uplift(r) trial. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 289–296. [Google Scholar] [CrossRef]
- Decramer, M.; Celli, B.; Kesten, S.; Lystig, T.; Mehra, S.; Tashkin, D.P. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (uplift): A prespecified subgroup analysis of a randomised controlled trial. Lancet 2009, 374, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Kunisaki, K.M.; Strand, M.J.; Anzueto, A.; Bhatt, S.P.; Bowler, R.P.; Criner, G.J.; Curtis, J.L.; Hanania, N.A.; Nath, H.; et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017, 195, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kohansal, R.; Martinez-Camblor, P.; Agusti, A.; Buist, A.S.; Mannino, D.M.; Soriano, J.B. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am. J. Respir. Crit. Care Med. 2009, 180, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.R.; Jones, P.W.; Calverley, P.M.; Celli, B.; Anderson, J.A.; Ferguson, G.T.; Yates, J.C.; Willits, L.R.; Vestbo, J. Efficacy of salmeterol/fluticasone propionate by gold stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled torch study. Respir. Res. 2009, 10, 59. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef]
- Galdiz, J.B.; Gomez, A.; Rodriguez, D.; Guell, R.; Cebollero, P.; Hueto, J.; Cejudo, P.; Ortega, F.; Sayago, I.; Chic, S.; et al. Telerehabilitation programme as a maintenance strategy for copd patients: A 12-month randomized clinical trial. Arch. Bronconeumol. 2021, 57, 195–204. [Google Scholar] [CrossRef]
- Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global initiative for chronic obstructive lung disease 2023 report: Gold executive summary. Arch. Bronconeumol. 2023, 59, 232–248. [Google Scholar] [CrossRef]
- Soriano, J.B.; Alfageme, I.; Miravitlles, M.; de Lucas, P.; Soler-Cataluna, J.J.; Garcia-Rio, F.; Casanova, C.; Rodriguez Gonzalez-Moro, J.M.; Cosio, B.G.; Sanchez, G.; et al. Prevalence and determinants of copd in Spain: Episcan ii. Arch. Bronconeumol. 2021, 57, 61–69. [Google Scholar] [CrossRef]
- James, M.D.; Milne, K.M.; Phillips, D.B.; Neder, J.A.; O’Donnell, D.E. Dyspnea and exercise limitation in mild copd: The value of cpet. Front. Med. 2020, 7, 442. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Mullerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; Macnee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Brusselle, G.; Dal Negro, R.W.; Ferrer, M.; Kardos, P.; Levy, M.L.; Perez, T.; Soler-Cataluna, J.J.; van der Molen, T.; Adamek, L.; et al. Health-related quality of life in patients by copd severity within primary care in europe. Respir. Med. 2011, 105, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Doherty, D.E.; Sonia Buist, A. Global initiative on obstructive lung disease (gold) classification of lung disease and mortality: Findings from the atherosclerosis risk in communities (aric) study. Respir. Med. 2006, 100, 115–122. [Google Scholar] [CrossRef]
- García-Sanz, M.T.; González-Barcala, F.J. Copd is more than just lung function: Let’s not forget depression. Arch. Bronconeumol. 2021, 57, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Banjade, P.; Kandel, K.; Itani, A.; Adhikari, S.; Basnet, Y.M.; Sharma, M.; Surani, S. The interplay between obstructive sleep apnea, chronic obstructive pulmonary disease, and congestive heart failure: Time to collectively refer to them as triple overlap syndrome? Medicina 2023, 59, 1374. [Google Scholar]
- Tashkin, D.P.; Li, N.; Kleerup, E.C.; Halpin, D.; Celli, B.; Decramer, M.; Elashoff, R. Acute bronchodilator responses decline progressively over 4 years in patients with moderate to very severe copd. Respir. Res. 2014, 15, 102. [Google Scholar] [CrossRef]
- Welte, T.; Vogelmeier, C.; Papi, A. Copd: Early diagnosis and treatment to slow disease progression. Int. J. Clin. Pract. 2015, 69, 336–349. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Jimenez-Ruiz, C.A.; Meneses Petersen, E.D.; Rabade Castedo, C.; Asensio Sanchez, S.; Vaquero Lozano, P.; Ferrer Espinosa, S.; Perez Soriano, M.D.P.; de Higes Martinez, E.; Garcia de Llanos, C.; et al. Smoking cessation units as a source of copd diagnoses: Project 1000-200. Arch. Bronconeumol. 2021, 58, 264–267. [Google Scholar] [CrossRef]
- Vestbo, J.; Lange, P. Can gold stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am. J. Respir. Crit. Care Med. 2002, 166, 329–332. [Google Scholar] [CrossRef]
- Agusti, A.; Noell, G.; Brugada, J.; Faner, R. Lung function in early adulthood and health in later life: A transgenerational cohort analysis. Lancet Respir. Med. 2017, 5, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Delgado Pecellin, I.; Quintana-Gallego, E.; Moreno Valera, M.J.; Moreno Ortega, M.; Carrasco Hernandez, L.; Lopez-Campos, J.L. Lung growth and aging: A complex and increasingly confounding network. Arch. Bronconeumol. 2019, 55, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Cosio, B.G.; Pascual-Guardia, S.; Borras-Santos, A.; Peces-Barba, G.; Santos, S.; Vigil, L.; Soler-Cataluna, J.J.; Martinez-Gonzalez, C.; Casanova, C.; Marcos, P.J.; et al. Phenotypic characterisation of early copd: A prospective case-control study. ERJ Open. Res. 2020, 6, 00047–2020. [Google Scholar] [CrossRef] [PubMed]
- Oelsner, E.C.; Smith, B.M.; Hoffman, E.A.; Folsom, A.R.; Kawut, S.M.; Kaufman, J.D.; Manichaikul, A.; Lederer, D.J.; Schwartz, J.E.; Watson, K.E.; et al. Associations between emphysema-like lung on ct and incident airflow limitation: A general population-based cohort study. Thorax 2018, 73, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Crim, C.; Celli, B.; Edwards, L.D.; Wouters, E.; Coxson, H.O.; Tal-Singer, R.; Calverley, P.M. Respiratory system impedance with impulse oscillometry in healthy and copd subjects: Eclipse baseline results. Respir. Med. 2011, 105, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, D.; Contoli, M.; Braido, F.; Maniscalco, M.; Micheletto, C.; Solidoro, P.; Santus, P.; Carone, M. Future perspectives of revaluating mild copd. Respiration 2022, 101, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Golpe, R.; Dacal-Rivas, D.; Blanco-Cid, N.; Castro-Añón, O. Need for epidemiological studies on chronic obstructive pulmonary disease in rural Spain. Arch. Bronconeumol. 2021, 57, 614–615. [Google Scholar] [CrossRef]
- Richmond, R.J.; Zellner, K.M. Alpha1-antitrypsin deficiency: Incidence and implications. Dimens. Crit. Care Nurs. 2005, 24, 255–260; quiz 252–261. [Google Scholar] [CrossRef]
- López-Campos, J.L.; Carrasco Hernández, L.; Ruiz-Duque, B.; Reinoso-Arija, R.; Caballero-Eraso, C. Step-up and step-down treatment approaches for copd: A holistic view of progressive therapies. Int. J. Chron. Obstruct Pulmon Dis. 2021, 16, 2065–2076. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Abed, A.; Jarrar, Y.B.; Alshammari, F.; Alshammari, B.; Basheti, I.A.; Zihlif, M. Alteration of the respiratory microbiome in hospitalized patients with asthma-copd overlap during and after an exacerbation. J. Clin. Med. 2023, 12, 2118. [Google Scholar] [CrossRef]
- Homętowska, H.; Klekowski, J.; Świątoniowska-Lonc, N.; Jankowska-Polańska, B.; Chabowski, M. Fatigue, depression, and anxiety in patients with copd, asthma and asthma-copd overlap. J. Clin. Med. 2022, 11, 7466. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Agusti, A.; Celli, B.R.; Han, M.K.; Allinson, J.P.; Bhatt, S.P.; Calverley, P.; Chotirmall, S.H.; Chowdhury, B.; Darken, P.; et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: Time to move forward. Am. J. Respir. Crit. Care Med. 2022, 205, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D. Copd and the response of the lung to tobacco smoke exposure. Pulm. Pharmacol. Ther. 2010, 23, 376–383. [Google Scholar] [CrossRef]
- Calzetta, L.; Rogliani, P.; Facciolo, F.; Rinaldi, B.; Cazzola, M.; Matera, M.G. N-acetylcysteine protects human bronchi by modulating the release of neurokinin a in an ex vivo model of copd exacerbation. Biomed. Pharmacother. 2018, 103, 1–8. [Google Scholar] [CrossRef]
- Martinez, F.J.; Han, M.K.; Allinson, J.P.; Barr, R.G.; Boucher, R.C.; Calverley, P.M.A.; Celli, B.R.; Christenson, S.A.; Crystal, R.G.; Fagerås, M.; et al. At the root: Defining and halting progression of early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1540–1551. [Google Scholar] [CrossRef]
- Petersen, H.; Sood, A.; Polverino, F.; Owen, C.A.; Pinto-Plata, V.; Celli, B.R.; Tesfaigzi, Y. The course of lung function in middle-aged heavy smokers: Incidence and time to early onset of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1449–1451. [Google Scholar] [CrossRef]
- Soriano, J.B.; Polverino, F.; Cosio, B.G. What is early copd and why is it important? Eur. Respir. J. 2018, 52, 1801448. [Google Scholar] [CrossRef]
- Díaz-Peña, R.; Silva, R.S.; Hosgood, H.D., 3rd; Jaime, S.; Miravitlles, M.; Olloquequi, J. Hla-drb1 alleles are associated with copd in a Latin American admixed population. Arch. Bronconeumol. 2021, 57, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Peña, R.; Julià, R.F.; Montes, J.F.; Silva, R.S.; Olloquequi, J. Polymorphisms in the frmd4a gene are associated with chronic obstructive pulmonary disease susceptibility in a Latin American population. Arch. Bronconeumol. 2022, 58, 454–456. [Google Scholar] [CrossRef]
- Díaz-Peña, R.; Silva, R.S.; Hosgood, H.D., 3rd; Agustí, À.; Olloquequi, J. Specific mirna profile in chronic obstructive pulmonary disease related to biomass smoke exposure. Arch. Bronconeumol. 2022, 58, 177–179. [Google Scholar] [CrossRef]
- Yang, W.; Li, F.; Li, C.; Meng, J.; Wang, Y. Focus on early copd: Definition and early lung development. Int. J. Chron. Obstruct Pulmon Dis. 2021, 16, 3217–3228. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Lange, P.; Vestbo, J. Importance of early copd in young adults for development of clinical copd: Findings from the Copenhagen general population study. Am. J. Respir. Crit. Care Med. 2021, 203, 1245–1256. [Google Scholar] [CrossRef]
- García-Quero, C.; García-Río, F. Smoking-induced small airway dysfunction. An early marker of future copd? Arch. Bronconeumol. 2021, 57, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Fazleen, A.; Wilkinson, T. Early copd: Current evidence for diagnosis and management. Ther. Adv. Respir. Dis. 2020, 14, 1753466620942128. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.M.G.; Roche, N.; Papi, A.; Stockley, R.A.; Wedzicha, J.; Vogelmeier, C.F. From gold 0 to pre-copd. Am. J. Respir. Crit. Care Med. 2021, 203, 414–423. [Google Scholar] [CrossRef]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future copd risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, C.H.; Hwang, H.G.; Kim, Y.I.; Kim, D.K.; Oh, Y.M.; Lee, S.H.; Kim, K.U.; Lee, S.D. Decline in carbon monoxide transfer coefficient in chronic obstructive pulmonary disease. J. Clin. Med. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Mohamed Hoesein, F.A.; de Jong, P.A.; Lammers, J.W.; Mali, W.P.; Schmidt, M.; de Koning, H.J.; van der Aalst, C.; Oudkerk, M.; Vliegenthart, R.; Groen, H.J.; et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur. Respir. J. 2015, 45, 644–651. [Google Scholar] [CrossRef]
- Celli, B.R.; Agusti, A. Copd: Time to improve its taxonomy? ERJ Open. Res. 2018, 4, 00132–2017. [Google Scholar] [CrossRef]
- Gogali, A.; Kostikas, K. Latent copd: A proposed new term in the disease nomenclature. Eur. Respir. J. 2023, 61, 2300535. [Google Scholar] [CrossRef]
- Cosío, B.G.; Casanova, C.; Soler-Cataluña, J.J.; Soriano, J.B.; García-Río, F.; de Lucas, P.; Alfageme, I.; Rodríguez González-Moro, J.M.; Sánchez, G.; Ancochea, J.; et al. Unravelling young copd and pre-copd in the general population. ERJ Open. Res. 2023, 9, 00334–2022. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.S.; Castaldi, P.J.; Cho, M.H.; Hokanson, J.E.; Regan, E.A.; Make, B.J.; Beaty, T.H.; Han, M.K.; Curtis, J.L.; Curran-Everett, D.; et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (prism) in copdgene. Respir. Res. 2014, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Wijnant, S.R.A.; De Roos, E.; Kavousi, M.; Stricker, B.H.; Terzikhan, N.; Lahousse, L.; Brusselle, G.G. Trajectory and mortality of preserved ratio impaired spirometry: The rotterdam study. Eur. Respir. J. 2020, 55, 1901217. [Google Scholar] [CrossRef]
- Wan, E.S. The clinical spectrum of prism. Am. J. Respir. Crit. Care Med. 2022, 206, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.S.; Balte, P.; Schwartz, J.E.; Bhatt, S.P.; Cassano, P.A.; Couper, D.; Daviglus, M.L.; Dransfield, M.T.; Gharib, S.A.; Jacobs, D.R., Jr.; et al. Association between preserved ratio impaired spirometry and clinical outcomes in us adults. JAMA 2021, 326, 2287–2298. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, T.; Park, H.J.; Cho, J.H.; Byun, M.K. Long-term clinical outcomes of patients with chronic obstructive pulmonary disease with sarcopenia. Life 2023, 13, 1628. [Google Scholar] [CrossRef]
- Higbee, D.H.; Granell, R.; Davey Smith, G.; Dodd, J.W. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: A uk biobank cohort analysis. Lancet Respir. Med. 2022, 10, 149–157. [Google Scholar] [CrossRef]
- Wan, E.S.; Fortis, S.; Regan, E.A.; Hokanson, J.; Han, M.K.; Casaburi, R.; Make, B.J.; Crapo, J.D.; DeMeo, D.L.; Silverman, E.K. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the copdgene study. Am. J. Respir. Crit. Care Med. 2018, 198, 1397–1405. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. Ers/ats technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Chen, C.; Jian, W.; Gao, Y.; Xie, Y.; Song, Y.; Zheng, J. Early copd patients with lung hyperinflation associated with poorer lung function but better bronchodilator responsiveness. Int. J. Chron. Obstruct Pulmon Dis. 2016, 11, 2519–2526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez-Campos, J.L.; Almagro, P.; Gomez, J.T.; Chiner, E.; Palacios, L.; Hernandez, C.; Navarro, M.D.; Molina, J.; Rigau, D.; Soler-Cataluna, J.J.; et al. Spanish copd guideline (gesepoc) update: Comorbidities, self-management and palliative care. Arch. Bronconeumol. 2022, 58, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Washio, Y.; Sakata, S.; Fukuyama, S.; Honda, T.; Kan, O.K.; Shibata, M.; Hata, J.; Inoue, H.; Kitazono, T.; Matsumoto, K.; et al. Risks of mortality and airflow limitation in Japanese individuals with preserved ratio impaired spirometry. Am. J. Respir. Crit. Care Med. 2022, 206, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Cui, M.; Nong, Y.; Qin, J.; Mo, S. A longitudinal study of trajectories and factors influencing patient-reported outcomes in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct Pulmon Dis. 2022, 17, 2945–2956. [Google Scholar] [CrossRef]
- Galera, R.; Casitas, R.; Martínez-Cerón, E.; Rodríguez-Fraga, O.; Utrilla, C.; Torres, I.; Cubillos-Zapata, C.; García-Río, F. Effect of dynamic hyperinflation on cardiac response to exercise of patients with chronic obstructive pulmonary disease. Arch. Bronconeumol. 2021, 57, 406–414. [Google Scholar] [CrossRef]
- Monteagudo, M.; Nuñez, A.; Barrecheguren, M.; Miravitlles, M. Effectiveness of treatment with dual bronchodilation (laba/lama) compared with combination therapy (laba/ics) for patients with copd: A population-based study. Arch. Bronconeumol. 2022, 58, 699–707. [Google Scholar] [CrossRef]
- Celli, B.; ZuWallack, R.; Wang, S.; Kesten, S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in copd patients with increased static lung volumes. Chest 2003, 124, 1743–1748. [Google Scholar] [CrossRef]
- Di Marco, F.; Sotgiu, G.; Santus, P.; O’Donnell, D.E.; Beeh, K.M.; Dore, S.; Roggi, M.A.; Giuliani, L.; Blasi, F.; Centanni, S. Long-acting bronchodilators improve exercise capacity in copd patients: A systematic review and meta-analysis. Respir. Res. 2018, 19, 18. [Google Scholar] [CrossRef]
- Nishimura, K.; Kusunose, M.; Mori, M.; Shibayama, A.; Nakayasu, K. The conceptual independence of health status, respiratory symptoms and dyspnea in chronic obstructive pulmonary disease in real clinical practice. Diagnostics 2023, 13, 2492. [Google Scholar] [CrossRef]
- Terzano, C.; Petroianni, A.; Conti, V.; Ceccarelli, D.; Graziani, E.; Sanduzzi, A.; D’Avelli, S. Rational timing of combination therapy with tiotropium and formoterol in moderate and severe copd. Respir. Med. 2008, 102, 1701–1707. [Google Scholar] [CrossRef][Green Version]
- Yamada, H.; Matsumoto, I.; Makita, N.; Arita, Y.; Hayashi, N.; Mitsuoka, K.; Tashiro, N.; Hizawa, N. Effect of timing of bronchodilator therapy initiation on exacerbations in patients with chronic obstructive pulmonary disease: A retrospective cohort study. Respir. Res. 2022, 23, 255. [Google Scholar] [CrossRef]
- Lin, C.H.; Cheng, S.L.; Chen, C.Z.; Chen, C.H.; Lin, S.H.; Wang, H.C. Current progress of copd early detection: Key points and novel strategies. Int. J. Chron. Obstruct Pulmon Dis. 2023, 18, 1511–1524. [Google Scholar] [CrossRef]
- Mukherjee, N.; Arathimos, R.; Chen, S.; Kheirkhah Rahimabad, P.; Han, L.; Zhang, H.; Holloway, J.W.; Relton, C.; Henderson, A.J.; Arshad, S.H.; et al. DNA methylation at birth is associated with lung function development until age 26 years. Eur. Respir. J. 2021, 57, 2003505. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agusti, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- McGeachie, M.J.; Yates, K.P.; Zhou, X.; Guo, F.; Sternberg, A.L.; Van Natta, M.L.; Wise, R.A.; Szefler, S.J.; Sharma, S.; Kho, A.T.; et al. Patterns of growth and decline in lung function in persistent childhood asthma. N. Engl. J. Med. 2016, 374, 1842–1852. [Google Scholar] [CrossRef]
- Ross, J.C.; Castaldi, P.J.; Cho, M.H.; Hersh, C.P.; Rahaghi, F.N.; Sánchez-Ferrero, G.V.; Parker, M.M.; Litonjua, A.A.; Sparrow, D.; Dy, J.G.; et al. Longitudinal modeling of lung function trajectories in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1033–1042. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Matera, M.G. The future of bronchodilators in copd and asthma. Arch. Bronconeumol. 2022, 58, 107–108. [Google Scholar] [CrossRef]
- José Soler-Cataluña, J.; Miravitlles, M.; Fernández-Villar, A.; Izquierdo, J.L.; García-Rivero, J.L.; Cosio, B.G.; López-Campos, J.L.; Agustí, A. Exacerbations in copd: A personalised approach to care. Lancet Respir. Med. 2023, 11, 224–226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doña, E.; Reinoso-Arija, R.; Carrasco-Hernandez, L.; Doménech, A.; Dorado, A.; Lopez-Campos, J.L. Exploring Current Concepts and Challenges in the Identification and Management of Early-Stage COPD. J. Clin. Med. 2023, 12, 5293. https://doi.org/10.3390/jcm12165293
Doña E, Reinoso-Arija R, Carrasco-Hernandez L, Doménech A, Dorado A, Lopez-Campos JL. Exploring Current Concepts and Challenges in the Identification and Management of Early-Stage COPD. Journal of Clinical Medicine. 2023; 12(16):5293. https://doi.org/10.3390/jcm12165293
Chicago/Turabian StyleDoña, Esperanza, Rocío Reinoso-Arija, Laura Carrasco-Hernandez, Adolfo Doménech, Antonio Dorado, and José Luis Lopez-Campos. 2023. "Exploring Current Concepts and Challenges in the Identification and Management of Early-Stage COPD" Journal of Clinical Medicine 12, no. 16: 5293. https://doi.org/10.3390/jcm12165293
APA StyleDoña, E., Reinoso-Arija, R., Carrasco-Hernandez, L., Doménech, A., Dorado, A., & Lopez-Campos, J. L. (2023). Exploring Current Concepts and Challenges in the Identification and Management of Early-Stage COPD. Journal of Clinical Medicine, 12(16), 5293. https://doi.org/10.3390/jcm12165293





