Microvascular Obstruction in Acute Myocardial Infarction, a Potential Therapeutic Target
Abstract
1. Introduction
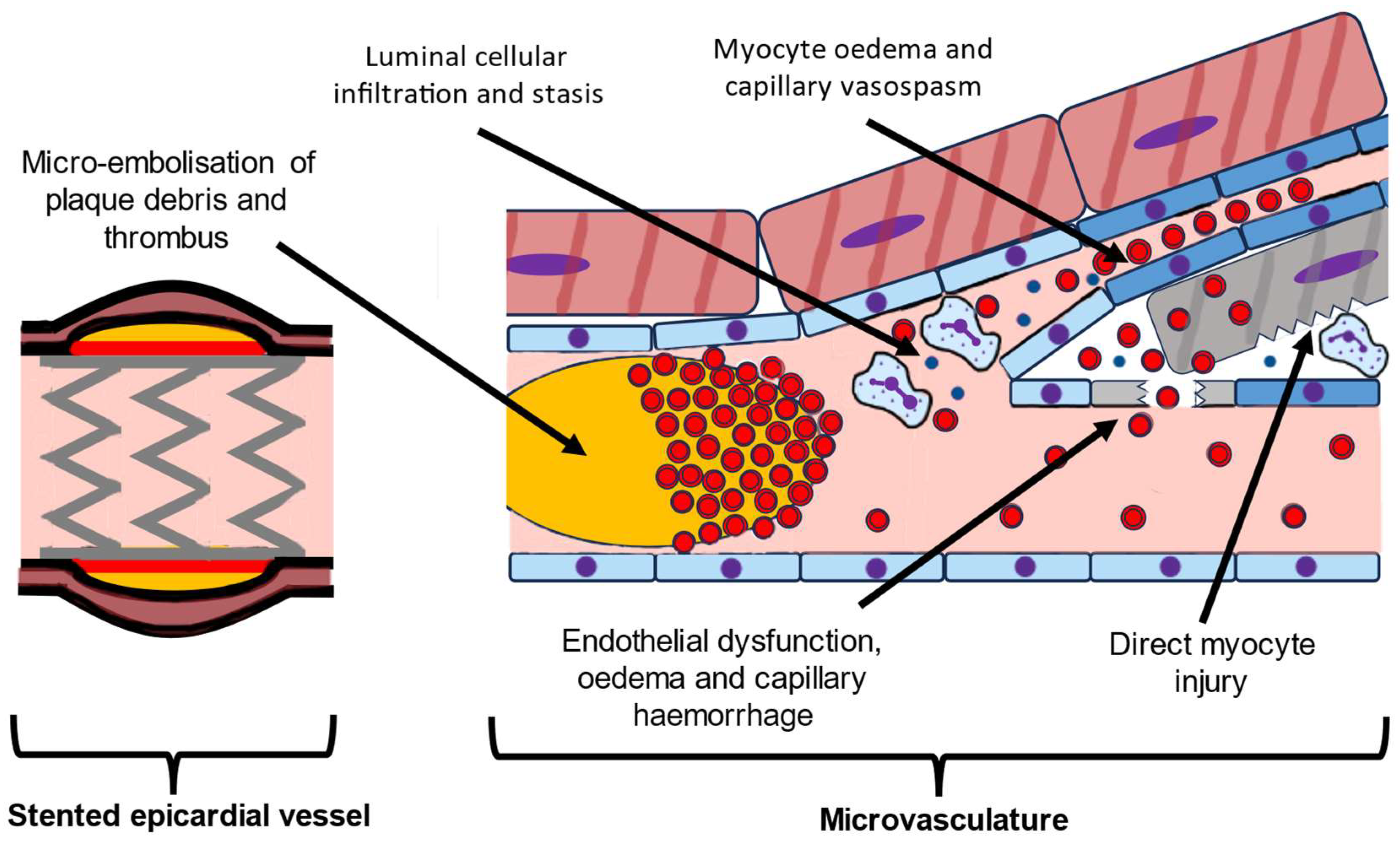
2. Pathophysiology of MVO
3. Prognosis of MVO
4. Treatments for Microvascular Obstruction
4.1. Pharmacological
4.1.1. Antiplatelet Treatment
4.1.2. Thrombolytic Therapy
4.1.3. Intracoronary Vasodilators
4.1.4. Intensive Statin Therapy
5. Non-Pharmacological
5.1. Thrombectomy
5.2. Selective Intracoronary Hypothermia (SIH)
5.3. Pressure-Controlled Intermittent Coronary Sinus Occlusion (PiCSO®)
5.4. Left Ventricular Unloading
5.5. Sonothrombylysis
5.6. Supersaturated Oxygen (SSO2)
5.7. Deferred Stenting
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Alkhalil, M.; De Maria, G.L.; Akbar, N.; Ruparelia, N.; Choudhury, R.P. Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair. J. Clin. Med. 2023, 12, 4668. [Google Scholar] [CrossRef]
- Kloner, R.A.; Ganote, C.E.; Jennings, R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J. Clin. Investig. 1974, 54, 1496–1508. [Google Scholar] [CrossRef]
- Judd, R.M.; Lugo-Olivieri, C.H.; Arai, M.; Kondo, T.; Croisille, P.; Lima, J.A.; Mohan, V.; Becker, L.C.; Zerhouni, E.A. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation 1995, 92, 1902–1910. [Google Scholar] [CrossRef]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial No-Reflow in Humans. J. Am. Coll. Cardiol. 2009, 54, 281–292. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Sabatine, M.S.; Gibson, C.M.; Roe, M.T.; Harrington, R.A.; Murphy, S.A.; Morrow, D.A.; Antman, E.M.; Braunwald, E. Combined assessment of thrombolysis in myocardial infarction flow grade, myocardial perfusion grade, and ST-segment resolution to evaluate epicardial and myocardial reperfusion. Am. J. Cardiol. 2004, 93, 1362–1367. [Google Scholar] [CrossRef]
- Jolly, S.S.; James, S.; Dzavik, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation 2017, 135, 143–152. [Google Scholar] [CrossRef]
- Sharma, V.; Jolly, S.S.; Hamid, T.; Sharma, D.; Chiha, J.; Chan, W.; Fuchs, F.; Bui, S.; Gao, P.; Kassam, S.; et al. Myocardial blush and microvascular reperfusion following manual thrombectomy during percutaneous coronary intervention for ST elevation myocardial infarction: Insights from the TOTAL trial. Eur. Heart J. 2016, 37, 1891–1898. [Google Scholar] [CrossRef]
- Alkhalil, M.; Kuzemczak, M.; Zhao, R.; Kavvouras, C.; Cantor, W.J.; Overgaard, C.B.; Lavi, S.; Sharma, V.; Chowdhary, S.; Stanković, G.; et al. Prognostic Role of Residual Thrombus Burden Following Thrombectomy: Insights from the TOTAL Trial. Circ. Cardiovasc. Interv. 2022, 15, e011336. [Google Scholar] [CrossRef]
- Alkhalil, M.; Oxford Acute Myocardial Infarction (OxAMI) Study; Borlotti, A.; De Maria, G.L.; Wolfrum, M.; Dawkins, S.; Fahrni, G.; Gaughran, L.; Langrish, J.P.; Lucking, A.; et al. Hyper-acute cardiovascular magnetic resonance T1 mapping predicts infarct characteristics in patients with ST elevation myocardial infarction. J. Cardiovasc. Magn. Reson. 2020, 22, 3. [Google Scholar] [CrossRef]
- Wamil, M.; Borlotti, A.; Liu, D.; e Gala, A.B.; Bracco, A.; Alkhalil, M.; De Maria, G.L.; Piechnik, S.K.; Ferreira, V.M.; Banning, A.P.; et al. Combined T1-mapping and tissue tracking analysis predicts severity of ischemic injury following acute STEMI—An Oxford Acute Myocardial Infarction (OxAMI) study. Int. J. Cardiovasc. Imaging 2019, 35, 1297–1308. [Google Scholar] [CrossRef]
- Alkhalil, M.; Borlotti, A.; De Maria, G.L.; Gaughran, L.; Langrish, J.; Lucking, A.; Ferreira, V.; Kharbanda, R.K.; Banning, A.P.; Channon, K.M.; et al. Dynamic changes in injured myocardium, very early after acute myocardial infarction, quantified using T1 mapping cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 82. [Google Scholar] [CrossRef]
- Ibanez, B.; Aletras, A.H.; Arai, A.E.; Arheden, H.; Bax, J.; Berry, C.; Bucciarelli-Ducci, C.; Croisille, P.; Dall’Armellina, E.; Dharmakumar, R.; et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef]
- Chang, X.; Lochner, A.; Wang, H.-H.; Wang, S.; Zhu, H.; Ren, J.; Zhou, H. Coronary microvascular injury in myocardial infarction: Perception and knowledge for mitochondrial quality control. Theranostics 2021, 11, 6766–6785. [Google Scholar] [CrossRef]
- Díez-Delhoyo, F.; Gutiérrez-Ibañes, E.; Sanz-Ruiz, R.; Vázquez-Álvarez, M.E.; Saldívar, H.G.; Juárez, A.R.; Sarnago, F.; Martínez-Sellés, M.; Bermejo, J.; Soriano, J.; et al. Prevalence of Microvascular and Endothelial Dysfunction in the Nonculprit Territory in Patients with Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2019, 12, e007257. [Google Scholar] [CrossRef]
- Bravo Baptista, S.; Faustino, M.; Brizida, L.; Loureiro, J.; Augusto, J.; Abecasis, J.; Monteiro, C.; Leal, P.; Nedio, M.; Farto, E.A.P.; et al. Early peripheral endothelial dysfunction predicts myocardial infarct extension and microvascular obstruction in patients with ST-elevation myocardial infarction. Rev. Port. Cardiol. 2017, 36, 731–742. [Google Scholar] [CrossRef]
- Akbar, N.; Braithwaite, A.T.; Corr, E.M.; Koelwyn, G.J.; van Solingen, C.; Cochain, C.; Saliba, A.-E.; Corbin, A.; Pezzolla, D.; Jørgensen, M.M.; et al. Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles. Cardiovasc. Res. 2022, 119, 236–251. [Google Scholar] [CrossRef]
- Alkhalil, M.; Kearney, A.; Hegarty, M.; Stewart, C.; Devlin, P.; Owens, C.G.; Spence, M.S. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting with Acute Myocardial Infarction. Am. J. Med. 2019, 132, e827–e834. [Google Scholar] [CrossRef]
- Ruparelia, N.; Godec, J.; Lee, R.; Chai, J.T.; Dall’Armellina, E.; McAndrew, D.; Digby, J.E.; Forfar, J.C.; Prendergast, B.D.; Kharbanda, R.K.; et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur. Heart J. 2015, 36, 1923–1934. [Google Scholar] [CrossRef]
- Moss, A.; Daghem, M.; Tzolos, E.; Meah, M.N.; Wang, K.-L.; Bularga, A.; Adamson, P.D.; Kwiecinski, J.; Fletcher, A.; Dawson, D.; et al. Coronary Atherosclerotic Plaque Activity and Future Coronary Events. JAMA Cardiol. 2023, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, M.; Edmond, E.; Edgar, L.; E Digby, J.; Omar, O.; Robson, M.D.; Choudhury, R.P. The relationship of perivascular adipose tissue and atherosclerosis in the aorta and carotid arteries, determined by magnetic resonance imaging. Diabetes Vasc. Dis. Res. 2018, 15, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.X.; Ekinci, Y.; Spray, L.; Jeyalan, V.; Olin, T.; Richardson, G.; Austin, D.; Alkhalil, M.; Spyridopoulos, I. Fractalkine Signalling (CX(3)CL1/CX(3)CR1 Axis) as an Emerging Target in Coronary Artery Disease. J. Clin. Med. 2023, 12, 4821. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients With STEMI Undergoing Primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef]
- Weir, R.A.; Murphy, C.A.; Petrie, C.J.; Martin, T.N.; Balmain, S.; Clements, S.; Steedman, T.; Wagner, G.S.; Dargie, H.J.; McMurray, J.J. Microvascular obstruction remains a portent of adverse remodeling in optimally treated patients with left ventricular systolic dysfunction after acute myocardial infarction. Circ. Cardiovasc. Imaging 2010, 3, 360–367. [Google Scholar] [CrossRef]
- Mather, A.N.; Fairbairn, T.A.; Ball, S.G.; Greenwood, J.P.; Plein, S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart 2011, 97, 453–459. [Google Scholar] [CrossRef]
- Liu, T.; Howarth, A.G.; Chen, Y.; Nair, A.R.; Yang, H.-J.; Ren, D.; Tang, R.; Sykes, J.; Kovacs, M.S.; Dey, D.; et al. Intramyocardial Hemorrhage and the “Wave Front” of Reperfusion Injury Compromising Myocardial Salvage. J. Am. Coll. Cardiol. 2022, 79, 35–48. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. The ATI score (age-thrombus burden-index of microcirculatory resistance) determined during primary percutaneous coronary intervention predicts final infarct size in patients with ST-elevation myocardial infarction: A cardiac magnetic resonance validation study. EuroIntervention 2017, 13, 935–943. [Google Scholar]
- De Maria, G.L.; Fahrni, G.; Alkhalil, M.; Cuculi, F.; Dawkins, S.; Wolfrum, M.; Choudhury, R.P.; Forfar, J.C.; Prendergast, B.D.; Yetgin, T.; et al. A tool for predicting the outcome of reperfusion in ST-elevation myocardial infarction using age, thrombotic burden and index of microcirculatory resistance (ATI score). EuroIntervention 2016, 12, 1223–1230. [Google Scholar] [CrossRef]
- Massalha, E.; Oren, D.; Goitein, O.; Brodov, Y.; Fardman, A.; Younis, A.; Berkovitch, A.; Raibman-Spector, S.; Konen, E.; Maor, E.; et al. Post–ST-Segment–Elevation Myocardial Infarction Platelet Reactivity Is Associated with the Extent of Microvascular Obstruction and Infarct Size as Determined by Cardiac Magnetic Resonance Imaging. J. Am. Heart Assoc. 2022, 11, e020973. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lo, S.; Mussap, C.J.; French, J.K.; Rajaratnam, R.; Kadappu, K.; Premawardhana, U.; Nguyen, P.; Juergens, C.P.; Leung, D.Y. Impact of Ticagrelor Versus Clopidogrel on Coronary Microvascular Function After Non–ST-Segment–Elevation Acute Coronary Syndrome. Circ. Cardiovasc. Interv. 2022, 15, e011419. [Google Scholar] [CrossRef] [PubMed]
- Janssens, G.N.; van Leeuwen, M.A.H.; van der Hoeven, N.W.; de Waard, G.A.; Nijveldt, R.; Diletti, R.; Zijlstra, F.; von Birgelen, C.; Escaned, J.; Valgimigli, M.; et al. Reducing Microvascular Dysfunction in Revascularized Patients with ST-Elevation Myocardial Infarction by Off-Target Properties of Ticagrelor versus Prasugrel. Rationale and Design of the REDUCE-MVI Study. J. Cardiovasc. Transl. Res. 2016, 9, 249–256. [Google Scholar] [CrossRef]
- Spigoni, V.; Fantuzzi, F.; Carubbi, C.; Pozzi, G.; Masselli, E.; Gobbi, G.; Solini, A.; Bonadonna, R.C.; Cas, A.D. Sodium-glucose cotransporter 2 inhibitors antagonize lipotoxicity in human myeloid angiogenic cells and ADP-dependent activation in human platelets: Potential relevance to prevention of cardiovascular events. Cardiovasc. Diabetol. 2020, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Schindler, K.; Friedenberger, J.; Eitel, I.; Furnau, G.; Grebe, E.; Erbs, S.; Linke, A.; Mobius-Winkler, S.; Kivelitz, D.; et al. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: The randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation 2008, 118, 49–57. [Google Scholar] [PubMed]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.H.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Faculty Opinions recommendation of Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- Desch, S.; Wohrle, J.; Hambrecht, R.; Rittger, H.; Birkemeyer, R.; Lauer, B.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Pauschinger, M.; et al. Intracoronary versus intravenous abciximab bolus in patients with ST-segment elevation myocardial infarction: 1-year results of the randomized AIDA STEMI trial. J. Am. Coll. Cardiol. 2013, 62, 1214–1215. [Google Scholar] [CrossRef]
- Sezer, M.; Oflaz, H.; Gören, T.; Okçular, I.; Umman, B.; Nişanci, Y.; Bilge, A.K.; Şanli, Y.; Meriç, M.; Umman, S. Intracoronary Streptokinase after Primary Percutaneous Coronary Intervention. N. Engl. J. Med. 2007, 356, 1823–1834. [Google Scholar] [CrossRef]
- McCartney, P.J.; Eteiba, H.; Maznyczka, A.M.; McEntegart, M.; Greenwood, J.P.; Muir, D.F.; Chowdhary, S.; Gershlick, A.H.; Appleby, C.; Cotton, J.M.; et al. Effect of Low-Dose Intracoronary Alteplase During Primary Percutaneous Coronary Intervention on Microvascular Obstruction in Patients with Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA 2019, 321, 56–68. [Google Scholar] [CrossRef]
- McCartney, P.J.; Maznyczka, A.M.; Eteiba, H.; McEntegart, M.; Oldroyd, K.G.; Greenwood, J.P.; Maredia, N.; Schmitt, M.; McCann, G.P.; Fairbairn, T.; et al. Low-Dose Alteplase During Primary Percutaneous Coronary Intervention According to Ischemic Time. J. Am. Coll. Cardiol. 2020, 75, 1406–1421. [Google Scholar] [CrossRef]
- Nazir, S.A.; Khan, J.N.; Mahmoud, I.Z.; Greenwood, J.P.; Blackman, D.J.; Kunadian, V.; Been, M.; Abrams, K.R.; Wilcox, R.; Adgey, A.A.J.; et al. The REFLO-STEMI (REperfusion Facilitated by LOcal adjunctive therapy in ST-Elevation Myocardial Infarction) trial: A randomised controlled trial comparing intracoronary administration of adenosine or sodium nitroprusside with control for attenuation of microvascular obstruction during primary percutaneous coronary intervention Southampton (UK). Effic. Mech. Eval. 2016, 3, 90. [Google Scholar]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Muçaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: The RESTORE observational study. Catheter. Cardiovasc. Interv. 2021, 97, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Afshar, E.J.; Samimisedeh, P.; Tayebi, A.; Hassani, N.S.; Rastad, H.; Yazdani, S. Efficacy and safety of intracoronary epinephrine for the management of the no-reflow phenomenon following percutaneous coronary interventions: A systematic-review study. Ther. Adv. Cardiovasc. Dis. 2023, 17, 17539447231154654. [Google Scholar] [CrossRef]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients with Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, e011408. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, J.; Bai, M.; Peng, Y.; Sun, S.; Zhang, Z. Effect of intracoronary agents on the no-reflow phenomenon during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction: A network meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 3. [Google Scholar] [CrossRef]
- Anayat, S.; Majid, K.; Nazir, H.S.; Nizami, A.A.; Mustafa, W.; Abbasi, M.S.R.; Ahsan, M.N.; Jadoon, S.K.; Ullah, I.; Asghar, M.S. Meta-Analysis on the Efficacy of High-Dose Statin Loading Before Percutaneous Coronary Intervention in Reducing No-Reflow Phenomenon in Acute Coronary Syndrome. Am. J. Cardiol. 2023, 195, 9–16. [Google Scholar] [CrossRef]
- Fujii, K.; Kawasaki, D.; Oka, K.; Akahori, H.; Iwasaku, T.; Fukunaga, M.; Eguchi, A.; Sawada, H.; Masutani, M.; Lee-Kawabata, M.; et al. The impact of pravastatin pre-treatment on periprocedural microcirculatory damage in patients undergoing percutaneous coronary intervention. JACC: Cardiovasc. Interv. 2011, 4, 513–520. [Google Scholar] [CrossRef][Green Version]
- Svilaas, T.; Vlaar, P.J.; van der Horst, I.C.; Diercks, G.F.; de Smet, B.J.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.S.; Suurmeijer, A.J.; et al. Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 2008, 358, 557–567. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized Trial of Primary PCI with or without Routine Manual Thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus aspiration during st-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef]
- Ali, A.; Cox, D.; Dib, N.; Brodie, B.; Berman, D.; Gupta, N.; Browne, K.; Iwaoka, R.; Azrin, M.; Stapleton, D.; et al. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J. Am. Coll. Cardiol. 2006, 48, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.J.; Parikh, S.A.; Wu, W.; Metzger, D.C.; Chambers, J.W.; Ghali, M.G.; Sumners, M.J.; Kolski, B.C.; Pinto, D.S.; Dohad, S. Sustained Mechanical Aspiration Thrombectomy for High Thrombus Burden Coronary Vessel Occlusion: The Multicenter CHEETAH Study. Circ. Cardiovasc. Interv. 2023, 16, e012433. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, D.; Loizzi, F.; Landi, A.; Utsch, L.; Araco, M.; Joner, M.; Valgimigli, M. EnVast Mechanical Thrombectomy After Thrombus Aspiration Failure in a STEMI With a Massive Thrombotic Burden. JACC Cardiovasc. Interv. 2023, 16, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Otterspoor, L.C.; Van ‘t Veer, M.; Van Nunen, L.X.; Brueren, G.R.G.; Tonino, P.A.L.; Wijnbergen, I.F.; Helmes, H.; Zimmermann, F.M.; Van Hagen, E.; Johnson, N.P.; et al. Safety and feasibility of selective intracoronary hypothermia in acute myocardial infarction. EuroIntervention 2017, 13, e1475–e1482. [Google Scholar] [CrossRef]
- El Farissi, M.; Good, R.; Engstrøm, T.; Oldroyd, K.G.; Karamasis, G.V.; Vlaar, P.J.; Lønborg, J.T.; Teeuwen, K.; Keeble, T.R.; Mangion, K.; et al. Safety of Selective Intracoronary Hypothermia During Primary Percutaneous Coronary Intervention in Patients with Anterior STEMI. JACC Cardiovasc. Interv. 2021, 14, 2047–2055. [Google Scholar] [CrossRef]
- VAN DE Hoef, T.P.; Nolte, F.; Delewi, R.; Henriques, J.P.; Spaan, J.A.; Tijssen, J.G.; Siebes, M.; Wykrzykowska, J.J.; Stone, G.W.; Piek, J.J. Intracoronary hemodynamic effects of pressure-controlled intermittent coronary sinus occlusion (picso): Results from the first-in-man prepare picso study. J. Interv. Cardiol. 2012, 25, 549–556. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction—Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, e352–e359. [Google Scholar]
- Egred, M.; Bagnall, A.; Spyridopoulos, I.; Purcell, I.F.; Das, R.; Palmer, N.; Grech, E.D.; Jain, A.; Stone, G.W.; Nijveldt, R.; et al. Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. IJC Heart Vasc. 2020, 28, 100526. [Google Scholar] [CrossRef]
- Kapur, N.K.; Paruchuri, V.; Urbano-Morales, J.A.; Mackey, E.E.; Daly, G.H.; Qiao, X.; Pandian, N.; Perides, G.; Karas, R.H. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013, 128, 328–336. [Google Scholar] [CrossRef]
- Al-Atta, A.; Zaidan, M.; Abdalwahab, A.; Asswad, A.G.; Egred, M.; Zaman, A.; Alkhalil, M. Mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock. Rev. Cardiovasc. Med. 2022, 23, 71. [Google Scholar] [CrossRef]
- Kapur, N.K.; Alkhouli, M.; DeMartini, T.J.; Faraz, H.; George, Z.H.; Goodwin, M.J.; Hernandez-Montfort, J.A.; Iyer, V.S.; Josephy, N.; Kalra, S.; et al. Unloading the Left Ventricle Before Reperfusion in Patients with Anterior ST-Segment–Elevation Myocardial Infarction. Circulation 2019, 139, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Mathias, W.; Tsutsui, J.M.; Tavares, B.G.; Xie, F.; Aguiar, M.O.; Garcia, D.R.; Oliveira, M.T.; Soeiro, A.; Nicolau, J.C.; Lemos, P.A.; et al. Diagnostic Ultrasound Impulses Improve Microvascular Flow in Patients with STEMI Receiving Intravenous Microbubbles. J. Am. Coll. Cardiol. 2016, 67, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Mathias, W.; Tsutsui, J.M.; Tavares, B.G.; Fava, A.M.; Aguiar, M.O.; Borges, B.C.; Oliveira, M.T.; Soeiro, A.; Nicolau, J.C.; Ribeiro, H.B.; et al. Sonothrombolysis in ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Bartorelli, A.L. Hyperoxemic perfusion for treatment of reperfusion microvascular ischemia in patients with myocardial infarction. Am. J. Cardiovasc. Drugs 2003, 3, 253–263. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef]
- Stone, G.W.; Martin, J.L.; de Boer, M.-J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef]
- David, S.W.; Khan, Z.A.; Patel, N.C.; Metzger, D.C.; Wood, F.O.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; et al. Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter. Cardiovasc. Interv. 2019, 93, 882–890. [Google Scholar] [CrossRef]
- Cafri, C.; Svirsky, R.; Zelingher, J.; Slutky, O.; Kobal, S.; Weinstein, J.M.; Ilia, R.; Gilutz, H. Improved procedural results in coronary thrombosis are obtained with delayed percutaneous coronary interventions. J. Invasive Cardiol. 2004, 16, 69–71. [Google Scholar]
- Carrick, D.; Oldroyd, K.G.; McEntegart, M.; Haig, C.; Petrie, M.C.; Eteiba, H.; Hood, S.; Owens, C.; Watkins, S.; Layland, J.; et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute st-segment elevation myocardial infarction (DEFER-STEMI). J. Am. Coll. Cardiol. 2014, 63, 2088–2098. [Google Scholar] [CrossRef]
- Mester, P.; Bouvaist, H.; Delarche, N.; Bouisset, F.; Abdellaoui, M.; Petiteau, P.-Y.; Dubreuil, O.; Boueri, Z.; Chettibi, M.; Souteyrand, G.; et al. At least seven days delayed stenting using minimalist immediate mechanical intervention (MIMI) in ST-segment elevation myocardial infarction: The SUPER-MIMI study. EuroIntervention 2017, 13, 390–396. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Oikonomou, E.K.; Wolfrum, M.; Choudhury, R.P.; Banning, A.P. Role of deferred stenting in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention: A systematic review and meta-analysis. J. Interv. Cardiol. 2017, 30, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kelbæk, H.; Høfsten, D.E.; Køber, L.; Helqvist, S.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; De Backer, O.; et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): An open-label, randomised controlled trial. Lancet 2016, 387, 2199–2206. [Google Scholar] [CrossRef] [PubMed]

| Intervention | Efficacy (a) | Current Level of Evidence (b) | Ongoing RCTs |
|---|---|---|---|
| Pharmacological | |||
| High-dose Statins | ++ | A | No |
| IC/IV Glycoprotein IIb/IIIa inhibitors | ++ | A | No |
| IC Thrombolytics | − | B | Yes |
| IC Epinephrine | + | B | No |
| IC Anisodamine | + | B | No |
| IC SNP | + | B | No |
| IC Adenosine | +/− | B | No |
| IC Non-DHP CCBs | +/− | B | No |
| IC Cangrelor | Unknown | C | Yes |
| SGLT2 inhibitors | Unknown | C | Yes |
| Intervention | Efficacy (a) | Current Level of Evidence (b) | Ongoing RCTs |
|---|---|---|---|
| Non-Pharmacological | |||
| Routine Manual Thrombectomy | − | A | No |
| Mechanical Thrombectomy | +/− | B | No |
| Selective Intracoronary Hypothermia | Unknown | C | Yes |
| PICSO® | ++ | B | Yes |
| Sonothrombolysis | ++ | B | Yes |
| Left Ventricular Unloading | + | B | Yes |
| Supersaturated Oxygen | ++ | B | No |
| Deferred Stenting | +/− | A | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghobrial, M.; Bawamia, B.; Cartlidge, T.; Spyridopoulos, I.; Kunadian, V.; Zaman, A.; Egred, M.; McDiarmid, A.; Williams, M.; Farag, M.; et al. Microvascular Obstruction in Acute Myocardial Infarction, a Potential Therapeutic Target. J. Clin. Med. 2023, 12, 5934. https://doi.org/10.3390/jcm12185934
Ghobrial M, Bawamia B, Cartlidge T, Spyridopoulos I, Kunadian V, Zaman A, Egred M, McDiarmid A, Williams M, Farag M, et al. Microvascular Obstruction in Acute Myocardial Infarction, a Potential Therapeutic Target. Journal of Clinical Medicine. 2023; 12(18):5934. https://doi.org/10.3390/jcm12185934
Chicago/Turabian StyleGhobrial, Mina, Bilal Bawamia, Timothy Cartlidge, Ioakim Spyridopoulos, Vijay Kunadian, Azfar Zaman, Mohaned Egred, Adam McDiarmid, Matthew Williams, Mohamed Farag, and et al. 2023. "Microvascular Obstruction in Acute Myocardial Infarction, a Potential Therapeutic Target" Journal of Clinical Medicine 12, no. 18: 5934. https://doi.org/10.3390/jcm12185934
APA StyleGhobrial, M., Bawamia, B., Cartlidge, T., Spyridopoulos, I., Kunadian, V., Zaman, A., Egred, M., McDiarmid, A., Williams, M., Farag, M., & Alkhalil, M. (2023). Microvascular Obstruction in Acute Myocardial Infarction, a Potential Therapeutic Target. Journal of Clinical Medicine, 12(18), 5934. https://doi.org/10.3390/jcm12185934








