Nasal High-Flow (NHF) Improves Ventilation in Patients with Interstitial Lung Disease (ILD)—A Physiological Study
Abstract
1. Introduction
2. Material and Methods
2.1. Devices
2.2. Subjects
2.3. Measurement of Airway Pressure
2.4. Measurement of Tidal Volume, Respiratory Rate, and Minute Volume
2.5. Comfort and Dyspnea Scale
2.6. Measurements in Hypercapnic ILD Subjects
2.7. Statistics
3. Results
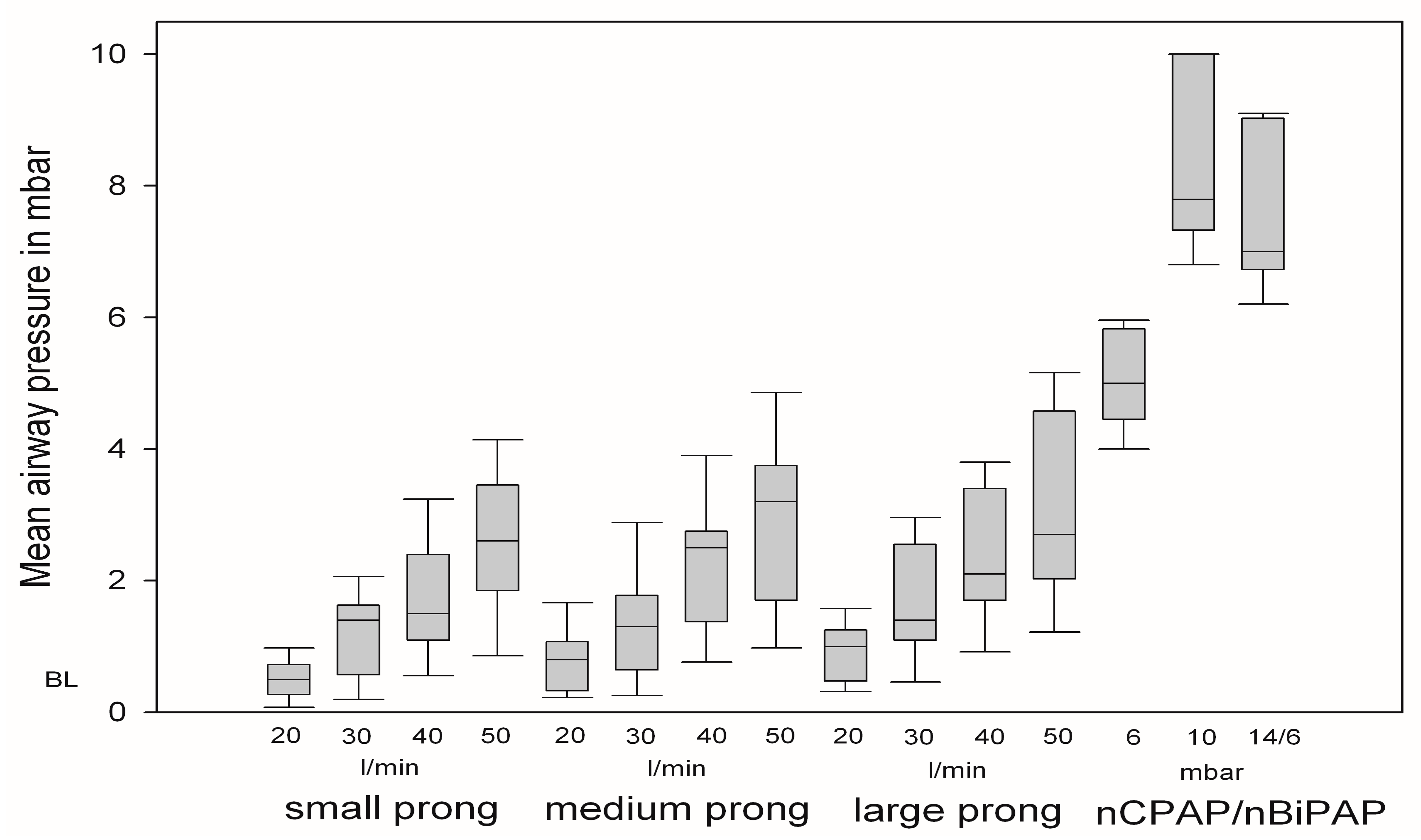
3.1. Changes in Mean Airway Pressure
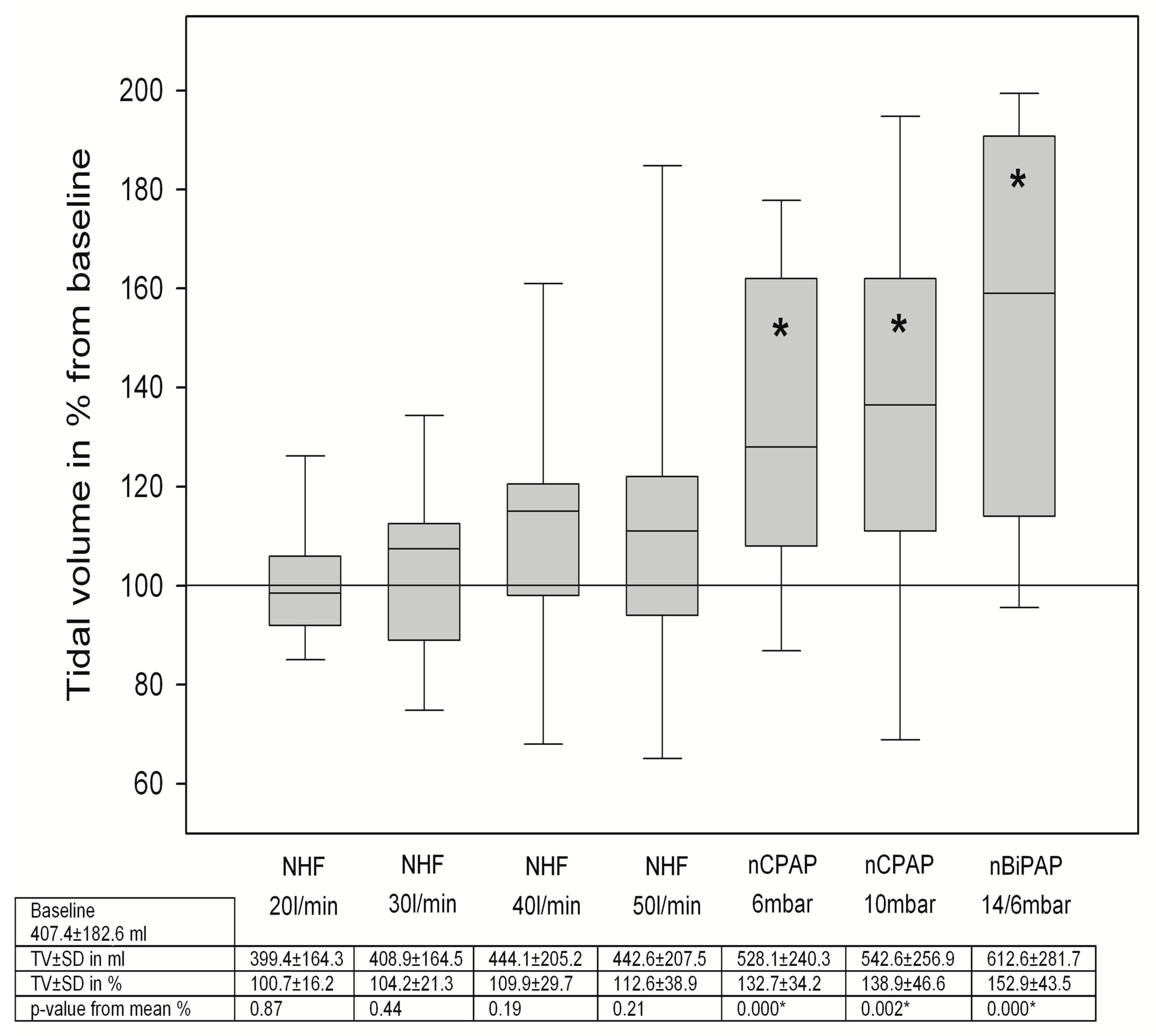
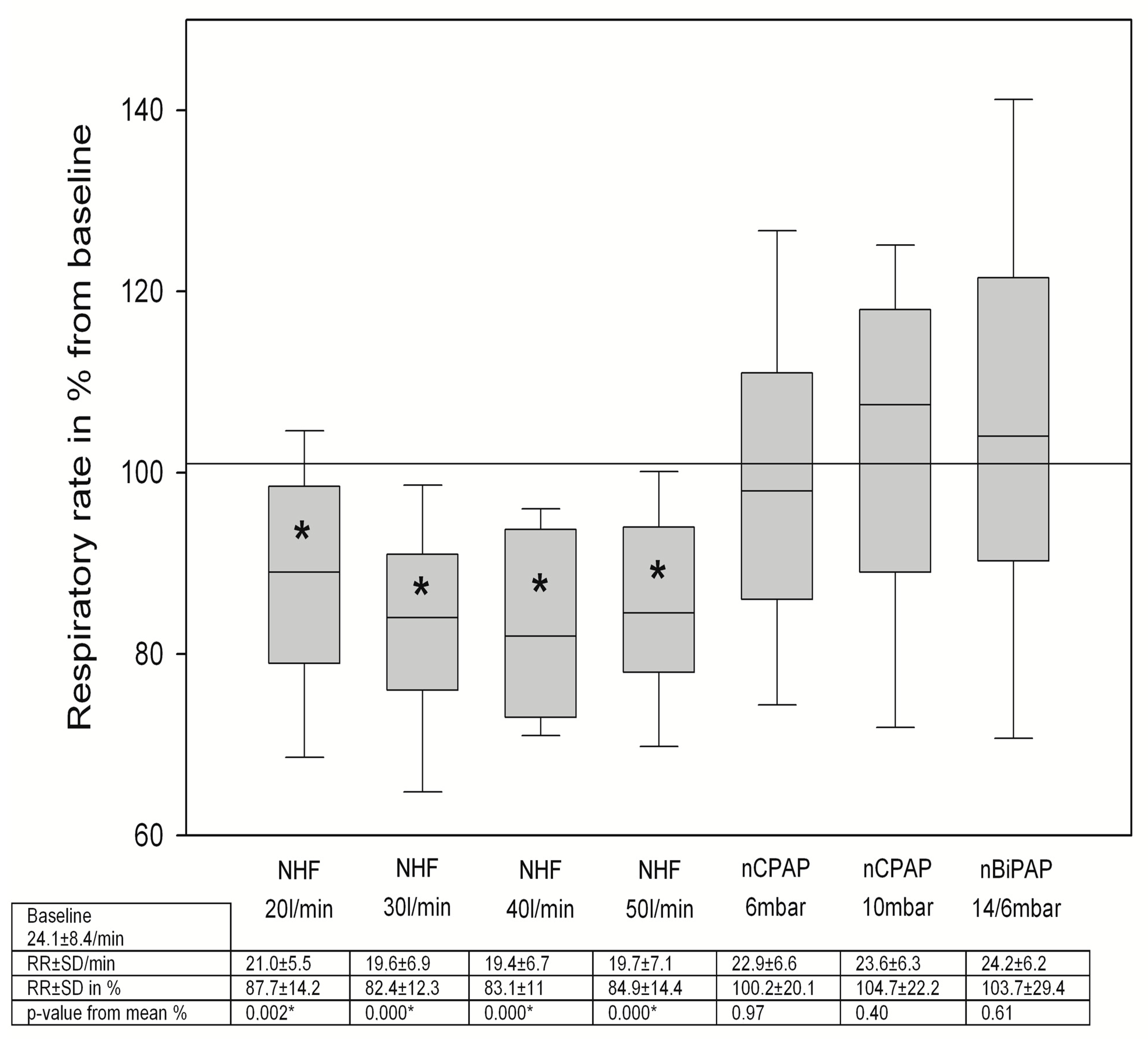
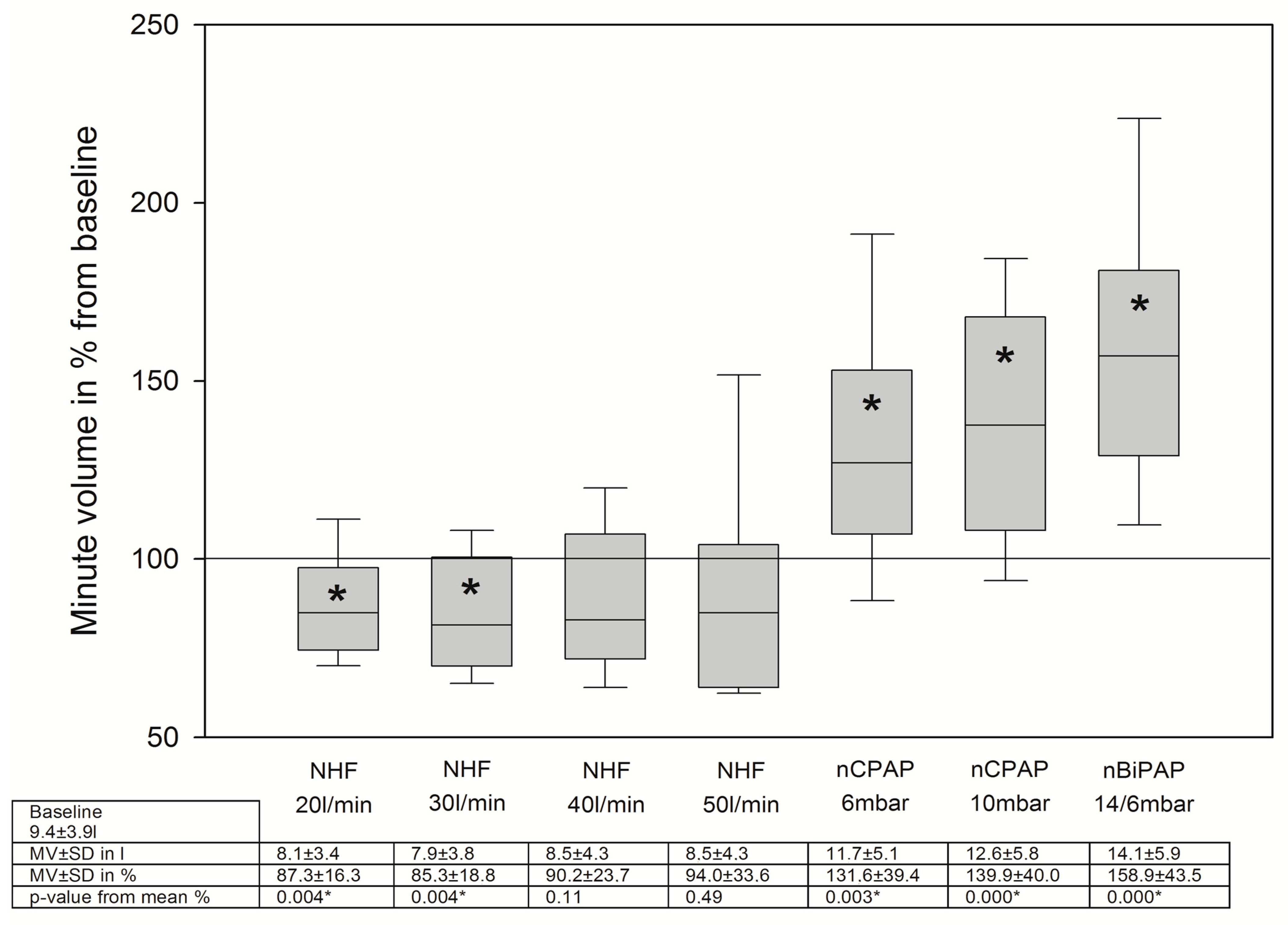
3.2. Changes in Breathing Patterns
3.3. Comfort and Dyspnea
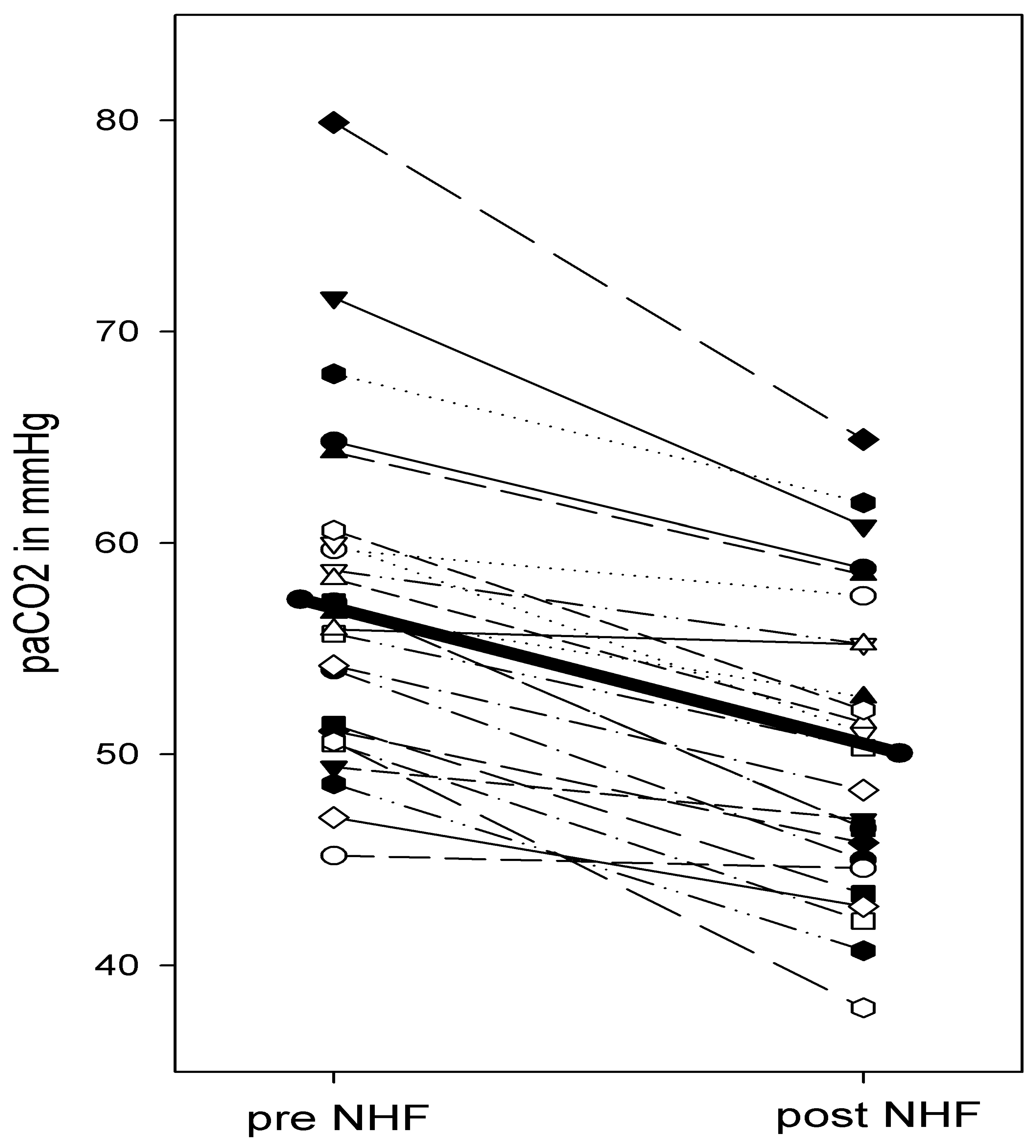
3.4. Changes in Partial Pressures of O2 and CO2
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Mollica, C.; Paone, G.; Conti, V.; Ceccarelli, D.; Schmid, G.; Mattia, P.; Terzano, C. Mechanical ventilation in patients with end-stage idio-pathic pulmonary fibrosis. Respiration 2010, 79, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Saydain, G.; Islam, A.; Afessa, B.; Ryu, J.H.; Scott, J.P.; Peters, S.G. Outcome of Patients with Idiopathic Pulmonary Fibrosis Admitted to the Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2002, 166, 839–842. [Google Scholar] [CrossRef]
- Nava, S.; Rubini, F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax 1999, 54, 390–395. [Google Scholar] [CrossRef]
- Blivet, S.; Philit, F.; Sab, J.M.; Langevin, B.; Paret, M.; Guérin, C.; Robert, D. Outcome of Patients with Idiopathic Pulmonary Fibrosis Admitted to the ICU for Respiratory Failure. Chest 2001, 120, 209–212. [Google Scholar] [CrossRef]
- Fumeaux, T.; Rothmeier, C.; Jolliet, P. Outcome of mechanical ventilation for acute respiratory failure in patients with pulmonary fibrosis. Intensive Care Med. 2001, 27, 1868–1874. [Google Scholar] [CrossRef]
- Tomii, K.; Tachikawa, R.; Chin, K.; Murase, K.; Handa, T.; Mishima, M.; Ishihara, K. Role of Non-invasive Ventilation in Managing Life-threatening Acute Exacerbation of Interstitial Pneumonia. Intern. Med. 2010, 49, 1341–1347. [Google Scholar] [CrossRef][Green Version]
- Yokoyama, T.; Tsushima, K.; Yamamoto, H.; Koizumi, T. Potential benefits of early continuous positive pressure ven-tilation in patients with rapidly progressive interstitial pneumonia. Respirology 2012, 17, 315–321. [Google Scholar] [CrossRef]
- Koschel, D.; Handzhiev, S.; Wiedemann, B.; Hoffken, G. Acute effects of NPPV in interstitial lung disease with chronic hypercapnic respiratory failure. Respir. Med. 2010, 104, 291–295. [Google Scholar] [CrossRef][Green Version]
- Frat, J.-P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A. Supplementary Appendix: High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Vaquero, C.; Colinas, L.; Cuena, R.; González, P.; Canabal, A.; Sanchez, S.; Rodriguez, M.L.; Villasclaras, A.; Fernández, R. Effect of Postextubation High-Flow Nasal Cannula vs. Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Ran-domized Clinical Trial. JAMA 2016, 316, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Vaquero, C.; González, P.; Subira, C.; Frutos-Vivar, F.; Rialp, G.; Laborda, C.; Colinas, L.; Cuena, R.; Fernández, R. Supplementary Online content 2: Effect of Postextubation High-Flow Nasal Cannula vs. Conventional Oxygen Therapy on Reintubation in Low-Risk Patients: A Ran-domized Clinical Trial. JAMA 2016, 315, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, S.M.; Idone, F.A.; Vaschetto, R.; Festa, R.; Cataldo, A.; Antonicelli, F.; Montini, L.; De Gaetano, A.; Navalesi, P.; Antonelli, M. Nasal High-Flow versus Venturi Mask Oxygen Therapy after Extubation. Effects on Oxygenation, Comfort, and Clinical Outcome. Am. J. Respir. Crit. Care Med. 2014, 190, 282–288. [Google Scholar] [CrossRef]
- Wagstaff, T.A.J.; Soni, N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia 2007, 62, 492–503. [Google Scholar] [CrossRef]
- Chikata, Y.; Onodera, M.; Oto, J.; Nishimura, M. FIO2 in an Adult Model Simulating High-Flow Nasal Cannula Therapy. Respir. Care 2016, 62, 193–198. [Google Scholar] [CrossRef]
- Bräunlich, J.; Beyer, D.; Mai, D. Hammerschmidt S, Seyfarth H-J, Wirtz H: Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration 2013, 85, 319–325. [Google Scholar] [CrossRef]
- Bräunlich, J.; Köhler, M.; Wirtz, H. Nasal highflow improves ventilation in patients with COPD. COPD 2016, 11, 1077–1085. [Google Scholar] [CrossRef]
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2016, 195, 1207–1215. [Google Scholar] [CrossRef]
- Delorme, M.; Bouchard, P.-A.; Simon, M.; Simard, S.; Lellouche, F. Effects of High-Flow Nasal Cannula on the Work of Breathing in Patients Recovering from Acute Respiratory Failure. Crit. Care Med. 2017, 45, 1981–1988. [Google Scholar] [CrossRef]
- Bräunlich, J.; Mauersberger, F.; Wirtz, H. Effectiveness of nasal highflow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm. Med. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Biselli, P.J.C.; Kirkness, J.P.; Grote, L.; Fricke, K.; Schwartz, A.R.; Smith, P.; Schneider, H. Nasal high-flow therapy reduces work of breathing compared with oxygen during sleep in COPD and smoking controls: A prospective observational study. J. Appl. Physiol. 2017, 122, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Fasano, L.; Corcione, N.; Comellini, V.; Musti, M.A.; Brandao, M.; Bottone, D.; Calderini, E.; Navalesi, P.; Nava, S. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017, 72, 373–375. [Google Scholar] [CrossRef]
- Fraser, J.F.; Spooner, A.J.; Dunster, K.R.; Anstey, C.M.; Corley, A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: A randomised crossover trial. Thorax 2016, 71, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Groves, N.; Tobin, A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust. Crit. Care 2007, 20, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, S.C.; Kang, C.; Lee, S.H.; Kang, T.-S.; Lee, S.B.; Jung, S.M.; Kim, D.S. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ED. Am. J. Emerg. Med. 2015, 33, 1344–1349. [Google Scholar] [CrossRef]
- Shah, N.M.; D’Cruz, R.F.; Murphy, P.B. Update: Non-invasive ventilation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2018, 10, S71–S79. [Google Scholar] [CrossRef]
- Stéphan, F.; Barrucand, B.; Petit, P.; Rézaiguia-Delclaux, S.; Médard, A.; Delannoy, B.; Cosserant, B.; Flicoteaux, G.; Imbert, A.; Pilorge, C.; et al. High-Flow Nasal Oxygen vs. Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery. JAMA 2015, 313, 2331–2339. [Google Scholar] [CrossRef]
| Physiological Group (n = 16) | All Patients (n = 25) | |
|---|---|---|
| Female | 10 | 18 |
| Age (years) | 59.1 ± 11.2 | 57.4 ± 8.3 |
| Height (cm) | 164.6 ± 8.2 | 166.3 ± 8.6 |
| Weight (kg) | 77.1 ± 17.7 | 80.3 ± 90.1 |
| diagnosis | 17 IPF, 4 sarcoidosis, 2 LAM, 1 PSS, 1 EAA | |
| Respiratory rate n/min | 24.1 ± 8.4 | 24.8 ± 6.5 |
| FVC % pred | 50.0 ± 14.3 * | 39.3 ± 10.8 * |
| FEV1/FVC % | 90.1 ± 11.0 | 97.6 ± 19.4 |
| TLC % pred | 60.8 ± 11.9 | 56.2 ± 15.3 |
| NHF Small Prong | NHF Medium Prong | NHF Large Prong | Nasal CPAP | Nasal BiPAP | |
|---|---|---|---|---|---|
| Comfort | 3.9 ± 1.8 | 4.0 ± 1.6 | 5.7 ± 2.1 | 5.9 ± 2.1 | 5.6 ± 2.5 |
| Dyspnoe | 2.9 ± 0.8 | 2.9 ± 0.8 | 3.1 ± 0.7 | 3.2 ± 0.9 | 3.4 ± 1.1 |
| pH | paCO2 mmHg | paO2 mmHg | |
|---|---|---|---|
| Pre NHF | 7.402 ± 0.07 | 57.2 ± 8.0 | 59.8 ± 10.5 |
| Post NHF | 7.434 ± 0.06 | 50.4 ± 7.2 | 58.7 ± 17.7 |
| p-values pre/post NHF | 0.17 | 0.003 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bräunlich, J.; Köhler, M.; Wirtz, H. Nasal High-Flow (NHF) Improves Ventilation in Patients with Interstitial Lung Disease (ILD)—A Physiological Study. J. Clin. Med. 2023, 12, 5853. https://doi.org/10.3390/jcm12185853
Bräunlich J, Köhler M, Wirtz H. Nasal High-Flow (NHF) Improves Ventilation in Patients with Interstitial Lung Disease (ILD)—A Physiological Study. Journal of Clinical Medicine. 2023; 12(18):5853. https://doi.org/10.3390/jcm12185853
Chicago/Turabian StyleBräunlich, Jens, Marcus Köhler, and Hubert Wirtz. 2023. "Nasal High-Flow (NHF) Improves Ventilation in Patients with Interstitial Lung Disease (ILD)—A Physiological Study" Journal of Clinical Medicine 12, no. 18: 5853. https://doi.org/10.3390/jcm12185853
APA StyleBräunlich, J., Köhler, M., & Wirtz, H. (2023). Nasal High-Flow (NHF) Improves Ventilation in Patients with Interstitial Lung Disease (ILD)—A Physiological Study. Journal of Clinical Medicine, 12(18), 5853. https://doi.org/10.3390/jcm12185853







