APOE ε4 Gene Carriers Demonstrate Reduced Retinal Capillary Densities in Asymptomatic Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Neuropsychological Examination
2.3. Apolipoprotein E Gene Analysis
2.4. Ophthalmological Examination
2.4.1. Visual Acuity (VA) Examination
2.4.2. Swept Source Optical Coherence Tomography Angiography (SS-OCTA) Imaging
2.5. Statistics Analysis
3. Results
3.1. Baseline Analysis
3.2. Retinal Vasculature Analysis of APOE ε4 Carriers vs. APOE ε4 Non-Carriers
3.3. Retinal Vasculature Analysis of CI vs. NCI
3.4. Subgroup Analysis
3.5. Interaction Analysis
3.6. Relative Importance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gharbi-Meliani, A.; Dugravot, A.; Sabia, S.; Regy, M.; Fayosse, A.; Schnitzler, A.; Kivimaki, M.; Singh-Manoux, A.; Dumurgier, J. The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimer’s Res. Ther. 2021, 13, 5. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Salloway, S.; Gur, T.; Berzin, T.; Tavares, R.; Zipser, B.; Correia, S.; Hovanesian, V.; Fallon, J.; Kuo-Leblanc, V.; Glass, D.; et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J. Neurol. Sci. 2002, 203–204, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; DeStefano, A.L.; Sachdev, P.S.; Choi, S.H.; Mather, K.A.; DeCarli, C.D.; Wen, W.; Hogh, P.; Raz, N.; Au, R.; et al. APOE genotype and MRI markers of cerebrovascular disease: Systematic review and meta-analysis. Neurology 2013, 81, 292–300. [Google Scholar] [CrossRef]
- Koizumi, K.; Hattori, Y.; Ahn, S.J.; Buendia, I.; Ciacciarelli, A.; Uekawa, K.; Wang, G.; Hiller, A.; Zhao, L.; Voss, H.U.; et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun. 2018, 9, 3816. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sajeev, G.; VanderWeele, T.J.; Viswanathan, A.; Sigurdsson, S.; Eiriksdottir, G.; Aspelund, T.; Betensky, R.A.; Grodstein, F.; Hofman, A.; et al. APOE ε4 and late-life cognition: Mediation by structural brain imaging markers. Eur. J. Epidemiol. 2022, 37, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Jiang, Y.; Wang, Y.; Wu, X.; Ren, J.; Lee, M.S.; Lee, S.; Huang, L. Modulation on brain gray matter activity and white matter integrity by APOE ε4 risk gene in cognitively intact elderly: A multimodal neuroimaging study. Behav. Brain Res. 2017, 322, 100–109. [Google Scholar] [CrossRef]
- Westlye, L.T.; Reinvang, I.; Rootwelt, H.; Espeseth, T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology 2012, 79, 1961–1969. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Albert, M.S.; Alonso, A.; Coker, L.H.; Coresh, J.; Davis, S.M.; Deal, J.A.; McKhann, G.M.; Mosley, T.H.; Sharrett, A.R.; et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017, 74, 1246–1254. [Google Scholar] [CrossRef]
- Fullerton, S.M.; Shirman, G.A.; Strittmatter, W.J.; Matthew, W.D. Impairment of the blood-nerve and blood-brain barriers in apolipoprotein e knockout mice. Exp. Neurol. 2001, 169, 13–22. [Google Scholar] [CrossRef]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimer’s Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Mok, V.; Foster, P.J.; Trucco, E.; Chen, C.; Wong, T.Y. Retinal imaging in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2021, 92, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Alber, J.; Goldfarb, D.; Thompson, L.I.; Arthur, E.; Hernandez, K.; Cheng, D.; DeBuc, D.C.; Cordeiro, F.; Provetti-Cunha, L.; den Haan, J.; et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimer’s Dement. 2020, 16, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tikellis, G.; Liew, G.; Klein, R.; Larsen, E.K.; Wong, T.Y. Apolipoprotein E gene and retinal microvascular signs in older people: The Cardiovascular Health Study. Mol. Vis. 2007, 13, 2105–2111. [Google Scholar]
- Frost, S.; Bhuiyan, A.; Offerman, D.; Doecke, J.D.; Macaulay, S.L.; Sohrabi, H.R.; Ames, D.; Masters, C.; Martins, R.N.; Kanagasingam, Y.; et al. Modulation of Retinal Arteriolar Central Reflection by APOE Genotype. Curr. Alzheimer Res. 2017, 14, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Lumbroso, B.; Rispoli, M. In Vivo Characterization of Retinal Vascularization Morphology Using Optical Coherence Tomography Angiography. Retina 2015, 35, 2196–2203. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Song, A.; Johnson, N.; Ayala, A.; Thompson, A.C. Optical Coherence Tomography in Patients with Alzheimer’s Disease: What Can It Tell Us? Eye Brain 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Moons, L.; De Groef, L. Multimodal retinal imaging to detect and understand Alzheimer’s and Parkinson’s disease. Curr. Opin. Neurobiol. 2022, 72, 1–7. [Google Scholar] [CrossRef]
- Robbins, C.B.; Thompson, A.C.; Bhullar, P.K.; Koo, H.Y.; Agrawal, R.; Soundararajan, S.; Yoon, S.P.; Polascik, B.W.; Scott, B.L.; Grewal, D.S.; et al. Characterization of Retinal Microvascular and Choroidal Structural Changes in Parkinson Disease. JAMA Ophthalmol. 2021, 139, 182–188. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, R.; Qin, R.; Chen, X.; Zou, J.; Jiang, Y.; Ye, Q.; Zhang, B.; Bai, F.; Xu, Y. Characteristic changes in the default mode network in hypertensive patients with cognitive impairment. Hypertens. Res. 2019, 42, 530–540. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Sun, W.; Chen, H.; Ye, Q.; Yang, D.; Li, M.; Luo, C.; Ma, J.; Shao, P.; et al. Early Segmental White Matter Fascicle Microstructural Damage Predicts the Corresponding Cognitive Domain Impairment in Cerebral Small Vessel Disease Patients by Automated Fiber Quantification. Front. Aging Neurosci. 2020, 12, 598242. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Bao, T.; Ou, R.; Wei, Q.; Chen, Y.; Yang, J.; Chen, X.; Shang, H. Effects of Sex on the Relationship Between Apolipoprotein E Gene and Serum Lipid Profiles in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 844066. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kwapong, W.R.; Tao, W.; Cao, L.; Ye, C.; Liu, J.; Zhang, S.; Wu, B. Association of retinal thickness and microvasculature with cognitive performance and brain volumes in elderly adults. Front. Aging Neurosci. 2022, 14, 1010548. [Google Scholar] [CrossRef]
- Lu, K.; Kwapong, W.R.; Jiang, S.; Zhang, X.; Xie, J.; Ye, C.; Yan, Y.; Cao, L.; Zhao, Y.; Wu, B. Differences in retinal microvasculature between large artery atherosclerosis and small artery disease: An optical coherence tomography angiography study. Front. Aging Neurosci. 2022, 14, 1053638. [Google Scholar] [CrossRef] [PubMed]
- Tewarie, P.; Balk, L.; Costello, F.; Green, A.; Martin, R.; Schippling, S.; Petzold, A. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 2012, 7, e34823. [Google Scholar] [CrossRef] [PubMed]
- Aytulun, A.; Cruz-Herranz, A.; Aktas, O.; Balcer, L.J.; Balk, L.; Barboni, P.; Blanco, A.A.; Calabresi, P.A.; Costello, F.; Sanchez-Dalmau, B.; et al. APOSTEL 2.0 Recommendations for Reporting Quantitative Optical Coherence Tomography Studies. Neurology 2021, 97, 68–79. [Google Scholar] [CrossRef]
- LaFleur, B.J.; Greevy, R.A. Introduction to permutation and resampling-based hypothesis tests. J. Clin. Child. Adolesc. Psychol. 2009, 38, 286–294. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- Liew, G.; Shankar, A.; Wang, J.J.; Klein, R.; Bray, M.S.; Couper, D.J.; Sharrett, A.R.; Wong, T.Y. Apolipoprotein E gene polymorphisms and retinal vascular signs: The atherosclerosis risk in communities (ARIC) study. Arch. Ophthalmol. 2007, 125, 813–818. [Google Scholar] [CrossRef]
- Wong, T.Y.; Mitchell, P. Hypertensive retinopathy. N. Engl. J. Med. 2004, 351, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.M.; Ashimatey, S.B.; Bennett, D.J.; Walters, S.M.; La Joie, R.; Jiang, X.; Wolf, A.; Cobigo, Y.; Staffaroni, A.M.; Rosen, H.J.; et al. Retinal imaging demonstrates reduced capillary density in clinically unimpaired APOE ε4 gene carriers. Alzheimer’s Dement. (Amst.) 2021, 13, e12181. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, Y.; Shi, Y.; Wright, C.B.; Sun, X.; Gregori, G.; Zheng, F.; Vanner, E.A.; Lam, B.L.; Rundek, T.; et al. Altered Macular Microvasculature in Mild Cognitive Impairment and Alzheimer Disease. J. Neuroophthalmol. 2018, 38, 292–298. [Google Scholar] [CrossRef]
- Zabel, P.; Kaluzny, J.J.; Wilkosc-Debczynska, M.; Gebska-Toloczko, M.; Suwala, K.; Zabel, K.; Zaron, A.; Kucharski, R.; Araszkiewicz, A. Comparison of Retinal Microvasculature in Patients With Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Murphy, O.; Caldito, N.G.; Calabresi, P.A.; Saidha, S. Emerging Applications of Optical Coherence Tomography Angiography (OCTA) in neurological research. Eye Vis. 2018, 5, 11. [Google Scholar] [CrossRef]
- Chua, J.; Hu, Q.; Ke, M.; Tan, B.; Hong, J.; Yao, X.; Hilal, S.; Venketasubramanian, N.; Garhöfer, G.; Cheung, C.Y.; et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimer’s Res. Ther. 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
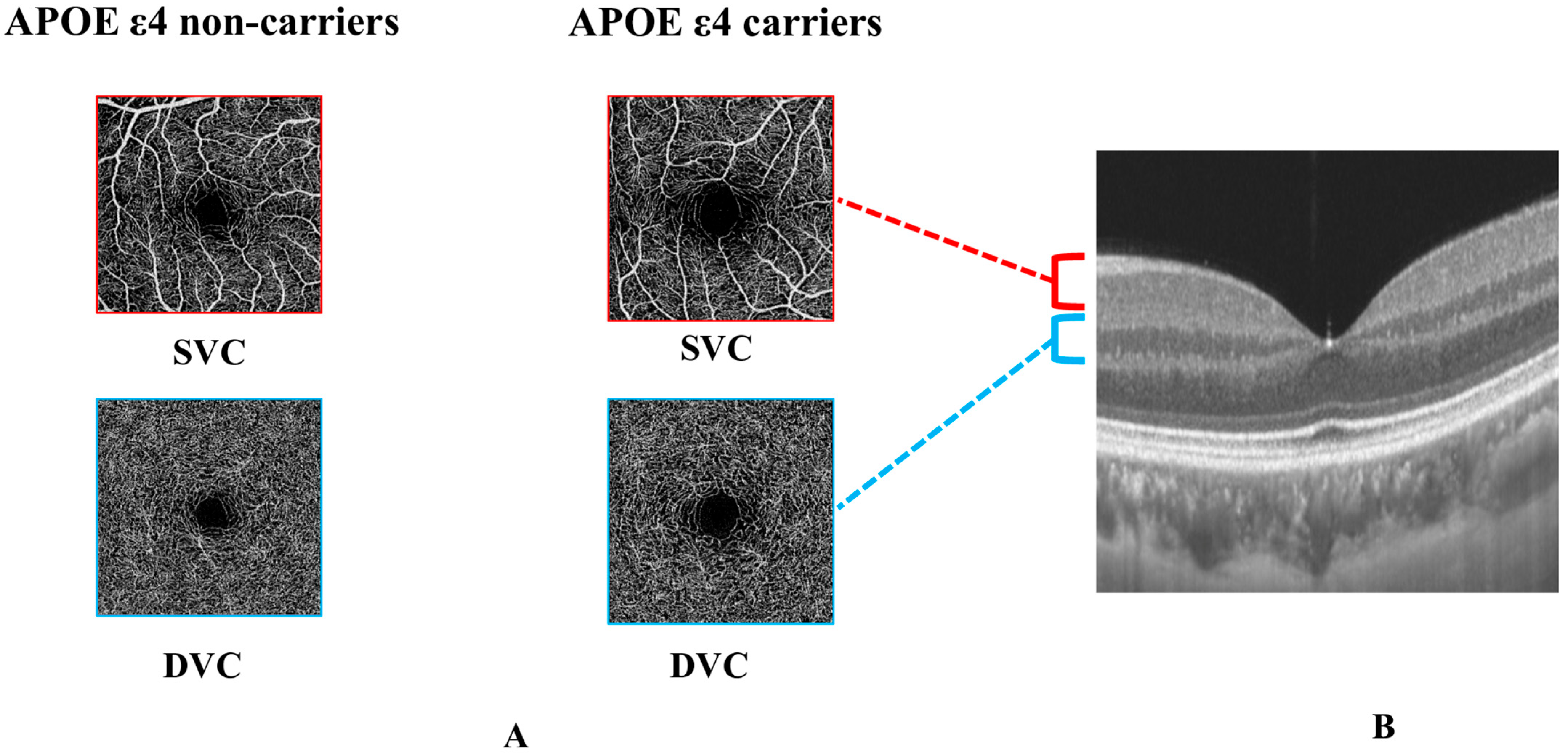

| All | APOE ε4 Non-Carriers | APOE ε4 Carriers | p-Value | |
|---|---|---|---|---|
| n | 163 | 128 | 35 | |
| Age, years | 59 (54–65) | 59 (54–65) | 58 (54–65) | 0.810 |
| Female, n (%) | 102 (62.58%) | 82 (64.06%) | 20 (57.14%) | 0.581 |
| Education, years | 9 (6–12) | 9 (6–12) | 9 (6–15) | 0.395 |
| Hypertension, n (%) | 43 (26.71%) | 35 (27.78%) | 8 (22.86%) | 0.714 |
| Diabetes, n (%) | 18 (11.18%) | 16 (12.70%) | 2 (5.71%) | 0.366 |
| Hyperlipidemia, n (%) | 59 (36.65%) | 50 (39.68%) | 9 (25.71%) | 0.187 |
| Smoking, n (%) | 13 (8.07%) | 9 (7.14%) | 4 (11.43%) | 0.482 |
| Drinkers, n (%) | 14 (8.70%) | 12 (9.52%) | 2 (5.71%) | 0.736 |
| MMSE | 29 (26–30) | 29 (26–30) | 29 (27–30) | 0.281 |
| MoCA | 23 (20–26) | 23 (20–26) | 23 (21–27) | 0.724 |
| VA, LogMAR | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.1 (0.1–0.2) | 0.304 |
| OCTA parameters | ||||
| Eyes, n | 311 | 242 | 69 | |
| SVC, % | 39.23 ± 5.42 | 39.61 ± 5.32 | 37.91 ± 5.58 | 0.021 * |
| DVC, % | 50.22 ± 4.12 | 50.39 ± 3.92 | 49.64 ± 4.75 | 0.182 |
| CI | NCI | p-Value | ε4 Carriers | ε4 Non-Carriers | p-Value | |
|---|---|---|---|---|---|---|
| SVC, % | 38.42 ± 5.32 | 39.91 ± 5.42 | 0.006 * | 37.91 ± 5.58 | 39.61 ± 5.32 | 0.023 † |
| DVC, % | 49.92 ± 3.97 | 50.49 ± 4.25 | 0.231 | 49.64 ± 4.75 | 50.39 ± 3.92 | 0.221 |
| ε4 Carriers | ε4 Non-Carriers | p-Value | ||
|---|---|---|---|---|
| NCI | SVC, % | 39.05 ± 5.84 | 40.18 ± 5.28 | 0.796 |
| DVC, % | 50.39 ± 4.84 | 50.51 ± 4.07 | 0.780 | |
| CI | SVC, % | 36.42 ± 4.92 | 38.96 ± 5.32 | 0.006 * |
| DVC, % | 48.66 ± 4.53 | 50.25 ± 3.76 | 0.048 * |
| Variables | SVC | DVC | ||||
|---|---|---|---|---|---|---|
| - | B | SE | p-Value | B | SE | p-Value |
| APOE ε4 carriers | −1.638 | 2.588 | 0.024 * | −0.772 | 0.579 | 0.183 |
| CI | −1.397 | 0.606 | 0.021 * | −0.674 | 0.485 | 0.166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kwapong, W.R.; Cao, L.; Feng, Z.; Wu, B.; Liu, J.; Zhang, S. APOE ε4 Gene Carriers Demonstrate Reduced Retinal Capillary Densities in Asymptomatic Older Adults. J. Clin. Med. 2023, 12, 5649. https://doi.org/10.3390/jcm12175649
Zhang Z, Kwapong WR, Cao L, Feng Z, Wu B, Liu J, Zhang S. APOE ε4 Gene Carriers Demonstrate Reduced Retinal Capillary Densities in Asymptomatic Older Adults. Journal of Clinical Medicine. 2023; 12(17):5649. https://doi.org/10.3390/jcm12175649
Chicago/Turabian StyleZhang, Ziyi, William Robert Kwapong, Le Cao, Zijuan Feng, Bo Wu, Junfeng Liu, and Shuting Zhang. 2023. "APOE ε4 Gene Carriers Demonstrate Reduced Retinal Capillary Densities in Asymptomatic Older Adults" Journal of Clinical Medicine 12, no. 17: 5649. https://doi.org/10.3390/jcm12175649
APA StyleZhang, Z., Kwapong, W. R., Cao, L., Feng, Z., Wu, B., Liu, J., & Zhang, S. (2023). APOE ε4 Gene Carriers Demonstrate Reduced Retinal Capillary Densities in Asymptomatic Older Adults. Journal of Clinical Medicine, 12(17), 5649. https://doi.org/10.3390/jcm12175649






