Phase Angle, Inflammation, and Sarcopenia in Late Postoperative Roux-En-Y Gastric Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Settings
2.2. Subjects
2.3. Anthropometric Measurements
2.4. Body Composition Analysis
2.5. Handgrip Strength Assessment (HGS)
2.6. Gait Speed Assessment
2.7. Appendicular Lean Mass Assessment (ALM)
2.8. Sarcopenia Classification
2.9. Assay Methods
2.10. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | Reactance Coefficient p Value | Resistance Coefficient p Value | PhA Coefficient p Value | Adiponectin Coefficient p Value | HOMA-IR Coefficient p Value | IL1-β Coefficient p Value |
|---|---|---|---|---|---|---|
| Men | ||||||
| Handgrip strength | −0.09133 0.7894 | −0.17273 0.6115 | 0.02727 0.9366 | 0.21818 0.5192 | 0.01818 0.9577 | 0.83636 0.00013 ** |
| Gait speed | 0.22375 0.5084 | 0.42727 0.1899 | 0.07273 0.8317 | 0.66364 0.0260 ** | −0.609 0.0467 ** | 0.23636 0.4841 |
| ALM | −0.16247 0.6332 | −0.44647 0.1686 | 0.03189 0.9258 | −0.68793 0.0193 ** | 0.20957 0.5363 | 0.53759 0.0881 |
| Women | ||||||
| Handgrip strength | −0.29998 0.1544 | −0.36894 0.0760 | −0.02958 0.8909 | −0.41654 0.0677 | 0.10568 0.6231 | 0.000308 0.9883 |
| Gait speed | −0.09145 0.6501 | −0.34574 0.0773 | 0.09881 0.6239 | 0.38246 0.0790 | −0.08718 0.6654 | −0.10012 0.6122 |
| ALM | −0.64009 0.0013 ** | −0.56538 0.006 ** | −0.19938 0.3737 | −0.12998 0.6190 | 0.20057 0.3708 | 0.09933 0.6520 |
| Parameters | Reactance Coefficient p Value | Resistance Coefficient p Value | PhA Coefficient p Value | Hs-CRP Coefficient p Value | HOMA-IR Coefficient p Value | Adiponectin Coefficient p Value |
|---|---|---|---|---|---|---|
| Men | ||||||
| Handgrip strength | −0.10858 0.7369 | −0.20280 0.5273 | 0.03497 0.9141 | −0.05406 0.9084 | 0.10490 0.7456 | 0.72727 0.0074 ** |
| Gait speed | −0.15993 0.6195 | 0.41053 0.1850 | 0.01404 0.9655 | 0.65455 0.1106 | 0.09474 0.7696 | 0.42807 0.1651 |
| ALM | −0.52254 0.2289 | −0.78571 0.0362 ** | −0.42857 0.3374 | 1.00000 | 0.39286 0.3833 | 0.67857 0.0938 |
| Women | ||||||
| Handgrip strength | −0.16762 0.5062 | 0.07537 0.7663 | −0.25697 0.3033 | 0.06007 0.8384 | −0.03818 0.8804 | −0.05882 0.8167 |
| Gait speed | −0.48030 0.0510 ** | −0.11548 0.6590 | −0.39411 0.1175 | 0.22409 0.4412 | 0.15838 0.5438 | −0.14487 0.5790 |
| ALM | −0.41180 0.0895 | −0.41507 0.0867 | −0.26316 0.2914 | −0.10679 0.7163 | −0.04025 0.8740 | −0.12074 0.6332 |
References
- Donini, L.M.; Poggiogalle, E.; Migliaccio, S.; Aversa, A.; Pinto, A. Body composition in sarcopenic obesity: Systematic review of the literature. Mediterr. J. Nutr. Metab. 2013, 6, 191–198. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Dufour, A.B.; Hannan, M.T.; Murabito, J.M.; Kiel, D.P.; McLean, R.R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 168–174. [Google Scholar] [CrossRef]
- Vittone, J.L.; Bailor, D.L.; Nair, K.S. Muscle wasting in the elderly. Age Nutr. 1996, 7, 96–105. [Google Scholar]
- Lexell, J. Human aging, muscle mass, and fiber type composition. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 11–16. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Waters, D.L.; Baumgartner, R.N. Sarcopenia and obesity. Clin. Geriatr. Med. 2011, 27, 401–421. [Google Scholar] [CrossRef]
- Sjöström, L. A computer-tomography based multicompartment body composition technique and anthropometric predictions of lean body mass, total and subcutaneous adipose tissue. Int. J. Obes. 1991, 15 (Suppl. S2), 19–30. [Google Scholar] [PubMed]
- Choi, Y.J. Dual-Energy X-ray Absorptiometry: Beyond Bone Mineral Density Determination. Endocrinol. Metab. 2016, 31, 25–30. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Stubbs, B.; Veronese, N.; Manzato, E. Measurement of lean body mass using bioelectrical impedance analysis: A consideration of the pros and cons. Aging Clin. Exp. Res. 2017, 29, 591–597. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Sun, J.S.; Lee, Y.H.; Lee, J.H.; Hong, J.; Lee, J.M. Comparative assessment of skeletal muscle mass using computerized tomography and bioelectrical impedance analysis in critically ill patients. Clin. Nutr. 2019, 38, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Aller, R.; Romero, E.; Dueñas, A.; Perez Castrillon, J.L. Relation of phase angle tertiles with blood adipocytokines levels, insulin resistance and cardiovascular risk factors in obese women patients. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 521–526. [Google Scholar]
- Basile, C.; Della-Morte, D.; Cacciatore, F.; Gargiulo, G.; Galizia, G.; Roselli, M.; Curcio, F.; Bonaduce, D.; Abete, P. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp. Gerontol. 2014, 58, 43–46. [Google Scholar] [CrossRef]
- Kilic, M.K.; Kizilarslanoglu, M.C.; Arik, G.; Bolayir, B.; Kara, O.; Dogan Varan, H.; Sumer, F.; Kuyumcu, M.E.; Halil, M.; Ulger, Z. Association of Bioelectrical Impedance Analysis-Derived Phase Angle and Sarcopenia in Older Adults. Nutr. Clin. Pract. 2017, 32, 103–109. [Google Scholar] [CrossRef]
- Kołodziej, M.; Ignasiak, Z. Changes in the bioelectrical impedance parameters estimating appendicular skeletal muscle mass in healthy older persons. Aging Clin. Exp. Res. 2019, 32, 1939–1945. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health. 2010. Available online: https://www.who.int/publications/i/item/9789241599979 (accessed on 31 January 2020).
- Batista, G.A.; Souza, A.L.; Marin, D.M.; Sider, M.; Melhado, V.C.; Fernandes, A.M.; Alegre, S.M. Body composition, resting energy expenditure and inflammatory markers: Impact in users of depot medroxyprogesterone acetate after 12 months follow-up. Arch. Endocrinol. Metab. 2017, 61, 70–75. [Google Scholar] [CrossRef]
- Ben-Noun, L.; Laor, A. Relationship of neck circumference to cardiovascular risk factors. Obes. Res. 2003, 11, 226–231. [Google Scholar] [CrossRef]
- World Health Organization. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. Tech. Rep. Ser. 2000, 894, 1–20.
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F. Bioelectrical impedance analysis: A review of principles and applications. J. Am. Coll. Nutr. 1992, 11, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.G.; Olson, B.; Palmiter-Thomas, P. How forearm position affects grip strength. Am. J. Occup. Ther. 1996, 50, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef]
- Steiger, U.; Lippuner, K.; Jensen, E.X.; Montandon, A.; Jaeger, P.; Horber, F.F. Body composition and fuel metabolism after kidney grafting. Eur. J. Clin. Investig. 1995, 25, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Geloneze, B.; Vasques, A.C.; Stabe, C.F.; Pareja, J.C.; Rosado, L.E.; Queiroz, E.C.; Tambascia, M.A.; Investigators, B. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef]
- Vaurs, C.; Diméglio, C.; Charras, L.; Anduze, Y.; Chalret du Rieu, M.; Ritz, P. Determinants of changes in muscle mass after bariatric surgery. Diabetes Metab. 2015, 41, 416–421. [Google Scholar] [CrossRef]
- Alba, D.L.; Wu, L.; Cawthon, P.M.; Mulligan, K.; Lang, T.; Patel, S.; King, N.J.; Carter, J.T.; Rogers, S.J.; Posselt, A.M.; et al. Changes in Lean Mass, Absolute and Relative Muscle Strength, and Physical Performance After Gastric Bypass Surgery. J. Clin. Endocrinol. Metab. 2019, 104, 711–720. [Google Scholar] [CrossRef]
- Otto, M.; Kautt, S.; Kremer, M.; Kienle, P.; Post, S.; Hasenberg, T. Handgrip strength as a predictor for post bariatric body composition. Obes. Surg. 2014, 24, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Samat, A.; Wolski, K.; Abood, B.; Pothier, C.E.; Bhatt, D.L.; Nissen, S.; Brethauer, S.A.; Schauer, P.R.; Kirwan, J.P.; et al. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: Effects of bariatric surgery vs standard medical therapy. Int. J. Obes. 2014, 38, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Mehaffey, J.H.; Mehaffey, R.L.; Mullen, M.G.; Turrentine, F.E.; Malin, S.K.; Schirmer, B.; Wolf, A.M.; Hallowell, P.T. Nutrient Deficiency 10 Years Following Roux-en-Y Gastric Bypass: Who’s Responsible? Obes. Surg. 2017, 27, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, G.; Hasenberg, T.; Krammer, J.; Kienle, P.; Ronellenfitsch, U.; Otto, M. The Phase Angle of the Bioelectrical Impedance Analysis as Predictor of Post-Bariatric Weight Loss Outcome. Obes. Surg. 2017, 27, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Danielzik, S.; Dörhöfer, R.P.; Later, W.; Wiese, S.; Müller, M.J. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. JPEN J. Parenter Enteral. Nutr. 2006, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martínez, M.; Rodríguez-García, W.; González-Islas, D.; Orea-Tejeda, A.; Keirns-Davis, C.; Salgado-Fernández, F.; Hernández-López, S.; Jiménez-Valentín, A.; Ríos-Pereda, A.V.; Márquez-Cordero, J.C.; et al. Impact of Body Composition and Sarcopenia on Mortality in Chronic Obstructive Pulmonary Disease Patients. J. Clin. Med. 2023, 12, 1321. [Google Scholar] [CrossRef]
- Maddocks, M.; Kon, S.S.; Jones, S.E.; Canavan, J.L.; Nolan, C.M.; Higginson, I.J.; Gao, W.; Polkey, M.I.; Man, W.D. Bioelectrical impedance phase angle relates to function, disease severity and prognosis in stable chronic obstructive pulmonary disease. Clin. Nutr. 2015, 34, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Inflammation versus host defense in obesity. Cell Metab. 2014, 20, 708–709. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am. J. Cardiol. 2008, 101, 11E–15E. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, R.B. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: Implications for glucose and fatty acids homeostasis. Int. J. Obes. 2005, 29, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Pahlavani, H. Exercise Therapy for People with Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Chen, W.L. Examining the Association Between Serum Leptin and Sarcopenic Obesity. J. Inflamm. Res. 2021, 14, 3481–3487. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
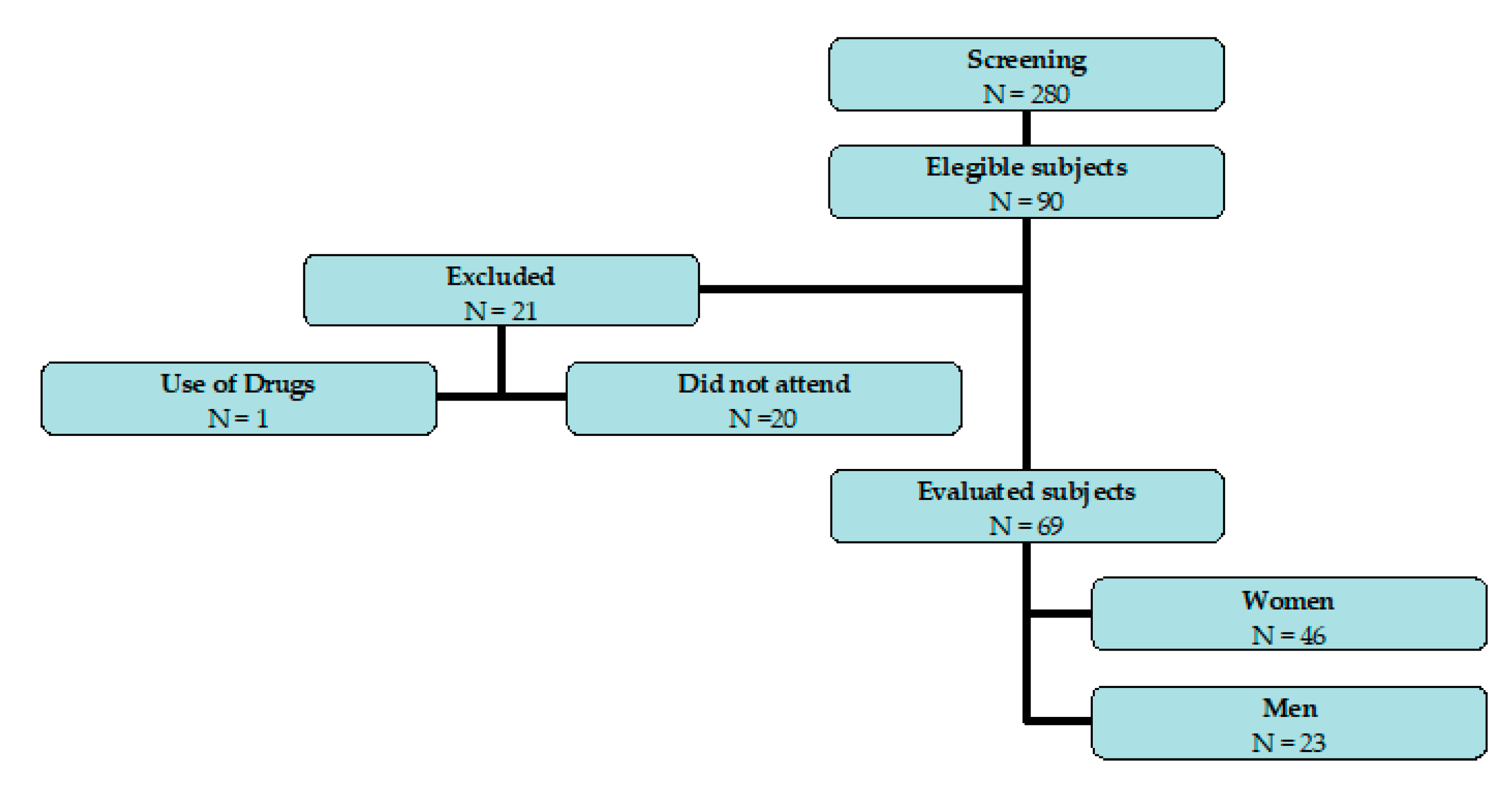

| Parameters | Preoperative | Postoperative | ||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | All | p Value | Women | Men | All | p Value | |
| Number of participants | 28 | 11 | 39 | - | 18 | 12 | 30 | - |
| Age (years) | 36.57 ± 6.75 | 41.82 ± 12.25 | 38.1 ± 8.81 | 0.9281 1 0.7435 a | 37.1 ± 5.58 | 42.17 ± 8.45 | 39.1 ± 7.19 | 0.9019 2 |
| Weight (kg) | 117.81 ± 17.21 | 131.11 ± 19.91 | 121.6 ± 18.75 | <0.0001 1,* <0.0001 a,* | 69.39 ± 10.52 | 83.67 ± 11.93 | 75.1 ± 13.02 | <0.0001 2,* |
| Height (m) | 1.62 ± 0.07 | 1.74 ± 0.06 | 1.66 ± 0.08 | 0.8745 1 0.7711 a | 1.62 ± 0.06 | 1.73 ± 0.06 | 1.70 ± 0.08 | 0.7808 2 |
| BMI (kg/m²) | 44.68 ± 5.68 | 42.93 ± 4.94 | 44.2 ± 5.47 | <0.0001 1,* <0.0001 a,* | 26.53 ± 3.04 | 27.9 ± 4.11 | 27.1 ± 3.51 | <0.0001 2,* |
| WC (cm) | 118.55 ± 9.99 | 129.95 ± 13.78 | 121.8 ± 12.16 | <0.0001 1,* <0.0001 a,* | 80.44 ± 9.27 | 91.75 ± 11.44 | 85 ± 11.48 | 0.0001 2,* |
| NC (cm) | 40.2 ± 2.43 | 47.65 ± 3.85 | 42.4 ± 4.46 | <0.0001 1,* <0.0001 a,* | 32.39 ± 2.07 | 37.67 ± 1.89 | 34.5 ± 3.28 | <0.0001 ²,* |
| SBP (mmHg) | 128.86 ± 14.54 | 138.36 ± 19.55 | 131.5 ± 16.42 | 0.0016 1,** 0.0002 a,** | 113.89 ± 12.38 | 119.67 ± 13.40 | 116.2 ± 12.89 | 0.0244 2 ** |
| DBP (mmHg) | 81.29 ± 18.10 | 89.82 ± 11.88 | 83.7 ± 16.88 | 0.0311 1,** 0.0077 a,** | 75 ± 10.90 | 79.83 ± 12.25 | 76.5 ± 11.5 | 0.0601 2 |
| Postoperative time (months) | - | - | - | - | 45.75 ± 10.47 | 44.5 ± 8.89 | 45.1 ± 9.5 | - |
| Weight loss postoperative (kg) | - | - | - | - | 56.64 ± 13.97 | 66.17 ± 50.90 | 61.0 ± 35.6 | - |
| Weight loss postoperative (%) | - | - | - | - | 55.27 ± 7.40 | 58.93 ± 10.98 | 57.0 ± 9.2 | - |
| Parameters | Preoperative | Postoperative | ||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | All | p Value | Women | Men | All | p Value | |
| Number of participants | 28 | 11 | 39 | - | 18 | 12 | 30 | - |
| FM (%) | 46.29 ± 3.64 | 35.93 ± 3.13 | 53 ± 11.49 | <0.0001 1,* <0.0001 a,* | 30.48 ± 6.61 | 23.96 ± 7.32 | 21.3 ± 7.93 | 0.0012 2,** |
| FM (kg) | 55.27 ± 11.24 | 47.49 ± 10.63 | 69.1 ± 12.29 | <0.0001 1,* <0.0001 a,* | 21.63 ± 7.30 | 20.68 ± 9.10 | 53.9 ± 9.15 | 0.0002 2,** |
| FFM (kg) | 63.21 ± 7.06 | 83.58 ± 10.24 | 43.3 ± 5.89 | <0.0001 1,* <0.0001 a,* | 47.76 ± 4.9 | 62.98 ± 5.67 | 27.9 ± 7.51 | 0.0001 2,* |
| PhA (degree) | 4.44 ± 1.53 | 4.93 ± 0.84 | 4.6 ± 1.37 | 0.0036 1,** <0.0007 a,** | 3.38 ± 0.92 | 4.10 ± 0.79 | 3.7 ± 0.93 | 0.0337 2,** |
| Resistance (Ohm Ω) | 497.56 ± 110.07 | 384.55 ± 50.15 | 464.8 ± 109 | 0.0199 1,** 0.0026 a,** | 559.56 ± 61.92 | 470.50 ± 47.25 | 523.9 ± 71.2 | 0.0012 2,** |
| Reactance (Ohm Ω) | 39.07 ± 22.52 | 33.45 ± 8.66 | 37.4 ± 19.6 | 0.5465 1 0.6652 a | 33.28 ± 10.11 | 33.83 ± 7.66 | 33.5 ± 9.1 | 0.8532 2 |
| BMR (kcal /d) | 1920.41 ± 215.95 | 2541.59 ± 311.52 | 2100.1 ± 374.7 | <0.0001 1,* <0.0001 a,* | 1452.17 ± 148.71 | 1915.17 ± 172.47 | 1637.4 ± 278.3 | 0.0002 2,** |
| Parameters | Preoperative | Postoperative | ||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Number of participants | 28 | 11 | 18 | 12 |
| Handgrip strength (<20 kgf; <30 kgf) % (n) | 17.86% (5) | 9.09 % (1) | 22.22% (4) | 16.66% (2) |
| Gait speed (<0.8 m/s) % (n) | 3.57% (1) | ¥ | ¥ | ¥ |
| ALM (kg/h²) % (n) | ¥ | ¥ | ¥ | ¥ |
| Parameters | Preoperative | Postoperative | ||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | All | p Value | Women | Men | All | p Value | |
| Number of participants | 28 | 11 | 39 | - | 18 | 12 | 30 | - |
| Insulin (mg/dL) | 17.53 ± 8.58 | 20.52 ± 5.23 | 18.4 ± 7.8 | <0.0001 1 <0.0001 a,* | 4.23 ± 2.29 | 4.88 ± 2.08 | 4.5 ± 2.2 | <0.0001 2,** |
| Glucose (mg/dL) | 88.20 ± 13.70 | 111.10 ± 56.10 | 94.8 ± 33.1 | 0.0003 1 <0.0001 a,* | 77.33 ± 4.94 | 75.80 ± 5.45 | 76.7 ± 5.1 | 0.0002 2,** |
| HOMA-IR | 3.81 ± 2.40 | 5.47 ± 2.43 | 4.4 ± 2.4 | <0.0001 1 <0.0001 a,* | 0.81 ± 0.45 | 0.91 ± 0.39 | 0.8 ± 0.4 | <0.0001 2,* |
| HbGli (%) | 5.69 ± 0.81 | 6.69 ± 2.12 | 6.0 ± 1.4 | 0.0627 ¹ 0.0003 a,** | 5.26 ± 0.40 | 5.13 ± 0.27 | 5.2 ± 0.4 | 0.0005 2,** |
| Total Cholesterol (mg/dL) | 174.04 ± 28.68 | 190.82 ± 35.17 | 179 ± 31.2 | <0.0001 1 <0.0001 a,* | 132.17 ± 25.52 | 146.00 ± 22.68 | 137.7 ± 25 | 0.0021 2,** |
| LDL–c (mg/dL) | 107.07 ± 26.06 | 122.00 ± 27.18 | 111.4 ± 26.9 | 0.0001 1 <0.0001 a,* | 72.83 ± 25.13 | 80.25 ± 24.21 | 75.8 ± 24.6 | 0.0019 2,** |
| HDL-c (mg/dL) | 40.12 ± 7.20 | 36.55 ± 6.67 | 39.1 ± 7.1 | 0.0010 1 <0.0001 a,* | 50.18 ± 10.10 | 54.08 ± 13.72 | 51.8 ± 11.7 | 0.0012 2,** |
| VLDL-c (mg/dL) | 26.70 ± 10.26 | 32.36 ± 8.55 | 28.3 ± 10 | <0.0001 1 <0.0001 a,* | 13.11 ± 4.16 | 32.36 ± 8.55 | 13.4 ± 3.9 | <0.0001 2,* |
| Triglyce-rides(mg/dL) | 129.74 ± 47.40 | 165.18 ± 50.81 | 140 ± 50.4 | <0.0001 1 <0.0001 a,* | 65.56 ± 20.54 | 69.58 ± 18.28 | 67.2 ± 19.4 | <0.0001 2,* |
| ALT (mg/dL) | 18.35 ± 7.54 | 32.67 ± 9.46 | 21.3 ± 9.8 | 0.0011 1,** 0.0007 a,** | 12.00 ± 3.46 | 16.17 ± 4.17 | 13.7 ± 4.3 | 0.0031 2 |
| AST (mg/dL) | 17.13 ± 3.45 | 25.17 ± 4.40 | 18.8 ± 4.9 | 0.1853 1 0.3023 a | 15.94 ± 4.05 | 20.50 ± 6.22 | 17.8 ± 5.5 | 0.1328 2 |
| Uric Acid (mg/dL) | 7.30 ± 5.76 | 8.92 ± 5.08 | 7.6 ± 5.6 | <0.0001 1 <0.0001 a,* | 3.48 ± 0.89 | 4.91 ± 1.04 | 4.1 ± 1.2 | 0.0077 2 |
| GGT (mg/dL) | 21.43 ± 11.02 | 40.52 ± 20.77 | 25.4 ± 15.3 | <0.0001 1 <0.0001 a,* | 9.07 ± 2.22 | 15.50 ± 5.54 | 11.9 ± 5.1 | 0.0274 2 |
| hs-CRP (mg/dL) | 4.87 ± 1.38 | 4.68 ± 1.63 | 4.82 ± 1.44 | <0.0001 1 <0.0001 a,* | 0.94 ± 1.68 | 1.50 ± 1.48 | 1.16 ± 1.60 | 0.0012 2 |
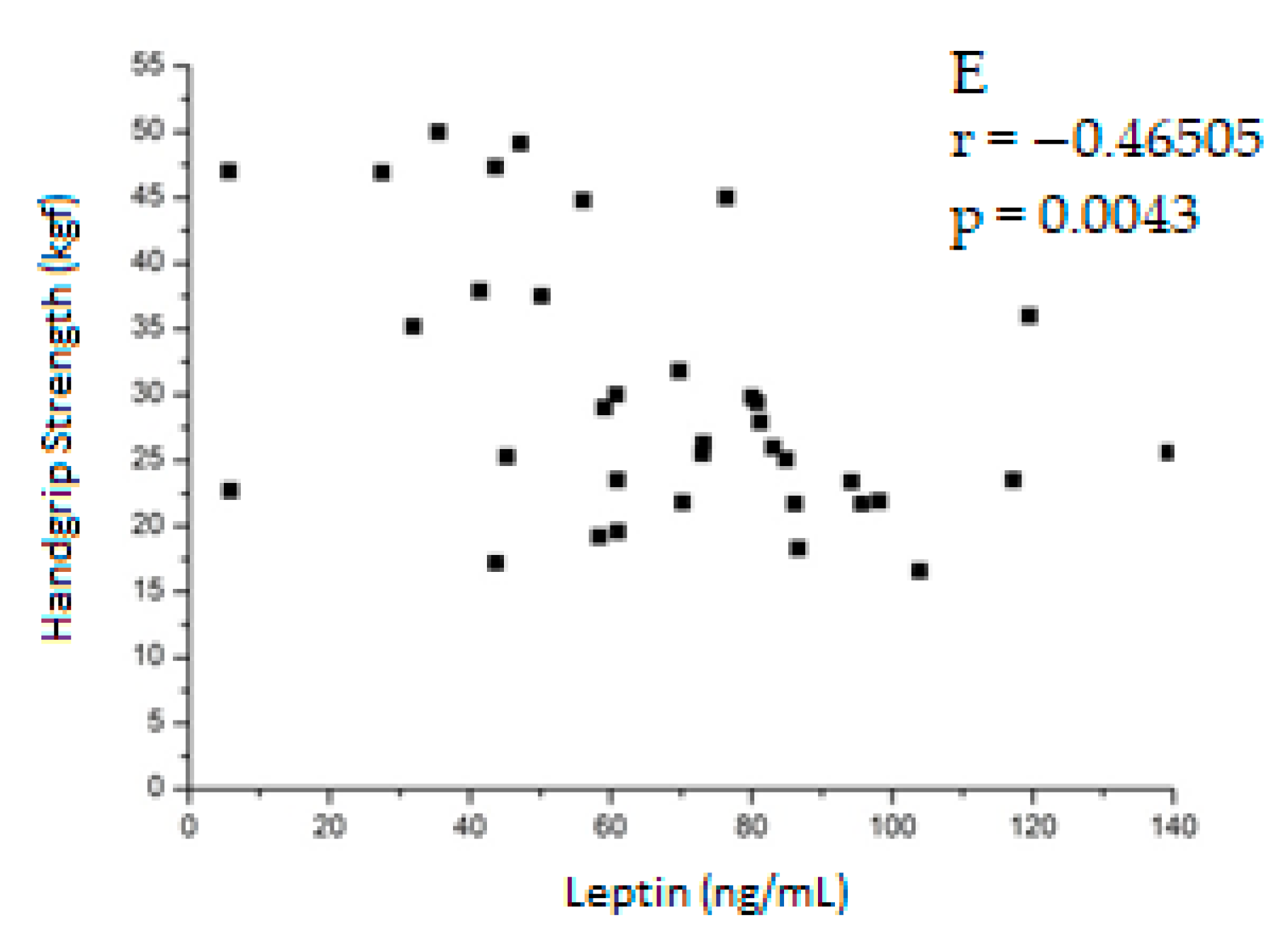
| Leptin (ng/mL) | 75.30 ± 29.22 | 41.87 ± 17.79 | 65.87 ± 30.37 | <0.0001 1 <0.0001 a | 19.50 ± 12.26 | 11.20 ± 8.28 | 16.18 ± 11.45 | 0.0004 2 |
| Parameters | Reactance Coefficient p Value | Resistance Coefficient p Value | PhA Coefficient p Value | Hs- CRP Coefficient p Value | Leptin Coefficient p Value | HOMA-IR Coefficient p Value |
|---|---|---|---|---|---|---|
| Preoperative | ||||||
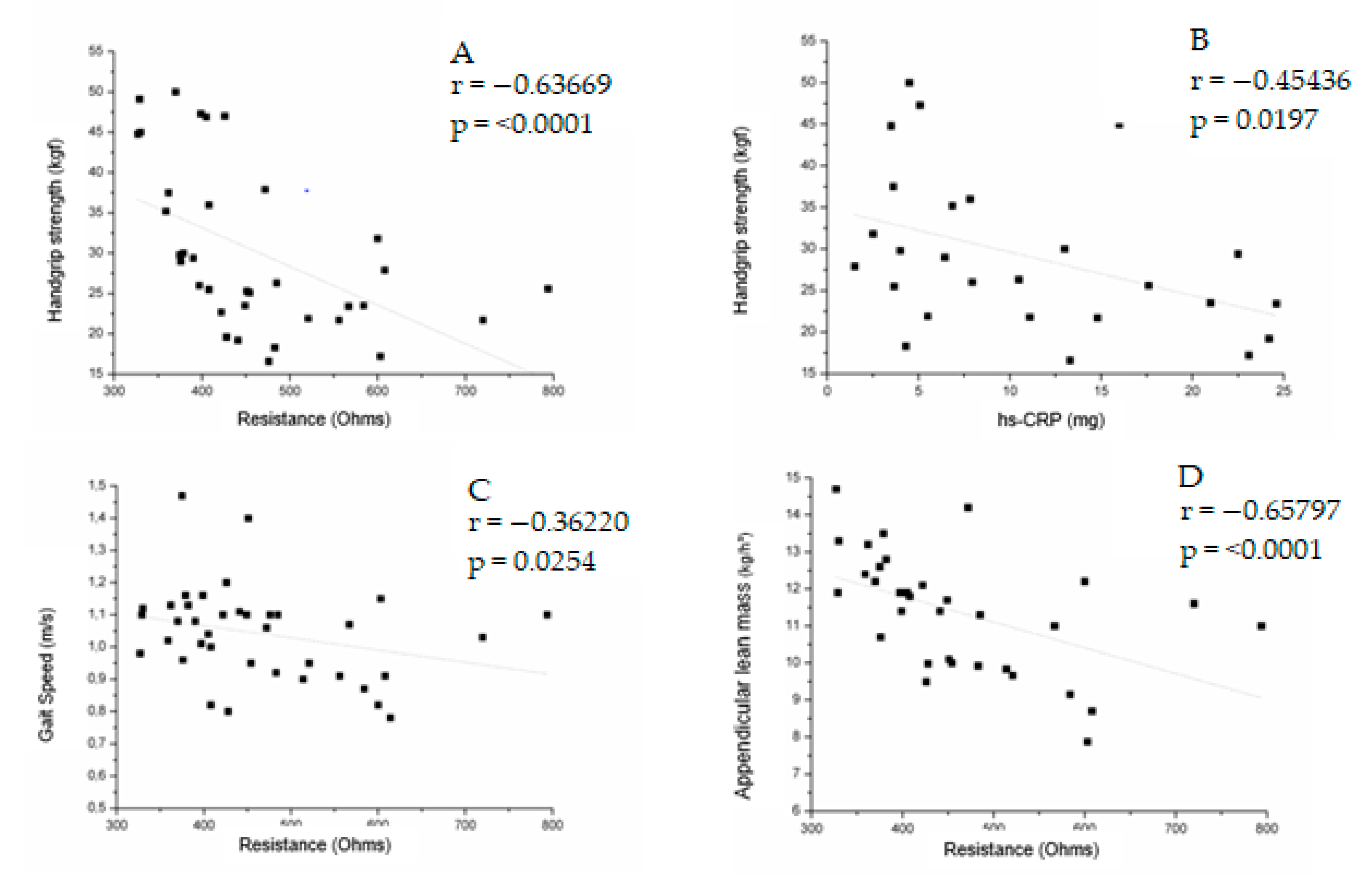
| Handgrip strength | −0.21901 0.2062 | −0.63669 <0.0001 * | 0.18351 0.2913 | −0.45436 0.0197 ** | −0.46505 0.0043 ** | 0.32455 0.0611 |
| Gait speed | −0.05695 0.7341 | −0.36220 0.0254 ** | 0.12684 0.4480 | 0.24500 0.2089 | −0.23532 0.1493 | −0.09166 0.5895 |
| ALM | −0.49485 0.0034 ** | −0.65797 <0.0001 * | −0.05702 0.7526 | −0.25562 0.2391 | −0.15701 0.3752 | 0.34941 0.0500 |
| Postoperative | ||||||
| Handgrip strength | −0.00669 0.9720 | −0.47147 0.0085 ** | 0.24538 0.1912 | 0.32571 0.1496 | −0.40200 0.0277 ** | 0.16796 0.3750 |
| Gait speed | −0.30006 0.1138 | −0.32540 0.0850 | −0.12580 0.5155 | −0.05474 0.8137 | 0.07770 0.6887 | 0.20128 0.2951 |
| ALM | −0.26003 0.2094 | −0.72668 <0.0001 * | 0.09385 0.6555 | −0.03735 0.8908 | −0.41000 0.00418 ** | 0.10692 0.6110 |
| Parameters | Preoperative | Postoperative | |
|---|---|---|---|
| (N = 6) | (N = 6) | p-Value | |
| Handgrip strength | 19.4 ± 3.1 | 21.5 ± 4.7 | 0.3358 |
| Gait speed (<0.8m/s) | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.9362 |
| ALM (kg/h²) | 9.9 ± 1.3 | 7.7 ± 1.2 | 0.0367 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florêncio, G.; Souza, A.; Chaim, E.; Santos, A.; Duran, L.; Carvalho, C.; Monte Alegre, S. Phase Angle, Inflammation, and Sarcopenia in Late Postoperative Roux-En-Y Gastric Bypass. J. Clin. Med. 2023, 12, 5124. https://doi.org/10.3390/jcm12155124
Florêncio G, Souza A, Chaim E, Santos A, Duran L, Carvalho C, Monte Alegre S. Phase Angle, Inflammation, and Sarcopenia in Late Postoperative Roux-En-Y Gastric Bypass. Journal of Clinical Medicine. 2023; 12(15):5124. https://doi.org/10.3390/jcm12155124
Chicago/Turabian StyleFlorêncio, Gisele, Aglécio Souza, Elinton Chaim, Allan Santos, Louise Duran, Camila Carvalho, and Sarah Monte Alegre. 2023. "Phase Angle, Inflammation, and Sarcopenia in Late Postoperative Roux-En-Y Gastric Bypass" Journal of Clinical Medicine 12, no. 15: 5124. https://doi.org/10.3390/jcm12155124
APA StyleFlorêncio, G., Souza, A., Chaim, E., Santos, A., Duran, L., Carvalho, C., & Monte Alegre, S. (2023). Phase Angle, Inflammation, and Sarcopenia in Late Postoperative Roux-En-Y Gastric Bypass. Journal of Clinical Medicine, 12(15), 5124. https://doi.org/10.3390/jcm12155124





