Free-Hand MIS TLIF without 3D Navigation—How to Achieve Low Radiation Exposure for Both Surgeon and Patient
Abstract
1. Introduction
2. Materials and Methods
2.1. Advanced Radiation Protection Principles
- Wearing protective gear (two-piece lead apron, thyroid lead collar, and protective goggles);
- Optimal use of a beam collimator whenever possible;
- Minimized distance from the patient to the flat panel detector;
- Maximized staff distance from the patient as source of Compton scattering;
- Removal of the hand from the X-ray beam when holding an instrument is necessary (use of surgical clamp to increase distance);
- No continuous fluoroscopy.
2.2. Radiation-Sparing Surgical Protocol
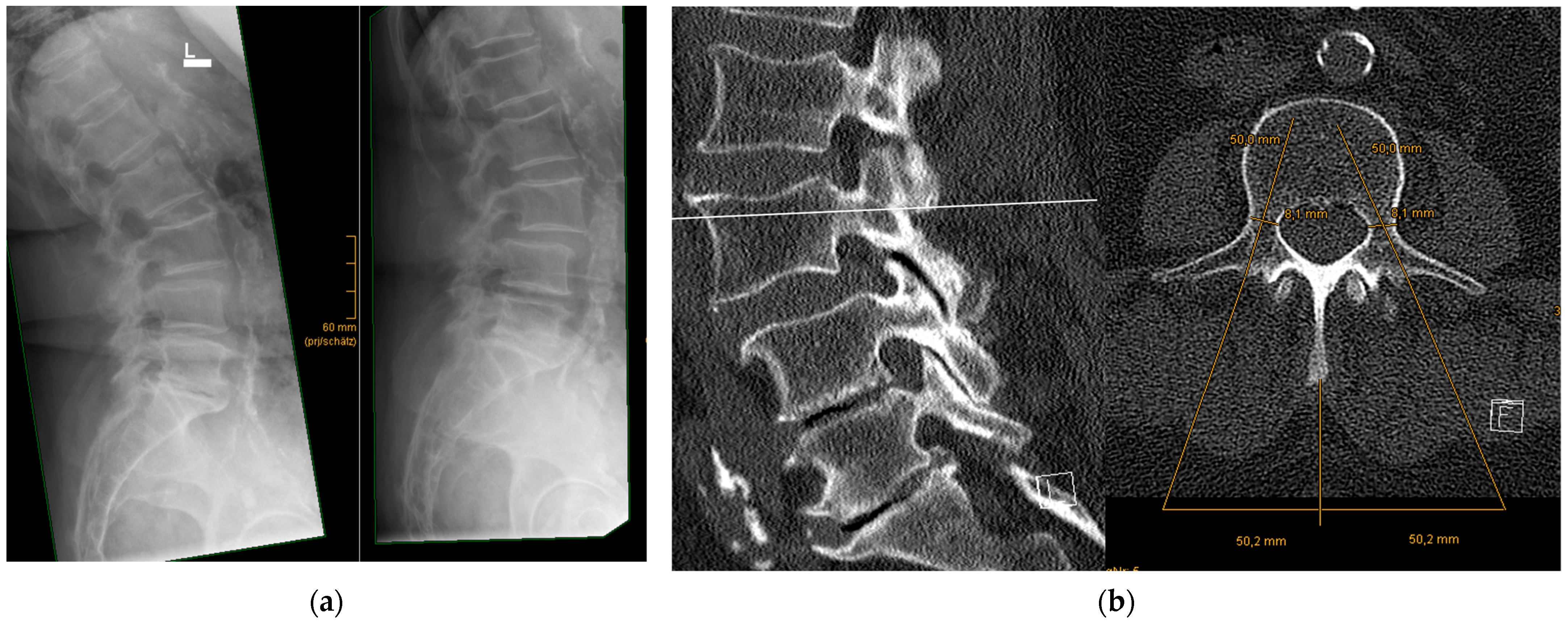
- Precise preoperative planning of screw trajectories and screw dimensions
- 2.
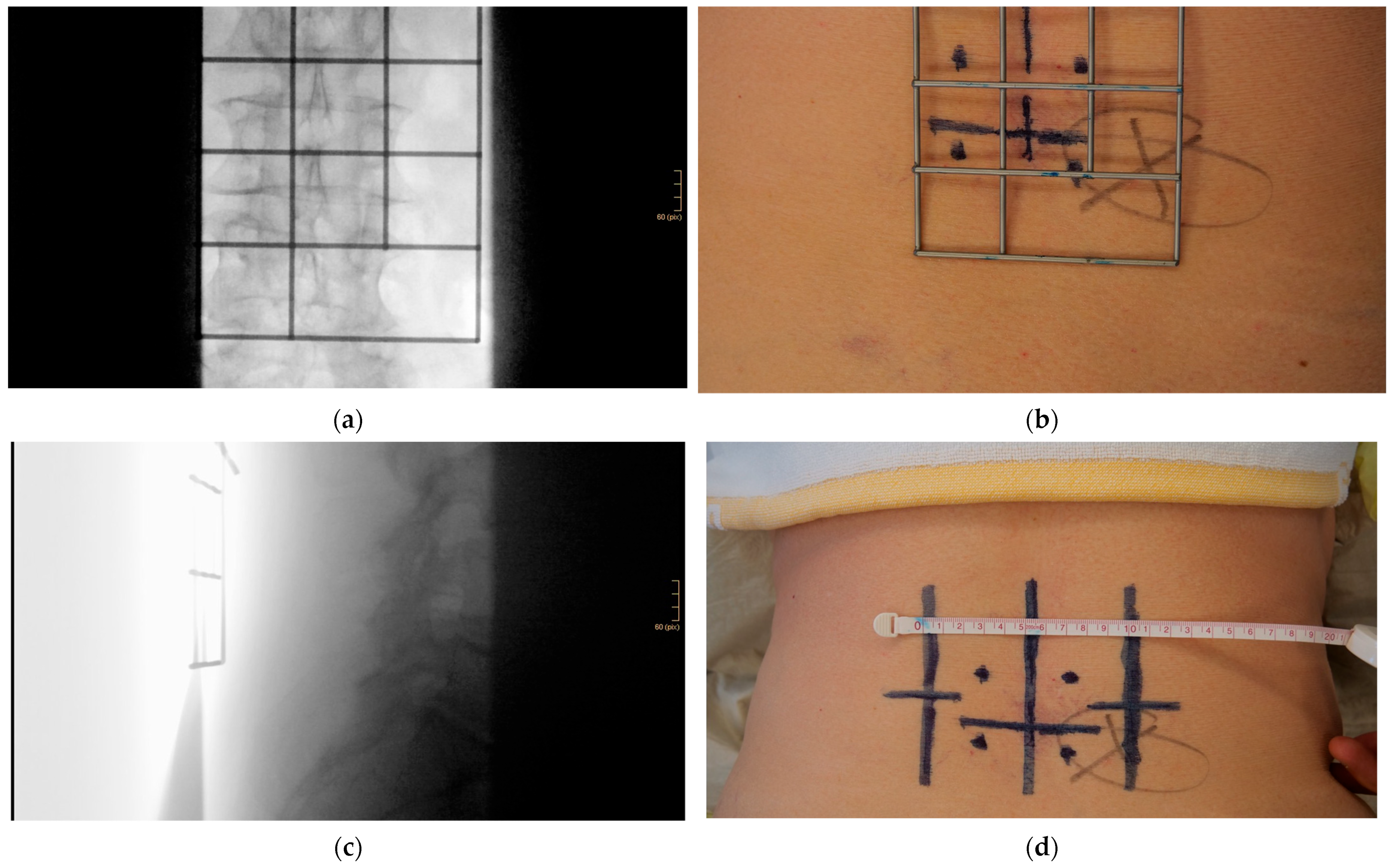
- Using a metal template for entry point identification
- 3.
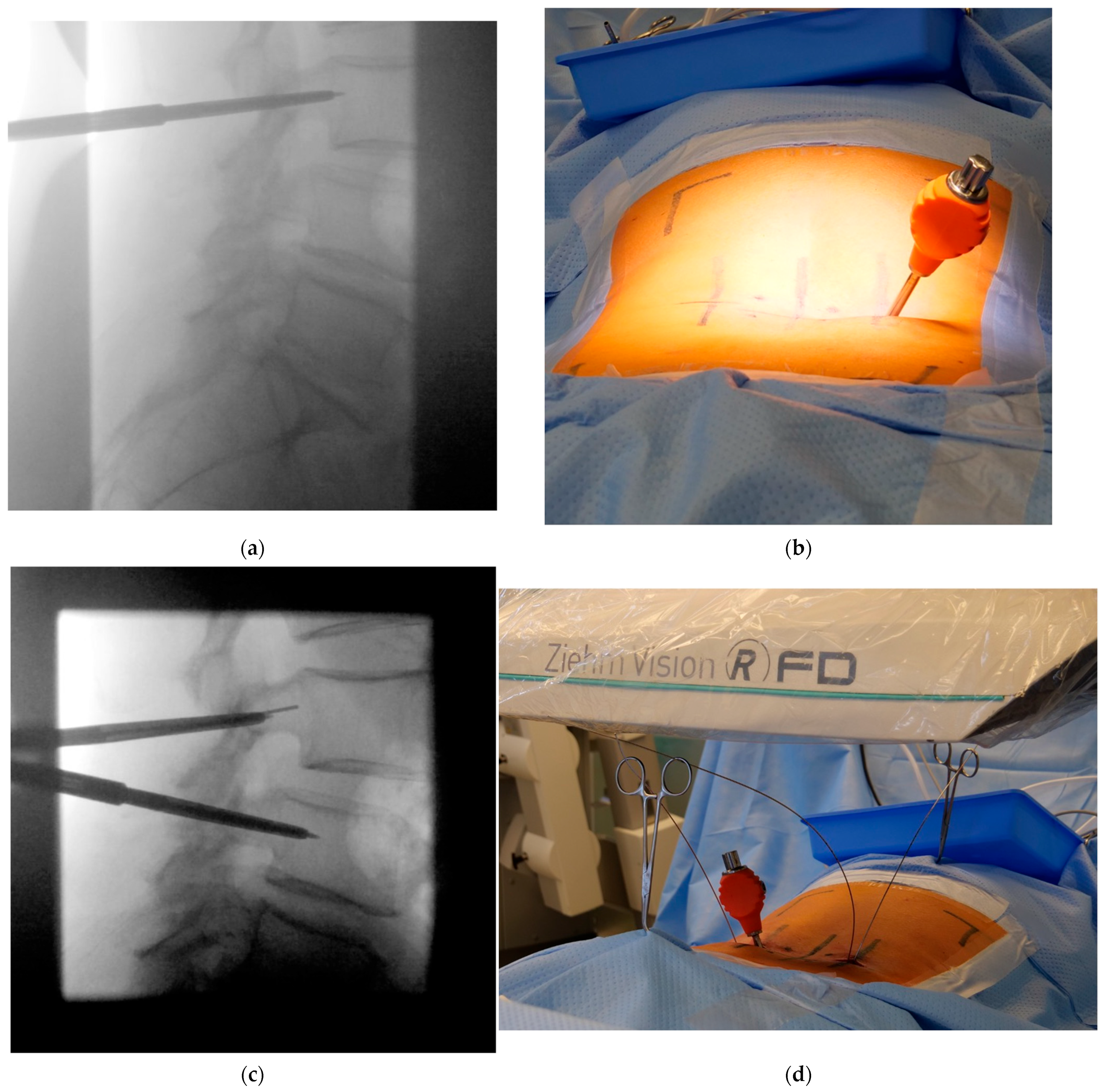
- Jamshidi needle positioning and K-wire placement
- 4.
- Confirmation of strictly transpedicular K-wire positioning
- 5.
- Ipsilateral placement of a tubular non-expandable retractor
- 6.
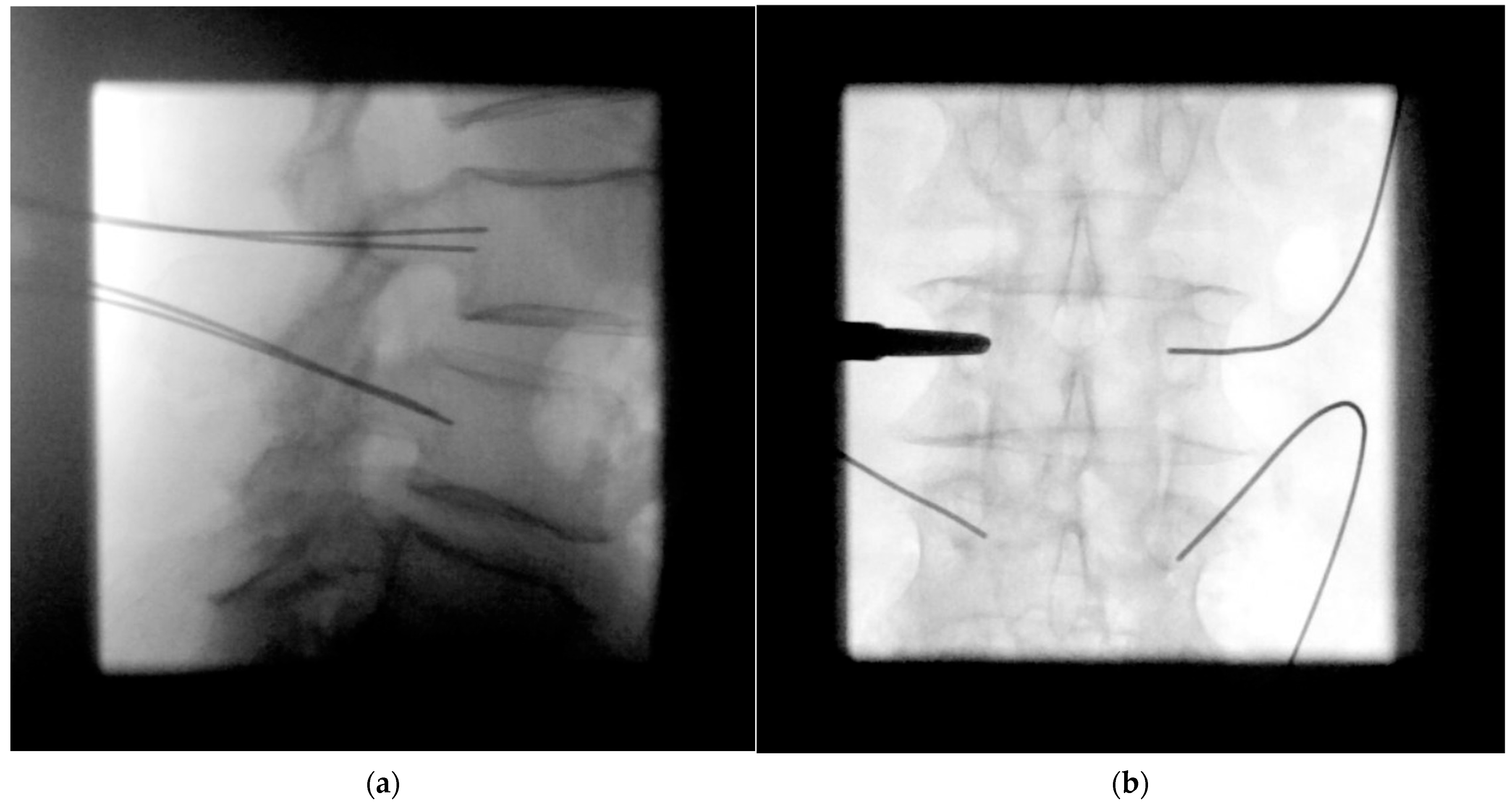
- Pedicle screw insertion
- 7.
- Facetectomy, decompression, discectomy, and distraction
- 8.
- Cage placement under lateral radiographic control
- 9.
- Ipsilateral screw placement usually without imaging
- 10.
- MIS rod insertion using haptic feedback and no or very sparse lateral radiographic control
3. Results
3.1. Clinical Characteristics
3.2. Radiation Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teng, I.; Han, J.; Phan, K.; Mobbs, R. A Meta-Analysis Comparing ALIF, PLIF, TLIF and LLIF. J. Clin. Neurosci. 2017, 44, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.T.; Gupta, S.K.; Justis, J.R.; Sherman, M.C. Percutaneous Pedicle Screw Fixation of the Lumbar Spine. Neurosurg. Focus 2001, 10, E10. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.T.; Lefkowitz, M.A. Advances in Minimally Invasive Spine Surgery. Clin. Neurosurg. 2002, 49, 499–517. [Google Scholar] [PubMed]
- Wong, A.P.; Smith, Z.A.; Stadler, J.A.; Hu, X.Y.; Yan, J.Z.; Li, X.F.; Lee, J.H.; Khoo, L.T. Minimally Invasive Transforaminal Lumbar Interbody Fusion (MI-TLIF): Surgical Technique, Long-Term 4-Year Prospective Outcomes, and Complications Compared with an Open TLIF Cohort. Neurosurg. Clin. N. Am. 2014, 25, 279–304. [Google Scholar] [CrossRef] [PubMed]
- Funao, H.; Ishii, K.; Momoshima, S.; Iwanami, A.; Hosogane, N.; Watanabe, K.; Nakamura, M.; Toyama, Y.; Matsumoto, M. Surgeons’ Exposure to Radiation in Single- and Multi-Level Minimally Invasive Transforaminal Lumbar Interbody Fusion; a Prospective Study. PLoS ONE 2014, 9, e95233. [Google Scholar] [CrossRef] [PubMed]
- Adogwa, O.; Carr, K.; Thompson, P.; Hoang, K.; Darlington, T.; Perez, E.; Fatemi, P.; Gottfried, O.; Cheng, J.; Isaacs, R.E. A Prospective, Multi-Institutional Comparative Effectiveness Study of Lumbar Spine Surgery in Morbidly Obese Patients: Does Minimally Invasive Transforaminal Lumbar Interbody Fusion Result in Superior Outcomes? World Neurosurg. 2015, 83, 860–866. [Google Scholar] [CrossRef]
- Jin-Tao, Q.; Yu, T.; Mei, W.; Xu-Dong, T.; Tian-Jian, Z.; Guo-Hua, S.; Lei, C.; Yue, H.; Zi-Tian, W.; Yue, Z. Comparison of MIS vs. Open PLIF/TLIF with Regard to Clinical Improvement, Fusion Rate, and Incidence of Major Complication: A Meta-Analysis. Eur. Spine J. 2015, 24, 1058–1065. [Google Scholar] [CrossRef]
- Krüger, M.T.; Naseri, Y.; Hohenhaus, M.; Hubbe, U.; Scholz, C.; Klingler, J.-H. Impact of Morbid Obesity (BMI > 40 Kg/M2) on Complication Rate and Outcome Following Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS TLIF). Clin. Neurol. Neurosurg. 2019, 178, 82–85. [Google Scholar] [CrossRef]
- Kim, C.H.; Easley, K.; Lee, J.-S.; Hong, J.-Y.; Virk, M.; Hsieh, P.C.; Yoon, S.T. Comparison of Minimally Invasive Versus Open Transforaminal Interbody Lumbar Fusion. Glob. Spine J. 2020, 10, 143S–150S. [Google Scholar] [CrossRef]
- Rampersaud, Y.R.; Foley, K.T.; Shen, A.C.; Williams, S.; Solomito, M. Radiation Exposure to the Spine Surgeon during Fluoroscopically Assisted Pedicle Screw Insertion. Spine 2000, 25, 2637–2645. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Fedeli, U.; Fadda, E.; Giovanazzi, A.; Scoizzato, L.; Saia, B. Increased Cancer Risk among Surgeons in an Orthopaedic Hospital. Occup. Med. Oxf. Engl. 2005, 55, 498–500. [Google Scholar] [CrossRef]
- Vano, E.; Gonzalez, L.; Fernández, J.M.; Haskal, Z.J. Eye Lens Exposure to Radiation in Interventional Suites: Caution Is Warranted. Radiology 2008, 248, 945–953. [Google Scholar] [CrossRef]
- Dauer, L.T.; Thornton, R.H.; Solomon, S.B.; St Germain, J. Unprotected Operator Eye Lens Doses in Oncologic Interventional Radiology Are Clinically Significant: Estimation from Patient Kerma-Area-Product Data. J. Vasc. Interv. Radiol. JVIR 2010, 21, 1859–1861. [Google Scholar] [CrossRef]
- Linet, M.S.; Slovis, T.L.; Miller, D.L.; Kleinerman, R.; Lee, C.; Rajaraman, P.; Berrington de Gonzalez, A. Cancer Risks Associated with External Radiation from Diagnostic Imaging Procedures. CA Cancer J. Clin. 2012, 62, 75–100. [Google Scholar] [CrossRef]
- Prasarn, M.L. Commentary on: Intraoperative Fluoroscopy, Portable X-Ray, and CT: Patient and Operating Room Personnel Radiation Exposure in Spinal Surgery. Spine J. 2014, 14, 2992–2994. [Google Scholar] [CrossRef]
- Villard, J.; Ryang, Y.-M.; Demetriades, A.K.; Reinke, A.; Behr, M.; Preuss, A.; Meyer, B.; Ringel, F. Radiation Exposure to the Surgeon and the Patient during Posterior Lumbar Spinal Instrumentation: A Prospective Randomized Comparison of Navigated versus Non-Navigated Freehand Techniques. Spine 2014, 39, 1004–1009. [Google Scholar] [CrossRef]
- Klingler, J.-H.; Sircar, R.; Scheiwe, C.; Kogias, E.; Krüger, M.T.; Scholz, C.; Hubbe, U. Comparative Study of C-Arms for Intraoperative 3-Dimensional Imaging and Navigation in Minimally Invasive Spine Surgery Part II: Radiation Exposure. Clin. Spine Surg. 2017, 30, E669–E676. [Google Scholar] [CrossRef]
- Klingler, J.-H.; Scholz, C.; Krüger, M.T.; Naseri, Y.; Volz, F.; Hohenhaus, M.; Brönner, J.; Hoedlmoser, H.; Sircar, R.; Hubbe, U. Radiation Exposure in Minimally Invasive Lumbar Fusion Surgery: A Randomized Controlled Trial Comparing Conventional Fluoroscopy and 3D Fluoroscopy-Based Navigation. Spine 2021, 46, 1–8. [Google Scholar] [CrossRef]
- Klingler, J.-H.; Naseri, Y.; Reinacher, P.C.; Hoedlmoser, H.; Urbach, H.; Hohenhaus, M. Patient Radiation Exposure from Intraoperative Computed Tomography in Spinal Surgery. Spine J. 2022, 22, 1576–1578. [Google Scholar] [CrossRef]
- Pennington, Z.; Cottrill, E.; Westbroek, E.M.; Goodwin, M.L.; Lubelski, D.; Ahmed, A.K.; Sciubba, D.M. Evaluation of Surgeon and Patient Radiation Exposure by Imaging Technology in Patients Undergoing Thoracolumbar Fusion: Systematic Review of the Literature. Spine J. 2019, 19, 1397–1411. [Google Scholar] [CrossRef]
- Hubbe, U.; Sircar, R.; Scheiwe, C.; Scholz, C.; Kogias, E.; Krüger, M.T.; Volz, F.; Klingler, J.-H. Surgeon, Staff, and Patient Radiation Exposure in Minimally Invasive Transforaminal Lumbar Interbody Fusion: Impact of 3D Fluoroscopy-Based Navigation Partially Replacing Conventional Fluoroscopy: Study Protocol for a Randomized Controlled Trial. Trials 2015, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- BIPM; IEC; IFCC; ILAC; ISO; IUPAC; IUPAP; OIML. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. Available online: https://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi_sYaeosGAAxWc0AIHHT8VDroQFnoECBUQAQ&url=https%3A%2F%2Fwww.bipm.org%2Fdocuments%2F20126%2F2071204%2FJCGM_100_2008_E.pdf&usg=AOvVaw0y6DQ4RJkdZh6KI6wpTtxg&opi=89978449 (accessed on 1 May 2023).
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar] [CrossRef]
- Gertzbein, S.D.; Robbins, S.E. Accuracy of Pedicular Screw Placement in Vivo. Spine 1990, 15, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Aoude, A.A.; Fortin, M.; Figueiredo, R.; Jarzem, P.; Ouellet, J.; Weber, M.H. Methods to Determine Pedicle Screw Placement Accuracy in Spine Surgery: A Systematic Review. Eur. Spine J. 2015, 24, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.-H.; Volz, F.; Krüger, M.T.; Kogias, E.; Rölz, R.; Scholz, C.; Sircar, R.; Hubbe, U. Accidental Durotomy in Minimally Invasive Transforaminal Lumbar Interbody Fusion: Frequency, Risk Factors, and Management. Sci. World J. 2015, 2015, 532628. [Google Scholar] [CrossRef]
- Klingler, J.-H.; Scholz, C.; Kogias, E.; Sircar, R.; Krüger, M.T.; Volz, F.; Scheiwe, C.; Hubbe, U. Minimally Invasive Technique for PMMA Augmentation of Fenestrated Screws. Sci. World J. 2015, 2015, 979186. [Google Scholar] [CrossRef]
- Härtl, R.; Lam, K.S.; Wang, J.; Korge, A.; Kandziora, F.; Audigé, L. Worldwide Survey on the Use of Navigation in Spine Surgery. World Neurosurg. 2013, 79, 162–172. [Google Scholar] [CrossRef]
- Lener, S.; Wipplinger, C.; Hernandez, R.N.; Hussain, I.; Kirnaz, S.; Navarro-Ramirez, R.; Schmidt, F.A.; Kim, E.; Härtl, R. Defining the MIS-TLIF: A Systematic Review of Techniques and Technologies Used by Surgeons Worldwide. Glob. Spine J. 2020, 10, 151S–167S. [Google Scholar] [CrossRef]
- Gullotti, D.M.; Soltanianzadeh, A.H.; Fujita, S.; Inserni, M.; Ruppel, E.; Franconi, N.G.; Zygourakis, C.; Protopsaltis, T.; Lo, S.-F.L.; Sciubba, D.M.; et al. Trends in Intraoperative Assessment of Spinal Alignment: A Survey of Spine Surgeons in the United States. Glob. Spine J. 2022, 12, 82S–86S. [Google Scholar] [CrossRef]
- Dabaghi Richerand, A.; Christodoulou, E.; Li, Y.; Caird, M.S.; Jong, N.; Farley, F.A. Comparison of Effective Dose of Radiation During Pedicle Screw Placement Using Intraoperative Computed Tomography Navigation Versus Fluoroscopy in Children With Spinal Deformities. J. Pediatr. Orthop. 2016, 36, 530–533. [Google Scholar] [CrossRef]
- Mendelsohn, D.; Strelzow, J.; Dea, N.; Ford, N.L.; Batke, J.; Pennington, A.; Yang, K.; Ailon, T.; Boyd, M.; Dvorak, M.; et al. Patient and Surgeon Radiation Exposure during Spinal Instrumentation Using Intraoperative Computed Tomography-Based Navigation. Spine J. 2016, 16, 343–354. [Google Scholar] [CrossRef]
- Bratschitsch, G.; Leitner, L.; Stücklschweiger, G.; Guss, H.; Sadoghi, P.; Puchwein, P.; Leithner, A.; Radl, R. Radiation Exposure of Patient and Operating Room Personnel by Fluoroscopy and Navigation during Spinal Surgery. Sci. Rep. 2019, 9, 17652. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, C.-H.; Lee, J.H.; Lee, S.-H.; Kim, J.-S. Radiation Exposure to the Surgeon during Percutaneous Endoscopic Lumbar Discectomy: A Prospective Study. Spine 2013, 38, 617–625. [Google Scholar] [CrossRef]
- Virk, S.; Qureshi, S. Navigation in Minimally Invasive Spine Surgery. J. Spine Surg. Hong Kong 2019, 5, S25–S30. [Google Scholar] [CrossRef]
- Mason, A.; Paulsen, R.; Babuska, J.M.; Rajpal, S.; Burneikiene, S.; Nelson, E.L.; Villavicencio, A.T. The Accuracy of Pedicle Screw Placement Using Intraoperative Image Guidance Systems. J. Neurosurg. Spine 2014, 20, 196–203. [Google Scholar] [CrossRef]
- Kim, T.T.; Drazin, D.; Shweikeh, F.; Pashman, R.; Johnson, J.P. Clinical and Radiographic Outcomes of Minimally Invasive Percutaneous Pedicle Screw Placement with Intraoperative CT (O-Arm) Image Guidance Navigation. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef]
- Dea, N.; Fisher, C.G.; Batke, J.; Strelzow, J.; Mendelsohn, D.; Paquette, S.J.; Kwon, B.K.; Boyd, M.D.; Dvorak, M.F.S.; Street, J.T. Economic Evaluation Comparing Intraoperative Cone Beam CT-Based Navigation and Conventional Fluoroscopy for the Placement of Spinal Pedicle Screws: A Patient-Level Data Cost-Effectiveness Analysis. Spine J. 2016, 16, 23–31. [Google Scholar] [CrossRef]
- Fan, G.; Fu, Q.; Zhang, J.; Zhang, H.; Gu, X.; Wang, C.; Gu, G.; Guan, X.; Fan, Y.; He, S. Radiation Reduction of Minimally Invasive Transforaminal Lumbar Interbody Fusion with Localisation System in Overweight Patients: Practical Technique. Bone Jt. J. 2017, 99, 944–950. [Google Scholar] [CrossRef]

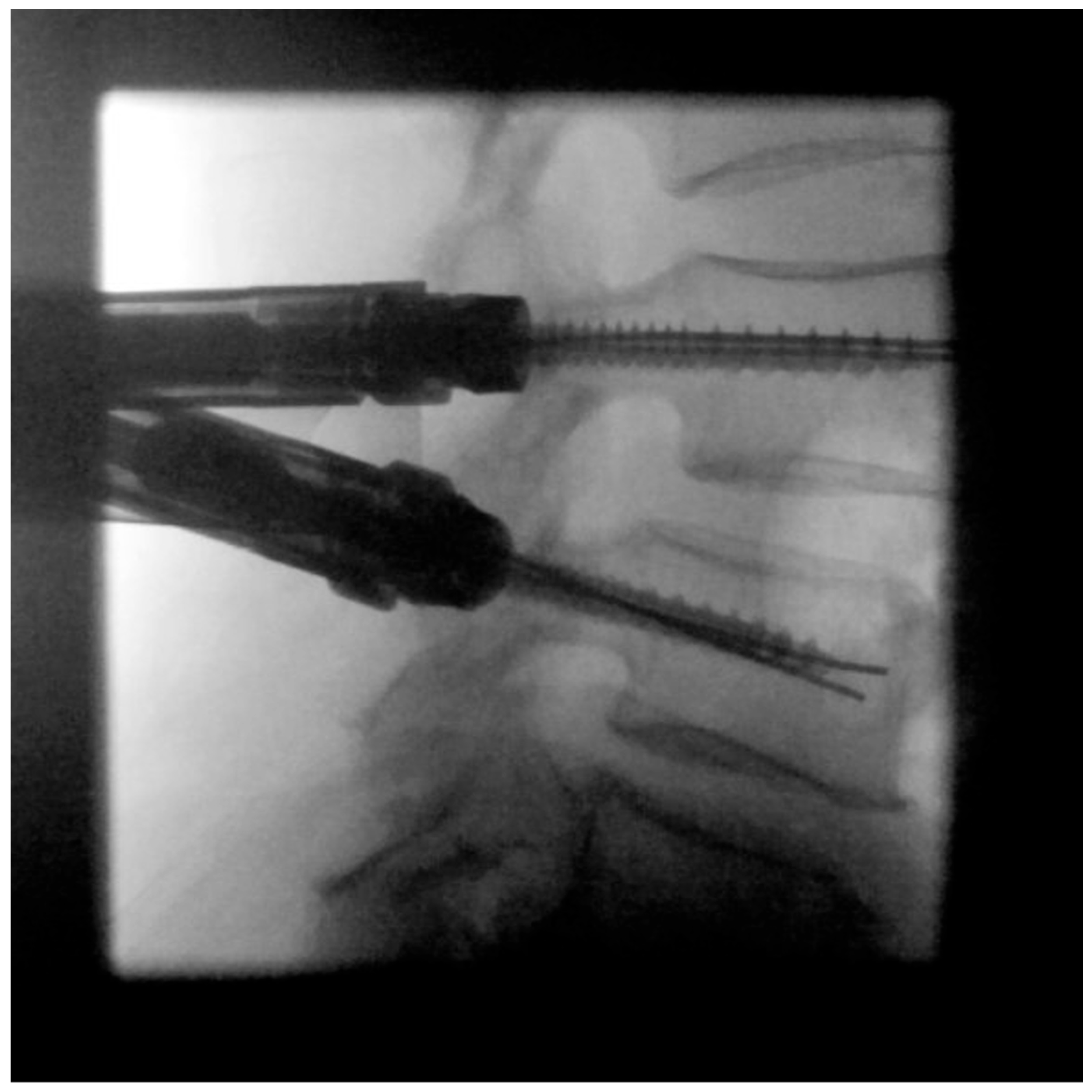

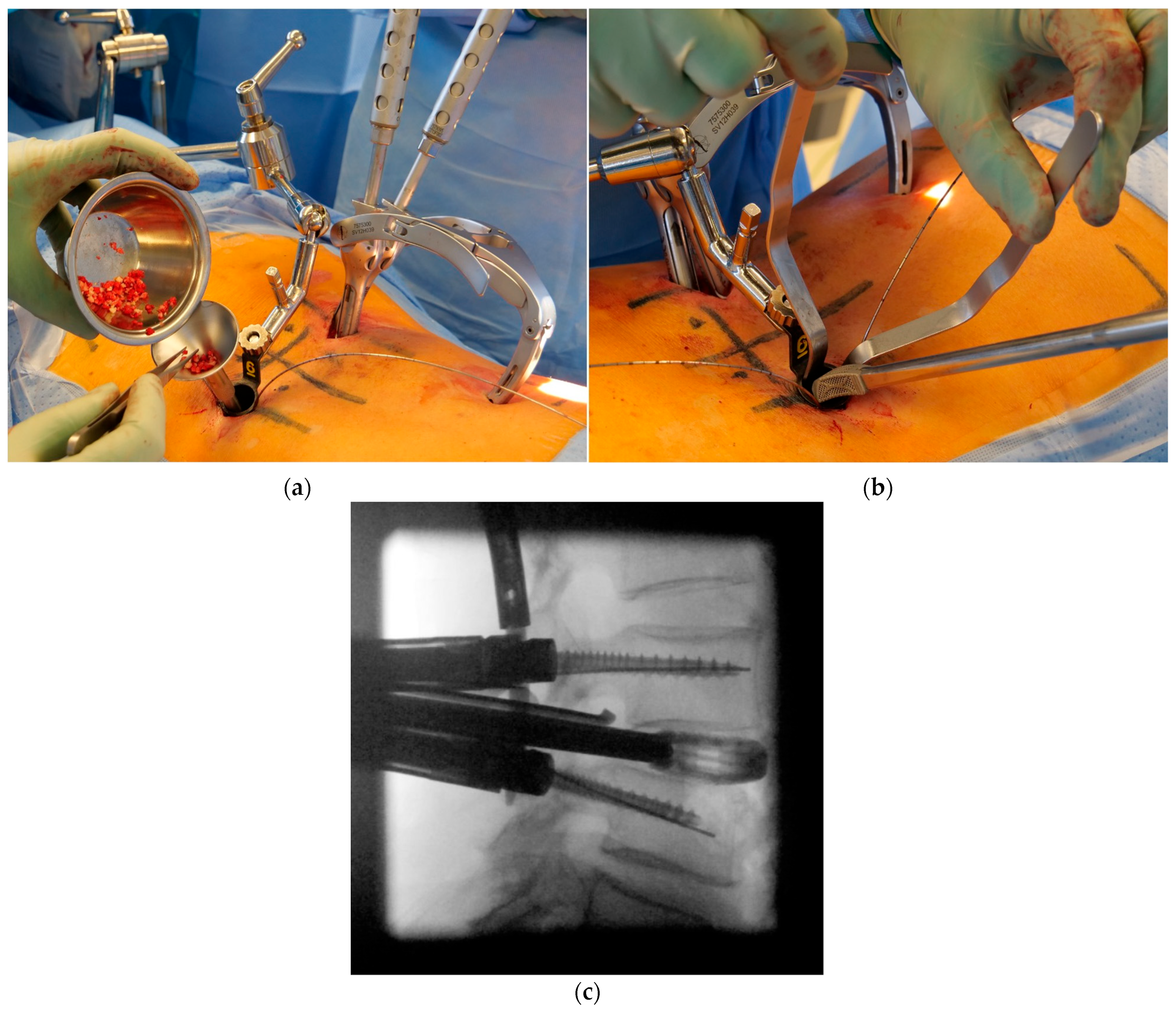
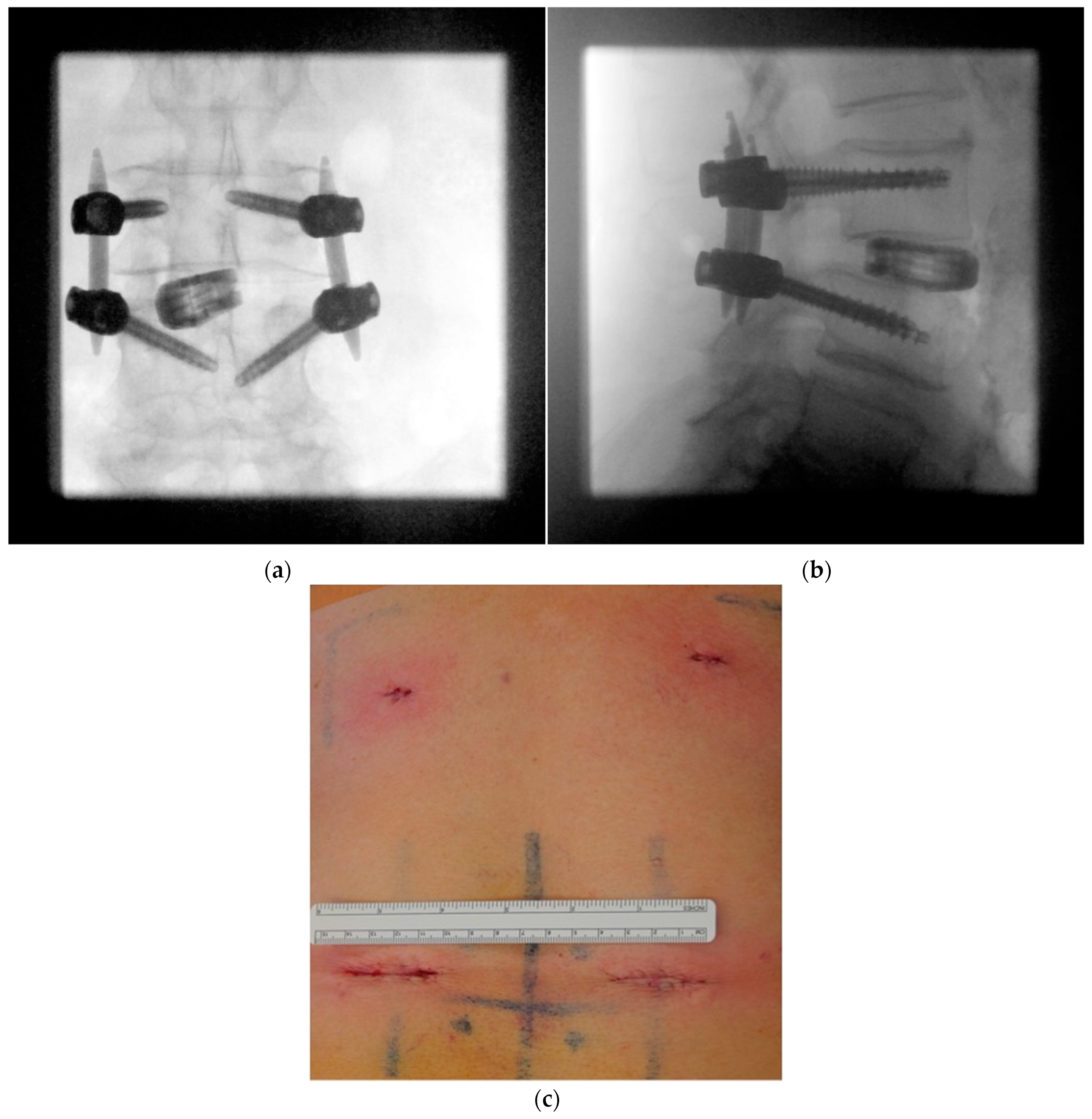
| Patient characteristics | |||
| Patients | 24 | ||
| Age (years) | 62.4 ± 14.0 | ||
| Female/male ratio | 12:12 | ||
| Body mass index (kg/m2) | 27.6 ± 5.3 | ||
| Characteristics of surgical segments (n = 27) | |||
| Spondylolisthesis, Meyerding grade I | 9 | ||
| Spondylolisthesis, Meyerding grade II | 16 | ||
| Spondylolysis | 15 | ||
| Segment operated | |||
| L3–L4 | 2 | ||
| L4–L5 | 12 | ||
| L5–S1 | 13 | ||
| Operative characteristics | |||
| Bilateral decompression | 11 | ||
| Monosegmental stabilization | 21 | ||
| Bisegmental stabilization | 3 | ||
| Total pedicle screws | 102 | ||
| No pedicle breach or pedicle breach ≤2 mm | 99 (97.1%) | ||
| Pedicle breach >2 mm | 3 (2.9%) | ||
| Estimated blood loss (mL) | 256 ± 328 | ||
| Operation time (min) | 184 ± 52 | ||
| All per Segment | ||
|---|---|---|
| Number of fluoroscopic images | 75 ± 43 | |
| Fluoroscopy time (s) | 64 ± 34 | |
| Dose–area product (cGycm2) | 526 ± 388 | |
| Effective radiation dose (µSv) | ||
| Surgeon | 41.4 ± 11.7 | |
| Total radiation dose (µSv) | ||
| Patient neck | 65 ± 40 | |
| Patient chest | 123 ± 116 | |
| Patient umbilical | 823 ± 862 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doria-Medina, R.; Hubbe, U.; Scholz, C.; Sircar, R.; Brönner, J.; Hoedlmoser, H.; Klingler, J.-H. Free-Hand MIS TLIF without 3D Navigation—How to Achieve Low Radiation Exposure for Both Surgeon and Patient. J. Clin. Med. 2023, 12, 5125. https://doi.org/10.3390/jcm12155125
Doria-Medina R, Hubbe U, Scholz C, Sircar R, Brönner J, Hoedlmoser H, Klingler J-H. Free-Hand MIS TLIF without 3D Navigation—How to Achieve Low Radiation Exposure for Both Surgeon and Patient. Journal of Clinical Medicine. 2023; 12(15):5125. https://doi.org/10.3390/jcm12155125
Chicago/Turabian StyleDoria-Medina, Roberto, Ulrich Hubbe, Christoph Scholz, Ronen Sircar, Johannes Brönner, Herbert Hoedlmoser, and Jan-Helge Klingler. 2023. "Free-Hand MIS TLIF without 3D Navigation—How to Achieve Low Radiation Exposure for Both Surgeon and Patient" Journal of Clinical Medicine 12, no. 15: 5125. https://doi.org/10.3390/jcm12155125
APA StyleDoria-Medina, R., Hubbe, U., Scholz, C., Sircar, R., Brönner, J., Hoedlmoser, H., & Klingler, J.-H. (2023). Free-Hand MIS TLIF without 3D Navigation—How to Achieve Low Radiation Exposure for Both Surgeon and Patient. Journal of Clinical Medicine, 12(15), 5125. https://doi.org/10.3390/jcm12155125







