Abstract
Placental histopathology provides insights, or “snapshots”, into relevant antenatal factors that could elevate the risk of perinatal brain injury. We present a systematic review and meta-analysis comparing frequencies of adverse neurological outcomes in infants born to women with placental abruption versus without abruption. Records were sourced from MEDLINE, Embase, and the CENTRAL Trials Registry from 1946 to December 2019. Studies followed the PRISMA guidelines and compared frequencies of neurodevelopmental morbidities in infants born to pregnant women with placental abruption (exposure) versus women without placental abruption (comparator). The primary endpoint was cerebral palsy. Periventricular and intraventricular (both severe and any grades of IVH) and any histopathological neuronal damage were the secondary endpoints. Study methodologic quality was assessed by the Ottawa–Newcastle scale. Estimated odds ratios (OR) and hazards ratio (HR) were derived according to study design. Data were meta-analyzed using a random effects model expressed as pooled effect sizes and 95% confidence intervals. We included eight observational studies in the review, including 1245 infants born to women with placental abruption. Results of the random effects meta-analysis show that the odds of infants born to pregnant women with placental abruption who experience cerebral palsy is higher than in infants born to pregnant women without placental abruption (OR 5.71 95% CI (1.17, 27.91); I2 = 84.0%). There is no statistical difference in the odds of infants born to pregnant women with placental abruption who experience severe IVH (grade 3+) (OR 1.20 95% CI (0.46, 3.11); I2 = 35.8%) and any grade of IVH (OR 1.20 95% CI (0.62, 2.32); I2 = 32.3%) vs. women without placental abruption. There is no statistically significant difference in the odds of infants born to pregnant women with placental abruption who experience PVL vs. pregnant women without placental abruption (OR 6.51 95% CI (0.94, 45.16); I2 = 0.0%). Despite our meta-analysis suggesting increased odds of cerebral palsy in infants born to pregnant women with placental abruption versus without abruption, this finding should be interpreted cautiously, given high heterogeneity and overall poor quality of the included studies.
Keywords:
infant; child; abruptio placentae; pregnant women; cerebral palsy; morbidity; delivery; obstetric hemorrhage 1. Introduction
Placental abruption can lead to bleeding at the decidual placental interface. The rate of placental abruption in several European countries has declined, while the trend in North America is increasing [1]. Placental abruption remains a critical concern for maternal and perinatal mortality, as well as morbidity. It can be associated with trauma and drug use. Maternal complications due to abruption vary based on its severity [2] and can include outcomes such as myocardial infarction, cardiomyopathy, heart failure, cerebrovascular disorders and even death. With respect to perinatal outcomes, lower gestational age can predict perinatal severity in pregnant women with abruption [3]. Pregnant women with abruption are more likely to have preterm births (i.e., live births earlier than 37 weeks of gestation) than women without abruption [4]. Intrauterine growth retardation (IUGR), congenital abnormalities [5], and complications of prematurity are additional neonatal consequences of placental abruption.
Placental histopathology provides insights, or “snapshots”, into relevant antenatal factors that could elevate the risk of perinatal brain injury [6]. In general, perinatal mortality and maternal complications occur in situations of severe placental abruption and intrauterine death [7]. In severe placental abruption cases, obstetricians must decide whether to intervene early and perform a caesarean delivery, which poses risks to hemodynamically unstable mothers, or delay fetal delivery, which increases the risk of fetal mortality and neurological morbidity. Evidence demonstrates that when fetal heart patterns are normal, delaying delivery when the maternal status is stable can be appropriate [8,9,10].
Several studies on long-term consequences of abruption have focused on non-neurological infant consequences [11,12,13,14] or maternal cardiac outcomes [15,16,17,18] with less focus on neonatal neurological impairment. There is a relationship between placental abruption and the possibility for fetal neurologic impairment. Greater than 50% of placentas from medicolegal cases with neurologic impairment had fetal thrombotic vasculopathy [6], which is a known etiology of placental abruption. Whitehead et al. [19] reported a significant association between abruption and childhood epilepsy (RR 4.6, 95% CI 3–6.9) in large, population-based cohorts, and this relationship holds true across the literature [20,21,22,23,24]. A small number of studies have investigated brain-related injuries, such as neonatal encephalopathy (NE)/hypoxic ischemic encephalopathy (HIE) [25,26,27]. No association was found between placental abruption and intraventricular and periventricular hemorrhage in babies born preterm [28,29,30]. Despite this, long-term risks such as cerebral palsy [21,31,32,33,34] and cognitive deficits [35] were elevated in surviving neonates. The association between abruption and cerebral palsy is, however, inconclusive. Additional studies show no difference in risk between neonates in pregnancies with and without abruption, although study populations differ (i.e., women without chronic hypertension vs. spastic cerebral palsy only vs. deliveries between 22 and 26 weeks) [30,36,37].
Although one systematic review has examined neonatal neurological outcomes in a setting of placental abruption [38], the number of included studies focusing on neonatal outcomes in relation to obstetric risks were minimal. In order to supplement this current body of knowledge, we performed a systematic investigation into the unique interplay between placental abruption and long-term neurological infant consequences.
Based on our search criteria, there is no current meta-analysis performed in 2021 that examines the neurological consequences of placental abruption. Therefore, the objective of this present study is to statistically review the primary literature examining infant neurological consequences among pregnant women with placental abruption versus without placental abruption.
2. Materials and Methods
This review followed the Cochrane Methodology to identify and select the studies [39] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to guide the reporting of this systematic review [40].
2.1. Eligibility Criteria, Information Sources, Search Strategy
A systematic search for relevant studies was performed between 1946 and December 2019, using the following databases: MEDLINE including Epub Ahead of Print, In-Process and Other Non-Indexed Citations (1946–December 2019) and Embase (1947–December 2019) and the CENTRAL Trials Registry of the Cochrane Collaboration (December 2019 Issue) using the Ovid interface. The rationale for including studies from 1946 to 2019 was to ensure all relevant data were captured, although we recognize that more recent studies are ideal given their greater clinical impact.
Searches were limited to English or French and full journal articles. Searches were designed, conducted, and pre-specified by a librarian experienced in systematic reviews, using a method designed to optimize term selection [41]. Prior to screening and data analysis, this review was registered in PROSPERO [42] on December 16, 2019 (registration number CRD42020161913).
After duplicate records were removed online, records retrieved by the electronic search were downloaded and imported into a reference manager, and then uploaded to a systematic review software InsightScope (InsightScope.ca) for title and abstract screening and full text review. Five reviewers (I.O., A.R., T.H., A.R., R.T.) screened at the title/abstract level and six reviewers (I.O., A.R., T.H., A.R., R.T., R.G.) at the full text review stages. Citations were excluded if at least two reviewers agreed to exclude.
2.2. Study Selection
Case-control and cohort studies examining our outcomes of interest, specifically infant hypoxic ischemic encephalopathy (HIE), periventricular leukomalacia (PVL), intraventricular hemorrhage (IVH), presence or absence of brain damage, and cerebral palsy among infants (<2 years) born to pregnant women with any stage of placental abruption in the first, second or third trimester versus without abruption, were included. Data were extracted by two reviewers (I.O. and A.R.) and verified by the opposite reviewer with no need for third-party consultation. Studies were excluded if our outcomes of interest were not mentioned (n = 90), or if there was ineligible exposure (i.e., absence of placental abruption) (n = 2), or other (i.e., cerebral palsy and death frequencies were combined and could not be teased separately) (n = 1).
2.3. Data Extraction
Two authors (I.O. and A.R.) extracted data using a pre-designed and piloted data abstraction sheet in REDCap (Research Electronic Data Capture) [43]. Briefly, the extracted information included: frequency of pregnant women with or without placental abruption, demographics such as maternal age, parity, smoking, gestational age, intraventricular hemorrhage (IVH), presence or absence of periventricular leukomalacia (PVL), cerebral palsy (CP), brain damage (histopathological evidence of neuronal damage in the brain), hypoxic ischemic encephalopathy (HIE), and neonatal mortality. Of note, we included all stages of IVH (not solely severe III and IV forms but I and II as well) to minimize selection bias and to capture cases of mild IVH and placental abruption as described in our included studies.
Our exposure variable was a diagnosis of any stage of placental abruption in the first, second or third trimester of pregnancy (based on gestational age in weeks). Four of the included studies [44,45,46,47] assessed mild, moderate, or severe abruption in the third trimester using the classification systems from Page [48] or Knab [49]. One study focused on grade 1 (external bleeding and mild uterine pain) and grade 2 (grade 1 symptoms, concealed hemorrhage, uterine tetany, and/or fetal distress but no maternal shock) placental abruptions occurring during the third trimester [50]. Another study examined any case of placental abruption among stillborn infants born at gestation of 24 or more weeks [51]. Two studies used clinical definition for placental abruption, in particular, the premature detachment of an implanted placenta from the uterine wall before delivery of the fetus, in the third trimester of pregnancy [24]. One study included any case of placental abruption, defined as the presence of retroplacental hematoma and clinical presentations (any, or the combination of genital bleeding, abdominal pain, pregnancy-induced hypertension, premature labor, premature rupture of membranes, intrauterine fetal death, or non-reassuring fetal heart rate pattern) in the second trimester of pregnancy [30].
The primary endpoint was cerebral palsy (CP). The secondary endpoints included IVH (both severe and any grades of IVH), PVL, and any histopathological neuronal damage (as defined by the study authors). Among our included studies, a child of at least 2 years at diagnosis, validated by physical findings and confirmed by a pediatrician, was considered a confirmed case of cerebral palsy [30,47]. A diagnosis of cerebral palsy could also have been made using the neurodevelopmental evaluation of the Bayley Scales of Infant Development at discharge and after 3, 6, 12 and 24 months. One study examined hospitalizations and further defined the diagnosis of cerebral palsy as the presence of unspecified infantile cerebral palsy, paraplegia, diplegia of upper limbs, or unspecified paralysis. All studies examining IVH used neonatal cranial ultrasound for diagnosis within 24 to 48 h on admission or at birth and weekly until discharge from the hospital [30] or cranial ultrasound at 3, 6, 12 or 24 months after birth (corrected gestational age) [45]. HIE was confirmed using CT or MRI and by clinical exam, and a severe diagnosis of HIE was made if the infant experienced frequent seizures, apnea, flaccid weakness, or coma [46]. Evidence of established brain damage included immunohistochemical staining of post-autopsy brain specimens to visualize antibodies to glial fibrillary acidic protein for astrocytes and to CD68 and major histocompatibility complex II for microglia [51]. We selected the above neurological outcomes because their consideration specifically appears to be lacking in the obstetric literature with respect to pregnant women with placental abruption.
2.4. Assessment of Risk of Bias
Three factors were considered to score the quality of included studies: (1) Selection, including representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that at the start of the study, the outcome of interest was not present. (2) Comparability, assessed on the basis of study design and analysis, and whether any confounding variables (not necessarily all) were adjusted for in a regression model (including gestational age, social class, or duration of mother’s education, in addition to other confounders such as previous or current abuse of substances and presence of medical comorbidities). Of note, we did not include birthweight as a potential confounder, as it may be a causal intermediate and thus contribute to collider bias [52]. Gestational age and birth weight are highly collinear, which makes it difficult to distinguish their individual effects on neurological outcome [53]. (3) Outcome, based on the follow-up period for when neurological outcome can be detected (minimum 24 months), cohort retention, and assessment of neurological outcome through independent blind assessment to the diagnosis of placental abruption. In other words, the pediatrician or neurologist who assesses the neurological outcome of the infant or child cannot be aware of the diagnosis of placental abruption in the mother. Conversely, the pathologist should not be aware of the clinical history of the case when making a diagnosis of neurological impairment.
We rated the quality of the studies (good, fair, and poor) by awarding stars in each domain following the guidelines of the Newcastle–Ottawa Scale [54]. A “good” quality score required 3 or 4 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcomes. A “fair” quality score required 2 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcomes. A “poor” quality score reflected 0 or 1 star(s) in selection, or 0 stars in comparability, or 0 or 1 star(s) in outcomes [55].
2.5. Data Synthesis
All data analyses were performed using the R statistical programming language (Version 4.0.3) [56]. Dichotomized variables were expressed as numbers and percentages. Data was meta-analyzed using a random effects model with R package “meta” [57]. Odds ratios (OR) were derived from case control studies. The estimated hazards ratio (HR) was used for one study whose HR could not be converted to OR [24]. When available in individual studies, control groups with placental previa were included. Statistical heterogeneity was determined using I2 tests. I2 is the proportion of total variation observed between studies attributable to differences between studies rather than sampling errors. We considered high heterogeneity if I2 > 75%.
3. Results
3.1. Study Selection
After initial screening, 101 articles were retained for full-text screening, of which 8 records met all the study criteria (i.e., captured at least one of our outcomes of interest) (Figure 1).
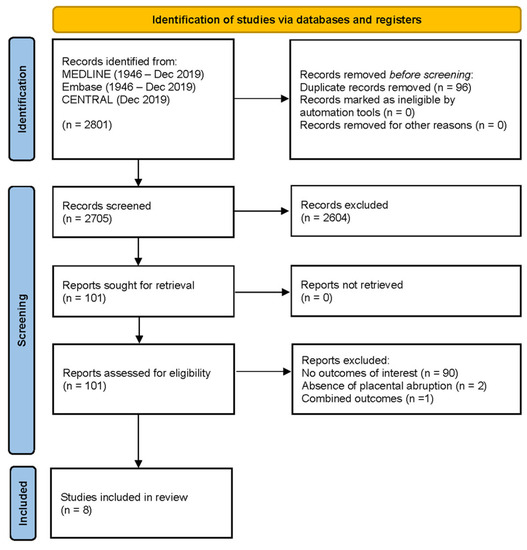
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics
Six of the eight studies were retrospective case-control studies, with sample sizes ranging from 53 to 217,910 (Table 1). There were 1245 infants born to women with placental abruption versus 217,608 without placental abruption. The mean maternal age of the participants with placental abruption was 29.4 ± 6.1 years vs. 28.2 ± 5.7 years in those without abruption. The mean gestational age of infants born to pregnant women with abruption was 35.4 ± 4.4 vs. 39.1 ± 1.8 weeks in those without abruption. Table 2 and Table 3 demonstrate the maternal and neonatal characteristics of the study population (depending on data availability).

Table 1.
Study characteristics.

Table 2.
Maternal characteristics of the study population.

Table 3.
Neonatal characteristics of the study population.
3.3. Risk of Bias of Included Studies
There were four poor, one fair, and three good-quality studies. Three studies adjusted for confounders in a regression model, awarding them a good evaluation [24,44,45]. Only two of the eight studies had a follow-up for at least 24 months of corrected gestational age [24,45]. Case-control studies should have clearly detailed when the neonatal examination occurred for adequate follow-up of a neurological diagnosis [44]. Pariente et al. [24] had completed follow-up of all the study participants, but in Spinillo et al. [45], the follow-up rate was less than 95% and with no description of those lost to follow-up. The presence of placental abruption in all studies was ascertained by referencing medical records or computer databases. In three studies, histopathological examination of the placenta was also used to confirm the presence of abruption [24,45,58], while only clinical confirmation was used in the others. None of the studies demonstrated any assessments to exclude the presence of study outcomes prior to commencement of the study. Blinding during outcome assessment was performed only in two studies, where pediatric neurologists were blinded during neurodevelopmental assessment of children with cerebral palsy [30] or where pathologists performing autopsies were blinded to clinical histories [51] (Table 4).

Table 4.
Assessment of study quality using the Ottawa–Newcastle scale. ★ Star denotes that the specific criteria are acceptable.
3.4. Synthesis of Results
Of the eight studies that included one of our outcomes of interest (i.e., cerebral palsy, IVH or PVL), only five were suitable for meta-analysis [24,30,45,50,58]. At least two of the five studies directly compared the risk of one of our outcomes of interest. Spinillo et al., 1993 [44], could not be added to avoid the possibility of patient overlap, and only one study measured brain damage at autopsy [51]. Four studies specifically reported on the association of abruption with cerebral palsy [24,30,45,58]. Since we had fewer than 10 studies in this meta-analysis, we did not perform a meta-regression based on the Cochrane handbook [59] to examine the effect of gestational age and placental abruption on cerebral palsy. Therefore, we could not examine the impact of decreasing gestational age on birth asphyxia and consequently the risk of cerebral palsy [60,61].
Results of the random effects meta-analysis show that the odds of infants born to pregnant women with placental abruption who experience cerebral palsy are higher than in infants born to pregnant women without placental abruption (OR 5.71 95% CI (1.17, 27.91); I2 = 84.0%). However, the confidence interval is very wide and imprecise (Figure 2). Pooled effect sizes were used to approximate the OR rather than the HR, since Pariente et al. had <50% weight in this meta-analysis.
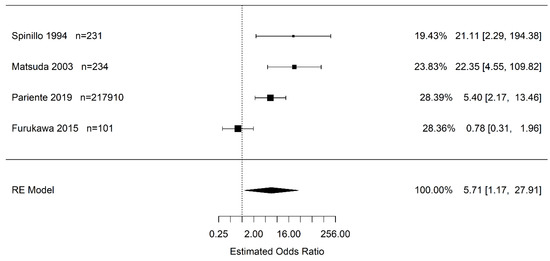
Figure 2.
Estimate combined odds and hazard ratio of placental abruption (vs. “controls”) for cerebral palsy calculated using random effects (RE) model [24,30,45,47].
Due to the confidence interval including one, there is no statistical difference in the odds of infants born to pregnant women with placental abruption who experience severe IVH (grade 3+) vs. women without placental abruption (OR 1.20 95% CI (0.46, 3.11); I2 = 35.8%) (Figure 3). Likewise, there is no statistical difference in the odds of infants born to pregnant women with placental abruption who experience any grade of IVH than in infants born to pregnant women without placental abruption (OR 1.20 95% CI (0.54, 2.68); I2 = 32.3%) (Figure 4).
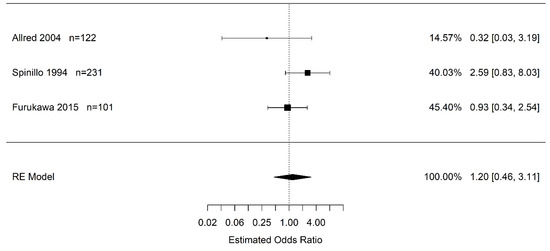
Figure 3.
Estimate odds ratio of placental abruption (vs. “controls”) for severe intraventricular hemorrhage (grade 3, 4) calculated using random effects (RE) model [30,45,50].
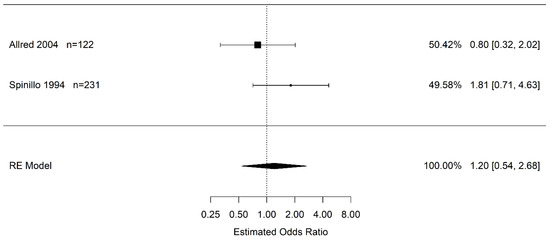
Figure 4.
Estimate odds ratio of placental abruption (vs. “controls”) for intraventricular hemorrhage (any grade) calculated using random effects (RE) model [45,50].
Only two studies reported on the association of abruption and PVL. Resulting from very wide, imprecise confidence intervals including one, there is no statistically significant difference in the odds of infants born to pregnant women with placental abruption who experience PVL vs. pregnant women without placental abruption (OR 6.51 95% CI (0.94, 45.16); I2 = 0.0%) (Figure 5).
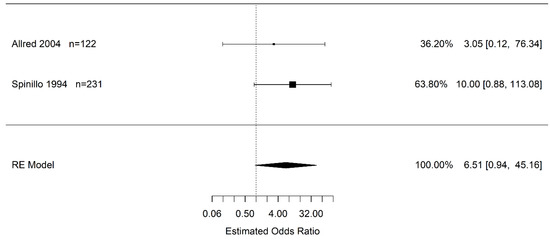
Figure 5.
Estimated odds ratio of placental abruption (vs. “controls”) for periventricular leukomalacia calculated using random effects (RE) model [45,50].
Excluded from the meta-analysis were neonatal mortality [30,58] and HIE [46] due to an insufficient number of studies. In Furukawa et al., there were no differences in the incidence of neonatal death in women with placental abruption versus without. Matsuda et al. determined that neonatal mortality was more frequent in cases of placental abruption (11/42, 26.2%) than in placenta previa (2/72, 2.8%) and preterm labor (1/120, 0.8%), although the difference was predominantly due to cerebral palsy. HIE was only reported in one study [46], with higher incidences of moderate and severe HIE in the placental cases than in healthy term newborns.
4. Discussion
4.1. Principal Findings
Our random effects meta-analysis showed statistically significant increased odds of cerebral palsy (CP) in infants born to pregnant women with abruption versus those born to mothers without abruption, which align with prior literature. Of note, we reported very high heterogeneity for this finding, likely due to variations in the measurement of placental abruption diagnosis (exposure) and cerebral palsy. In light of these variations, this particular finding should be interpreted with caution. Our meta-analysis also shows no statistical difference in the increased odds of any grade and severe IVH, respectively, in infants born to pregnant women with placental abruption. We noted no difference in the odds of PVL in infants born to pregnant women with placental abruption. However, this result was not statistically significant and imprecise.
4.2. Comparison with Existing Literature
In line with our meta-analysis, other studies have shown a similar increase in CP [38,62]. After adjusting for confounding factors, Spinillo et al. [45] found that infants from pregnancies complicated by placental abruption were 3.9 times more likely than controls to have neonatal death or cerebral palsy. This finding was echoed in the long-term follow-up study conducted by Pariente et al. [24]. Infants born to mothers with placental abruption showed higher rates of cerebral palsy (0.73 vs. 0.10 per 1000 person-years) than the control group without abruption. Of all the selected studies, only Furukawa et al. [30] showed no significant difference in the incidence of cerebral palsy between the abruption and control groups.
Several studies in the literature have shown a direct relationship between placental abruption and cerebral palsy [7,21]. The precipitating and pathologic factor contributing to the development of cerebral palsy is fetal acidemia, resulting from impaired gas exchange and endothelial dysfunction, as reflected by abnormal umbilical pH/cord gases. Placental abruption is associated with acidosis, impaired gas exchange and endothelial dysfunction [63]. Since neuronal tissue is sensitive to metabolic disturbances [64], placental abruption could lead to hypoxia, hypercapnia, and in turn, CP. Moreover, studies have shown lower umbilical cord blood pH after placental abruption in the CP group than in the non-CP group [22]. In cases with umbilical pH < 7.0, neonates are at a higher risk of HIE due to acidemia, which in turn, elevates their risk of CP [65,66]. Acidemia (pH ≤ 7.0) [67] occurred in 114 of 168 (69.2%) cases of severe CP, and a majority of these were also pregnancies complicated by placental abruption [68]. Another report documented that more than half of all CP cases showed umbilical cord blood pH < 7.0, further supporting the link between fetal acidemia, abruption, and CP [69].
Risk factors for abruption include maternal habits such as alcohol consumption and smoking during pregnancy, as well as multiparity and hypertensive disorders in pregnancy [70,71,72]. In particular, alcohol consumption (OR 3.38, 95% CI (2.01–5.68)), smoking during pregnancy (OR 3.50, 95% CI (1.32–9.25)), number of deliveries (OR 1.28, 95% CI (1.05–1.56)), and hypertensive disorders in pregnancy (OR 2.25, 95% CI (1.27–4.07) are significant risk factors for CP following placental abruption [22]. Moreover, heavy alcohol consumption may cause neurodevelopmental abnormalities, such as fetal alcohol syndrome and CP [73]. Smoking and hypertensive disorders also contribute to endothelial dysfunction [74,75] and uteroplacental insufficiency [76], potentially leading to higher rates of maternal, fetal, and infant mortality, and severe morbidity [77].
Our meta-analysis did not show a difference in the odds of IVH. It is possible that this meta-analysis did not have sufficient power to detect statistical differences. Spinillo et al. [45] showed significantly higher risk of grades III and IV intraventricular hemorrhage in comparison with control subjects (OR 3.5; 95% CI, 1.01 to 12.2), and even after adjusting for confounding factors, the difference remained statistically significant (OR 4.8; 95% CI 1.2 to 19.3). However, two additional studies [30,50] that assessed severe IVH did not show a significant difference between the abruption and absence of abruption groups.
Despite these findings, a previous study demonstrated a simultaneously high prevalence of low Apgar scores, prematurity, acidosis, and perinatal asphyxia, among IVH infants [45]. Moreover, among preterm infants born to mothers with placental abruption, there could have been differences in prenatal care received, differences in gestational age at birth, or complex vascular placental diseases, which could heighten the risk of IVH [78]. IVH can result from the interruption of maternal–fetal exchange, which impairs blood flow [79,80]. The presence of coagulation abnormalities associated with fetal asphyxia could also elevate the risk of IVH [79].
We did not note any statistical difference in the odds of PVL between women with placental abruption. Two of our included studies reported no significant difference in PVL rates between the cases and controls [45,50]. However, histopathological lesions show disturbance of uteroplacental circulation, including placental abruption, and are more common in infants with PVL [81]. Only one study [46] documented HIE and showed that the development quotient of children in the study in their Gesell Developmental Scale was significantly lower in the placental abruption group than in the no-abruption group, indicative of greater neurological impairment.
In contrast, a previous study reported higher occurrence of severe asphyxia in infants with PVL born to mothers with placental abruption, explaining how episodes of prolonged hypoxia result in severe metabolic acidosis, followed by higher rates of motor and cognitive deficits in surviving infants [82]. Gonen et al. found that severe neonatal morbidity (≥ 1 severe neonatal complications: seizures, IVH, HIE, PVL, blood transfusion, NEC, or death) was independently associated with early abruption (aOR = 5.3, 95% CI = 3.9–7.6), placental maternal vascular malperfusion (MVM) (aOR = 1.5, 95% CI = 1.2–1.9), and placental maternal inflammatory response (MIR) lesions (aOR = 1.9, 95% CI = 1.4–2.3) [83]. Early occurrence of abruption leads to higher rates of MVM, MIR and placental hemorrhage. In a setting of preeclampsia, other studies have suggested that MVM and MIR lesions are independently associated with lower gestational age at delivery and adverse neonatal outcomes, such as small-for-gestational age, cerebral morbidity or death [84,85]. Moreover, Sehgal et al. found that maternal vascular malperfusion, accelerated villous maturation, and fetal vascular malperfusion were features that were significantly more common in preterm fetal growth restriction (FGR) placentae than in preterm appropriately grown infants [86]. Preterm births have in turn been associated with long-term adverse neonatal outcomes, such as autism, HIE, and other neurodevelopmental disabilities [87,88,89,90].
4.3. Limitations
All studies in the meta-analysis were retrospective in nature and primarily relied on clinical diagnosis of placental abruption. Hence, exploration of additional variables or potential confounders, is not possible. In addition, there is no true gold standard for diagnosing placental abruption. As such, abruption was diagnosed differently across the studies, including referencing clinical diagnoses, ultrasonography, and histopathology findings. Hence, variation in the field makes it challenging to compare findings across studies and to make definitive clinical recommendations. In general, the prevalence of placental abruption is low [91], and the incidence of CP and neurological complication appears even lower. The attributable fraction for the abruption–neurological outcome association is likely very small with minimal population impact, given that abruption is unpreventable, and no existing interventions can treat it [38].
There were more preterm births in women with placental abruption than without abruption; therefore, adjusting for lower gestational age (and thus prematurity) would have been ideal to accurately examine the impact of placental abruption on cerebral palsy. Only two of the eight studies had any follow-up on infants from pregnancies complicated by placental abruption. Therefore, these studies had a poor score on the Newcastle–Ottawa scale. The absence of follow-up is also a limitation since there is evidence of increased risk of neurodevelopmental abnormalities in childhood due to prenatal hypoxia during pregnancy [92]. Hence, it is likely that these studies may have underreported cerebral palsy or other neurodevelopmental outcomes. There was overlap of data (double counting) in two articles authored by the same research group [44,45]; however, we did not include both studies in the meta-analysis, as that would have skewed numbers and led to biased results. Instead, we only included one article [45], which had a robust number of controls, and whose cases of abruption were not divided according to clinical grade of IVH. Since our sample size was small, we were unable to perform subgroup analysis to examine confounders of interest such as maternal alcohol consumption, smoking, prematurity, and other parameters. Most of the data reported in our included studies were too variable, as indicated by high I2 in the meta-analysis. Therefore, more consistent measures to comprehensively analyze neurodevelopmental outcomes will be needed in future placental abruption studies.
4.4. Conclusions and Implications
Our meta-analysis loosely suggests increased odds of cerebral palsy in infants born to pregnant women with placental abruption as compared to pregnant women without abruption. Whether this finding suggests a need for timely obstetric delivery is not presently known, given the complexity of cerebral palsy, the multiple conditions contributing to placental abruption, and the range of presentation of placental abruption at different gestational ages. Moreover, most studies do not provide an adequate follow-up period for cerebral palsy diagnosis. Our systematic review shares a promising result in that it is the first of its kind to investigate the role of placental abruption and neurodevelopmental outcomes, in a systematic manner. This is an area where there is a paucity of evidence that needs to be rectified to ensure better quality of evidence-based care for abruption patients. Future studies should further evaluate differences in neonatal neurodevelopmental outcomes in the settings of acute and chronic abruption.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Katie O’Hearn, (Children’s Hospital of Eastern Ontario Research Institute) for methodological assistance and Margaret Sampson, (Children’s Hospital of Eastern Ontario) for developing the electronic search strategies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ananth, C.V.; Keyes, K.M.; Hamilton, A.; Gissler, M.; Wu, C.; Liu, S.; Luque-Fernandez, M.A.; Skjærven, R.; Williams, M.A.; Tikkanen, M.; et al. An international contrast of rates of placental abruption: An age-period-cohort analysis. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Lavery, J.A.; Vintzileos, A.M.; Skupski, D.W.; Varner, M.; Saade, G.; Biggio, J.; Williams, M.A.; Wapner, R.J.; Wright, J.D. Severe placental abruption: Clinical definition and associations with maternal complications. Am. J. Obstet. Gynecol. 2016, 214, 272.e1–272.e9. [Google Scholar] [CrossRef] [PubMed]
- Oyelese, Y.; Ananth, C.V. Placental Abruption. Obstet. Gynecol. 2006, 108, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.; Gissler, M.; Saari, J.; Kramer, M.; Heinonen, S. Contribution of Risk Factors to Extremely, Very and Moderately Preterm Births—Register-Based Analysis of 1,390,742 Singleton Births. PLoS ONE 2013, 8, e60660. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, O.; Metsäranta, M.; Ritvanen, A.; Gissler, M.; Luukkaala, T.; Paavonen, J.; Nuutila, M.; Andersson, S.; Tikkanen, M. Increased Prevalence of Major Congenital Anomalies in Births with Placental Abruption. Obstet. Gynecol. 2013, 122, 268–274. [Google Scholar] [CrossRef]
- Redline, R.W. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am. J. Obstet. Gynecol. 2005, 192, 452–457. [Google Scholar] [CrossRef]
- Kayani, S.I.; Walkinshaw, S.A.; Preston, C. Pregnancy outcome in severe placental abruption. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 679–683. [Google Scholar] [CrossRef]
- Bonnar, J. Massive obstetric haemorrhage. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 1–18. [Google Scholar] [CrossRef]
- López-Llera, M.; de la Luz Espinosa, M.; Arratia, C. Eclampsia and placental abruption: Basic patterns, management and morbidity. Int. J. Gynecol. Obstet. 1988, 27, 335–342. [Google Scholar] [CrossRef]
- Maye, J.P. Placental abruption: A case study. CRNA 1994, 5, 20–21. [Google Scholar]
- Downes, K.L.; Shenassa, E.D.; Grantz, K.L. Neonatal Outcomes Associated with Placental Abruption. Am. J. Epidemiol. 2017, 186, 1319–1328. [Google Scholar] [CrossRef]
- Pariente, G.; Wiznitzer, A.; Sergienko, R.; Mazor, M.; Holcberg, G.; Sheiner, E. Placental abruption: Critical analysis of risk factors and perinatal outcomes. J. Matern. Neonatal Med. 2011, 24, 698–702. [Google Scholar] [CrossRef]
- Riihimäki, O.; Metsäranta, M.; Paavonen, J.; Luukkaala, T.; Gissler, M.; Andersson, S.; Nuutila, M.; Tikkanen, M. Placental Abruption and Child Mortality. Pediatrics 2018, 142, e20173915. [Google Scholar] [CrossRef]
- Ananth, C.V. Placental Abruption and Adverse Perinatal Outcomes. JAMA 1999, 282, 1646. [Google Scholar] [CrossRef]
- DeRoo, L.; Skjærven, R.; Wilcox, A.; Klungsøyr, K.; Wikström, A.-K.; Morken, N.-H.; Cnattingius, S. Placental abruption and long-term maternal cardiovascular disease mortality: A population-based registry study in Norway and Sweden. Eur. J. Epidemiol. 2016, 31, 501–511. [Google Scholar] [CrossRef]
- Adams, T.; Yeh, C.; Bennett-Kunzier, N.; Kinzler, W.L. Long-term maternal morbidity and mortality associated with ischemic placental disease. Semin. Perinatol. 2014, 38, 146–150. [Google Scholar] [CrossRef]
- Pariente, G.; Shoham-Vardi, I.; Kessous, R.; Sherf, M.; Sheiner, E. Placental Abruption as a Significant Risk Factor for Long-term Cardiovascular Mortality in a Follow-up Period of More Than a Decade. Paediatr. Perinat. Epidemiol. 2014, 28, 32–38. [Google Scholar] [CrossRef]
- Ananth, C.V.; Hansen, A.V.; Elkind, M.S.V.; Williams, M.A.; Rich-Edwards, J.W.; Nybo Andersen, A.-M. Cerebrovascular disease after placental abruption. Neurology 2019, 93, e1148–e1158. [Google Scholar] [CrossRef]
- Whitehead, E.; Dodds, L.; Joseph, K.S.; Gordon, K.E.; Wood, E.; Allen, A.C.; Camfield, P.; Dooley, J.M. Relation of Pregnancy and Neonatal Factors to Subsequent Development of Childhood Epilepsy: A Population-Based Cohort Study. Pediatrics 2006, 117, 1298–1306. [Google Scholar] [CrossRef]
- Hasegawa, J.; Ikeda, T.; Toyokawa, S.; Jojima, E.; Satoh, S.; Ichizuka, K.; Tamiya, N.; Nakai, A.; Fujimori, K.; Maeda, T.; et al. Relevant obstetric factors associated with fetal heart rate monitoring for cerebral palsy in pregnant women with hypertensive disorder of pregnancy. J. Obstet. Gynaecol. Res. 2018, 44, 647–654. [Google Scholar] [CrossRef]
- Trønnes, H.; Wilcox, A.J.; Lie, R.T.; Markestad, T.; Moster, D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: A national cohort study. Dev. Med. Child Neurol. 2014, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Ichizuka, K.; Toyokawa, S.; Ikenoue, T.; Satoh, S.; Hasegawa, J.; Ikeda, T.; Tamiya, N.; Nakai, A.; Fujimori, K.; Maeda, T.; et al. Risk factors for cerebral palsy in neonates due to placental abruption. J. Obstet. Gynaecol. Res. 2021, 47, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Himmelmann, K.; Ahlin, K.; Jacobsson, B.; Cans, C.; Thorsen, P. Risk factors for cerebral palsy in children born at term. Acta Obstet. Gynecol. Scand. 2011, 90, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Pariente, G.; Wainstock, T.; Walfisch, A.; Landau, D.; Sheiner, E. Placental abruption and long-term neurological hospitalisations in the offspring. Paediatr. Perinat. Epidemiol. 2019, 33, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Lucke, A.M.; McIntire, D.D.; Sánchez, P.J.; Leveno, K.J.; Chalak, L.F. Obstetric antecedents to body-cooling treatment of the newborn infant. Am. J. Obstet. Gynecol. 2014, 211, 155.e1–155.e6. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, A.; Incerti, M.; Paterlini, G.; Doria, V.; Consonni, S.; Provero, C.; Ghidini, A. Antepartum and Intrapartum Risk Factors for Neonatal Encephalopathy at Term. Am. J. Perinatol. 2010, 27, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Nasiell, J.; Papadogiannakis, N.; Löf, E.; Elofsson, F.; Hallberg, B. Hypoxic ischemic encephalopathy in newborns linked to placental and umbilical cord abnormalities. J. Matern. Neonatal Med. 2016, 29, 721–726. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wei, K.-L.; Zhou, C.-L.; Yao, Y.-J.; Yang, Y.-J.; Fan, X.-F.; Gao, X.-R.; Liu, X.-H.; Qian, J.-H.; Wu, B.-Q.; et al. Incidence of brain injuries in premature infants with gestational age ≤34 weeks in ten urban hospitals in China. World J. Pediatr. 2013, 9, 17–24. [Google Scholar] [CrossRef]
- Dani, C.; Poggi, C.; Bertini, G.; Pratesi, S.; Di Tommaso, M.; Scarselli, G.; Rubaltelli, F.F. Method of delivery and intraventricular haemorrhage in extremely preterm infants. J. Matern. Neonatal Med. 2010, 23, 1419–1423. [Google Scholar] [CrossRef]
- Furukawa, S.; Doi, K.; Furuta, K.; Sameshima, H. The effect of placental abruption on the outcome of extremely premature infants. J. Matern. Neonatal Med. 2015, 28, 705–708. [Google Scholar] [CrossRef]
- Hjern, A.; Thorngren-Jerneck, K. Perinatal complications and socio-economic differences in cerebral palsy in Sweden—A national cohort study. BMC Pediatr. 2008, 8, 49. [Google Scholar] [CrossRef]
- Stelmach, T.; Pisarev, H.; Talvik, T. Ante- and perinatal factors for cerebral palsy: Case-control study in Estonia. J. Child Neurol. 2005, 20, 654–661. [Google Scholar] [CrossRef]
- Kułak, W.; Okurowska-Zawada, B.; Sienkiewicz, D.; Paszko-Patej, G.; Krajewska-Kułak, E. Risk factors for cerebral palsy in term birth infants. Adv. Med. Sci. 2010, 55, 216–221. [Google Scholar] [CrossRef]
- Hasegawa, J.; Toyokawa, S.; Ikenoue, T.; Asano, Y.; Satoh, S.; Ikeda, T.; Ichizuka, K.; Tamiya, N.; Nakai, A.; Fujimori, K.; et al. Relevant Obstetric Factors for Cerebral Palsy: From the Nationwide Obstetric Compensation System in Japan. PLoS ONE 2016, 11, e0148122. [Google Scholar] [CrossRef]
- Ananth, C.; Friedman, A.; Lavery, J.; VanderWeele, T.; Keim, S.; Williams, M. Neurodevelopmental outcomes in children in relation to placental abruption. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 463–472. [Google Scholar] [CrossRef]
- Love, E.R.; Crum, J.; Bhattacharya, S. Independent effects of pregnancy induced hypertension on childhood development: A retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 219–224. [Google Scholar] [CrossRef]
- Nielsen, L.; Schendel, D.; Grove, J.; Hvidtjørn, D.; Jacobsson, B.; Josiassen, T.; Vestergaard, M.; Uldall, P.; Thorsen, P. Asphyxia-related risk factors and their timing in spastic cerebral palsy. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1518–1528. [Google Scholar] [CrossRef]
- Downes, K.L.; Grantz, K.L.; Shenassa, E.D. Maternal, Labor, Delivery, and Perinatal Outcomes Associated with Placental Abruption: A Systematic Review. Am. J. Perinatol. 2017, 34, 935–957. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane. 2022. Available online: www.training.cochrane.org/handbook (accessed on 15 December 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Bramer, W.M.; De Jonge, G.B.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. A systematic approach to searching: An efficient and complete method to develop literature searches. J. Med. Libr. Assoc. 2018, 106, 531–541. [Google Scholar] [CrossRef]
- Prospero International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 20 January 2021).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Fazzi, E.; Stronati, M.; Ometto, A.; Iasci, A.; Guaschino, S. Severity of abruptio placentae and neurodevelopmental outcome in low birth weight infants. Early Hum. Dev. 1993, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Fazzi, E.; Stronati, M.; Ometto, A.; Capuzzo, E.; Guaschino, S. Early Morbidity and Neurodevelopmental Outcome in Low-Birthweight Infants Born after Third Trimester Bleeding. Am. J. Perinatol. 1994, 11, 85–90. [Google Scholar] [CrossRef]
- Lv, H.Y.; Wang, Q.L.; Chen, H.Y.; You, Y.J.; Ren, P.S.; Li, L.X. Study on serum Tau protein level and neurodevelopmental outcome of placental abruption with neonatal hypoxic-ischemic encephalopathy. J. Matern. Neonatal Med. 2019, 33, 3887–3893. [Google Scholar] [CrossRef]
- Matsuda, Y.; Maeda, T.; Kouno, S. Comparison of neonatal outcome including cerebral palsy between abruptio placentae and placenta previa. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 106, 125–129. [Google Scholar] [CrossRef]
- Page, E.W.; King, E.B.; Merrill, J.A. Abruptio placentae; dangers of delay in delivery. Obstet. Gynecol. 1954, 3, 385–393. [Google Scholar]
- Knab, D.R. Abruptio placentae. An assessment of the time and method of delivery. Obstet. Gynecol. 1978, 52, 625–629. [Google Scholar]
- Allred, L.S.; Batton, D. The Effect of Placental Abruption on the Short-Term Outcome of Premature Infants. Am. J. Perinatol. 2004, 21, 157–162. [Google Scholar] [CrossRef]
- Becher, J.C.; Bell, J.E.; Keeling, J.W.; Liston, W.A.; McIntosh, N.; Wyatt, B. The Scottish Perinatal Neuropathology Study—Clinicopathological correlation in stillbirths. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 310–317. [Google Scholar] [CrossRef]
- Lee, H.; Aronson, J.; Nunan, D.; Collider Bias. Catalogue of Bias. Available online: https://catalogofbias.org/biases/collider-bias/ (accessed on 27 July 2021).
- Joseph, L. Confounding and Collinearity in Multiple Linear Regression. Available online: http://www.medicine.mcgill.ca/epidemiology/joseph/courses/epib-621/confounding.pdf (accessed on 15 December 2022).
- Wells, G.A.; Shea, B.; O’Connell, D.A.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 December 2022).
- Sharmin, S.; Kypri, K.; Khanam, M.; Wadolowski, M.; Bruno, R.; Attia, J.; Holliday, E.; Palazzi, K.; Mattick, R.P. Effects of parental alcohol rules on risky drinking and related problems in adolescence: Systematic review and meta-analysis. Drug Alcohol Depend. 2017, 178, 243–256. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org (accessed on 15 December 2022).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based. Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Maeda, T.; Kouno, S. Fetal/neonatal outcome in abruptio placentae during preterm gestation. Semin. Thromb. Hemost. 2005, 31, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 241–284. ISBN 9781119536604. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119536604 (accessed on 15 December 2022).
- May, H.J.; Fasheun, J.A.; Bain, J.M.; Baugh, E.H.; Bier, L.E.; Revah-Politi, A.; Roye, D.P.; Goldstein, D.B.; Carmel, J.B.; Lippa, N.; et al. Genetic testing in individuals with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Sjölander, A.; Johansson, S.; Lu, D.; Razaz, N.; Tedroff, K.; Villamor, E.; Cnattingius, S. Impact of gestational age on risk of cerebral palsy: Unravelling the role of neonatal morbidity. Int. J. Epidemiol. 2021, 50, 1852–1863. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yamada, T.; Morikawa, M.; Minakami, H. Clinical features of abruptio placentae as a prominent cause of cerebral palsy. Early Hum. Dev. 2012, 88, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol. 2012, 64, 309–320. [Google Scholar]
- du Plessis, A.J.; Volpe, J.J. Perinatal brain injury in the preterm and term newborn. Curr. Opin. Neurol. 2002, 15, 151–157. [Google Scholar] [CrossRef]
- Williams, K.; Singh, A. The correlation of seizures in newborn infants with significant acidosis at birth with umbilical artery cord gas values. Obstet. Gynecol. 2002, 100, 557–560. [Google Scholar] [CrossRef]
- Graham, E.M.; Ruis, K.A.; Hartman, A.L.; Northington, F.J.; Fox, H.E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 2008, 199, 587–595. [Google Scholar] [CrossRef]
- Low, J.A.; Simpson, L.L.; Tonni, G.; Chamberlain, S. Limitations in the clinical prediction of intrapartum fetal asphyxia. Am. J. Obstet. Gynecol. 1995, 172, 801–804. [Google Scholar] [CrossRef]
- Matsuda, Y.; Umezaki, H.; Ogawa, M.; Ohwada, M.; Satoh, S.; Nakai, A. Umbilical arterial pH in patients with cerebral palsy. Early Hum. Dev. 2014, 90, 131–135. [Google Scholar] [CrossRef]
- Japan Council for Quality Health Care The Japan Obstetric Compensation System for Cerebral Palsy. In the 6th Recurrence Prevention Report. In Japanese; Tokyo. 2016. Available online: http://www.sanka-hp.jcqhc.or.jp/documents/english/pdf/looking_back_over10years_after_system_was_launched201905.pdf (accessed on 15 December 2022).
- Ananth, C.V.; Wilcox, A.J. Placental abruption and perinatal mortality in the United States. Am. J. Epidemiol. 2001, 153, 332–337. [Google Scholar] [CrossRef]
- Ananth, C.V. Placental Abruption among Singleton and Twin Births in the United States: Risk Factor Profiles. Am. J. Epidemiol. 2001, 153, 771–778. [Google Scholar] [CrossRef]
- Ananth, C.V.; Oyelese, Y.; Prasad, V.; Getahun, D.; Smulian, J.C. Evidence of placental abruption as a chronic process: Associations with vaginal bleeding early in pregnancy and placental lesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 15–21. [Google Scholar] [CrossRef]
- O’Leary, C.M.; Watson, L.; D’Antoine, H.; Stanley, F.; Bower, C. Heavy maternal alcohol consumption and cerebral palsy in the offspring. Dev. Med. Child Neurol. 2012, 54, 224–230. [Google Scholar] [CrossRef]
- Rasmussen, S.; Irgens, L.M. The effects of smoking and hypertensive disorders on fetal growth. BMC Pregnancy Childbirth 2006, 6, 16. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Tairy, D.; Weiner, E.; Kovo, M.; Zamir, A.M.; Gandelsman, E.; Levy, M.; Herman, H.G.; Volpert, E.; Schreiber, L.; Bar, J.; et al. Fetal Growth Restriction in Hypertensive vs. Heavy Smoking Women—Placental Pathology, Ultrasound Findings, and Pregnancy Outcomes. Reprod. Sci. 2021, 28, 819–827. [Google Scholar] [CrossRef]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef]
- Chevallier, M.; Debillon, T.; Pierrat, V.; Delorme, P.; Kayem, G.; Durox, M.; Goffinet, F.; Marret, S.; Ancel, P.Y.; Arnaud, C.; et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: Results of the EPIPAGE 2 cohort study. Am. J. Obstet. Gynecol. 2017, 216, 518.e1–518.e12. [Google Scholar] [CrossRef]
- Ballabh, P. Pathogenesis and Prevention of Intraventricular Hemorrhage. Clin. Perinatol. 2014, 41, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M. Interruption of Placental Blood Flow during Labor: Potential Systemic and Cerebral Organ Consequences. J. Pediatr. 2011, 158, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, K.; Nakayama, M.; Sumida, Y.; Ozono, K.; Mushiake, S.; Suehara, N.; Wada, Y.; Fujimura, M. Placental features in preterm infants with periventricular leukomalacia. Pediatrics 2002, 109, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Low, J.A.; Galbraith, R.S.; Muir, D.W.; Killen, H.L.; Pater, E.A.; Karchmar, E.J. Factors associated with motor and cognitive deficits in children after intrapartum fetal hypoxia. Am. J. Obstet. Gynecol. 1984, 148, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Gonen, N.; Levy, M.; Kovo, M.; Schreiber, L.; Noy, L.K.; Volpert, E.; Bar, J.; Weiner, E. Placental Histopathology and Pregnancy Outcomes in “Early” vs. “Late” Placental Abruption. Reprod. Sci. 2021, 28, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Weiner, E.; Feldstein, O.; Tamayev, L.; Grinstein, E.; Barber, E.; Bar, J.; Schreiber, L.; Kovo, M. Placental histopathological lesions in correlation with neonatal outcome in preeclampsia with and without severe features. Pregnancy Hypertens. 2018, 12, 6–10. [Google Scholar] [CrossRef]
- Paules, C.; Youssef, L.; Rovira, C.; Crovetto, F.; Nadal, A.; Peguero, A.; Figueras, F.; Eixarch, E.; Crispi, F.; Miranda, J.; et al. Distinctive patterns of placental lesions in pre-eclampsia vs small-for-gestational age and their association with fetoplacental Doppler. Ultrasound Obstet. Gynecol. 2019, 54, 609–616. [Google Scholar] [CrossRef]
- Sehgal, A.; Dahlstrom, J.E.; Chan, Y.; Allison, B.J.; Miller, S.L.; Polglase, G.R. Placental histopathology in preterm fetal growth restriction. J. Paediatr. Child Health 2019, 55, 582–587. [Google Scholar] [CrossRef]
- Raghavan, R.; Helfrich, B.B.; Cerda, S.R.; Ji, Y.; Burd, I.; Wang, G.; Hong, X.; Fu, L.; Pearson, C.; Daniele Fallin, M.; et al. Preterm birth subtypes, placental pathology findings, and risk of neurodevelopmental disabilities during childhood. Placenta 2019, 83, 17–25. [Google Scholar] [CrossRef]
- Bingham, A.; Gundogan, F.; Rand, K.; Laptook, A.R. Placental findings among newborns with hypoxic ischemic encephalopathy. J. Perinatol. 2019, 39, 563–570. [Google Scholar] [CrossRef]
- Straughen, J.K.; Misra, D.P.; Divine, G.; Shah, R.; Perez, G.; VanHorn, S.; Onbreyt, V.; Dygulska, B.; Schmitt, R.; Lederman, S.; et al. The association between placental histopathology and autism spectrum disorder. Placenta 2017, 57, 183–188. [Google Scholar] [CrossRef]
- Tokuhisa, T.; Ibara, S.; Minakami, H.; Maede, Y.; Ishihara, C.; Matsui, T. Outcome of infants with hypoxic ischemic encephalopathy treated with brain hypothermia. J. Obstet. Gynaecol. Res. 2015, 41, 229–237. [Google Scholar] [CrossRef]
- Broers, T.; King, W.D.; Arbuckle, T.E.; Liu, S. The occurrence of abruptio placentae in Canada: 1990 to 1997. Chronic Dis. Can. 2004, 25, 16–20. [Google Scholar]
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of Prenatal Hypoxia in Brain Development, Cognitive Functions, and Neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).