An Osteotomy Tool That Preserves Bone Viability: Evaluation in Preclinical and Clinical Settings
Abstract
:1. Introduction
2. Materials and Methods
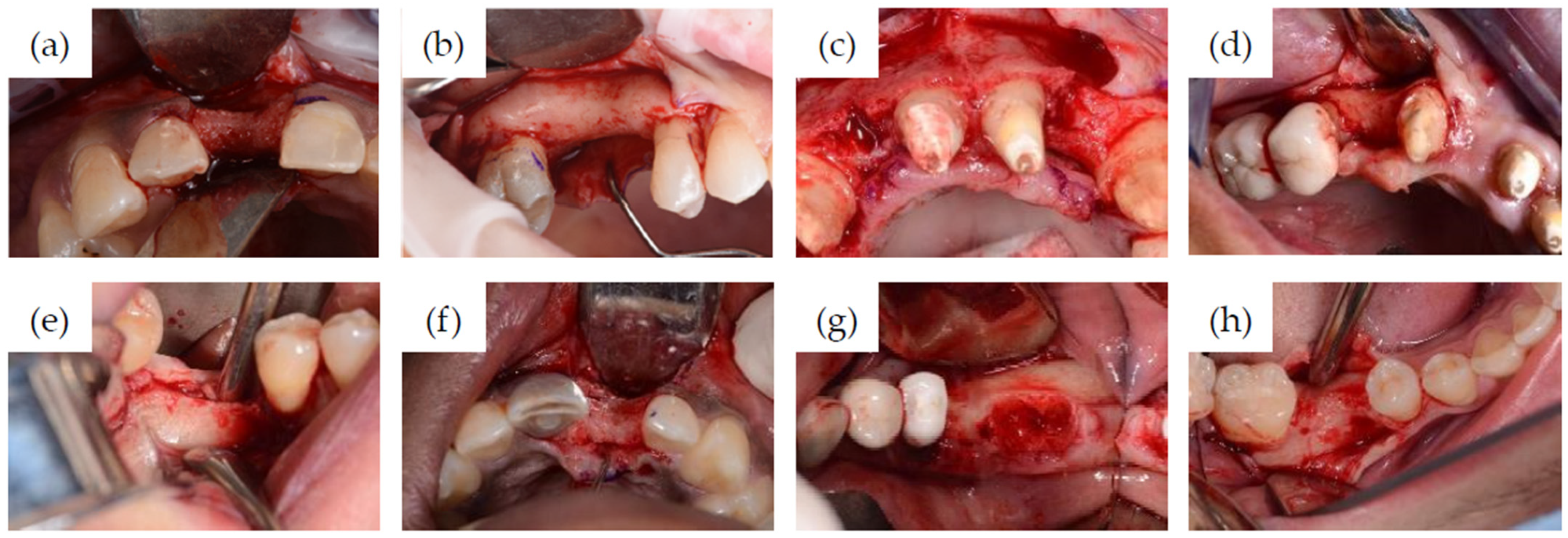
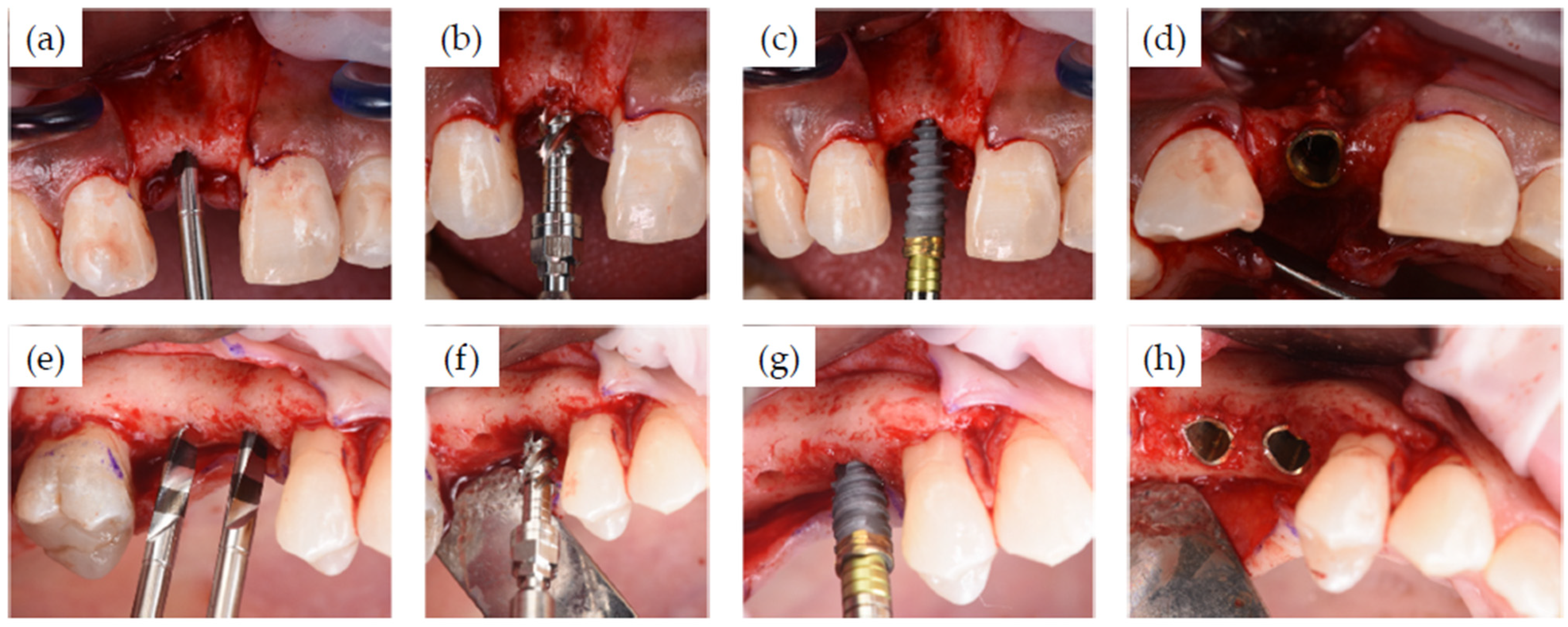
2.1. Clinical Case Series
Surgical and Restorative Procedures
2.2. Animal Model Experiments
2.2.1. Rodent Information
2.2.2. Micro-Computed Tomography (µCT) in Rodents
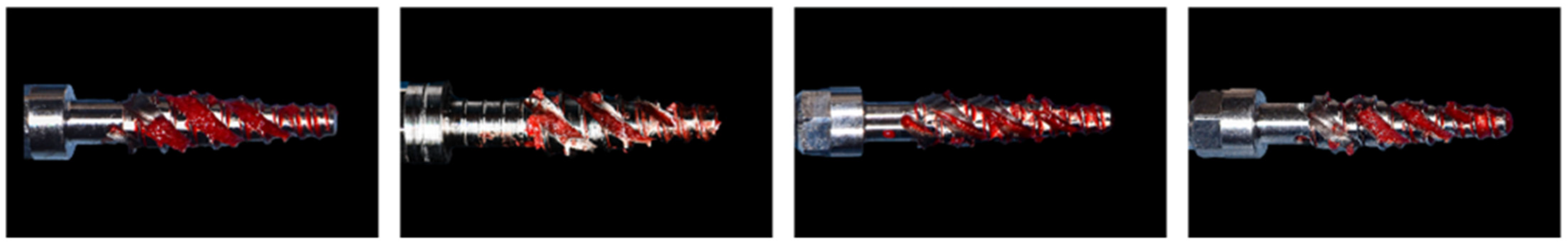
2.2.3. Osteotomy Site Preparation in Rodents
2.2.4. Rodent Tissue Collection and Processing
2.2.5. Histology
2.2.6. Programmed Cell Death
2.2.7. Tartrate-Resistant Acid Phosphatase (TRAP) Activity
2.2.8. Immunostaining
2.2.9. Histomorphometric Analyses
2.2.10. Statistical Analyses
3. Results
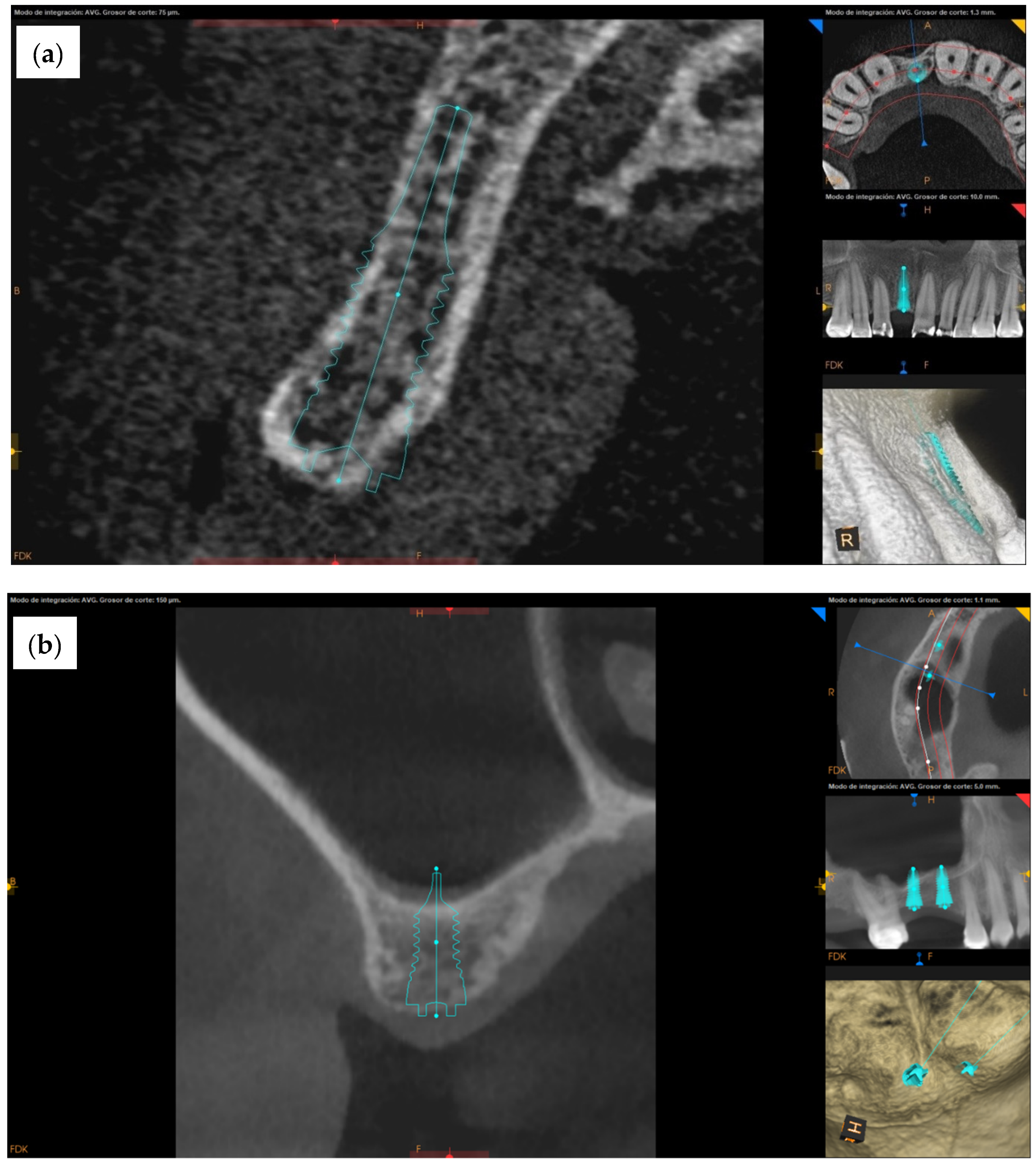
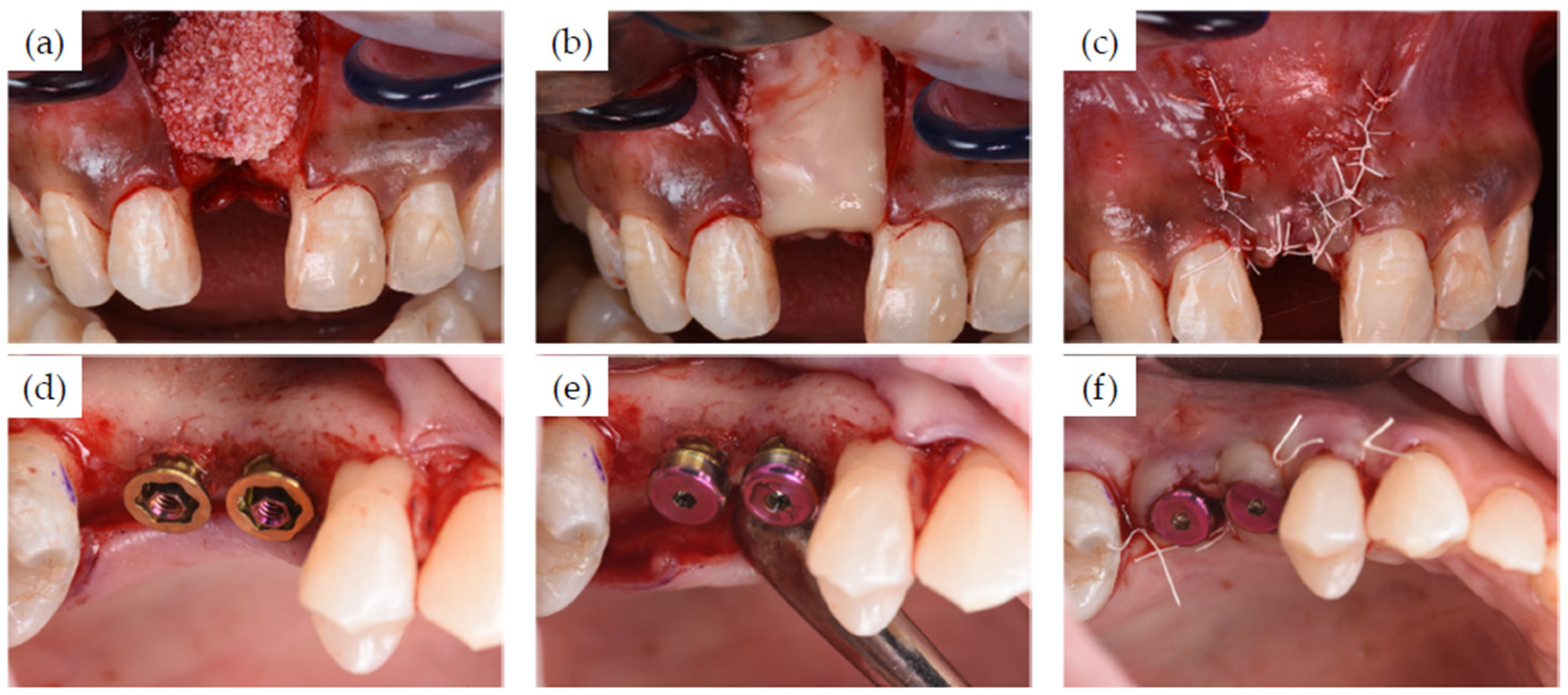
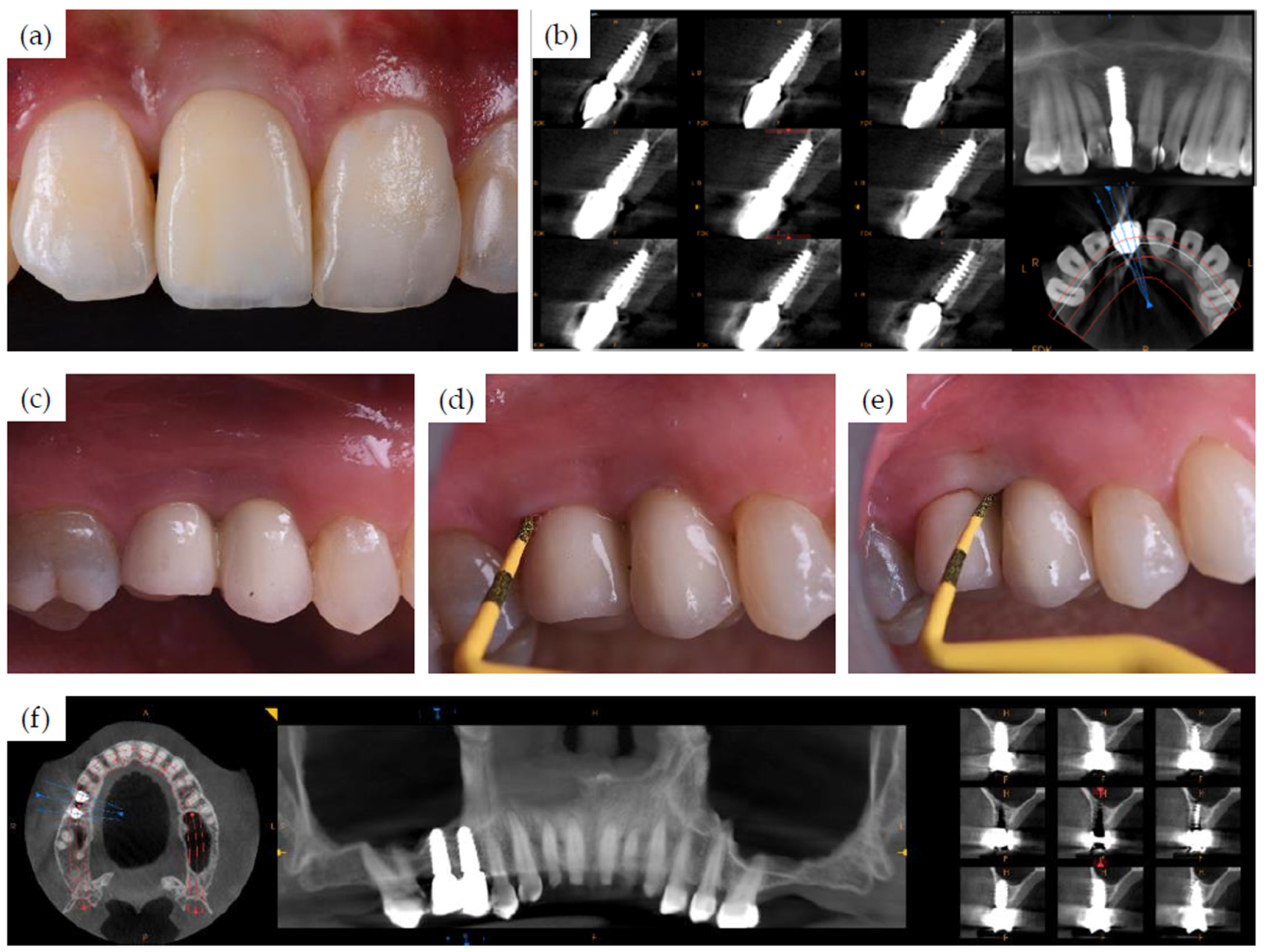
3.1. Clinical Cases
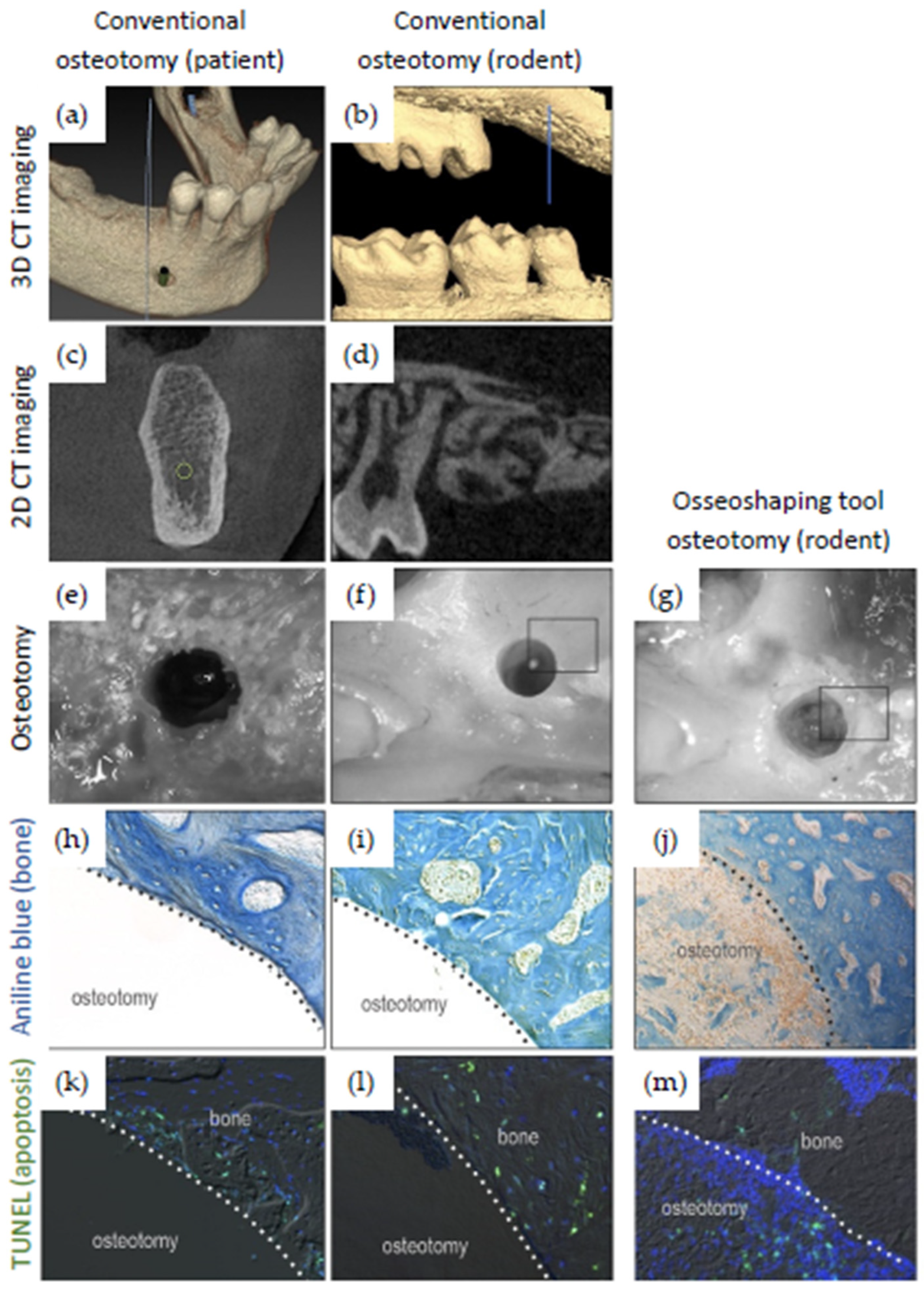
3.2. Evaluation of Rodent Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivan Cardoso, M.; Vandamme, K.; Chaudhari, A.; De Rycker, J.; Van Meerbeek, B.; Naert, I.; Duyck, J. Dental Implant Macro-Design Features Can Impact the Dynamics of Osseointegration. Clin. Implant Dent. Relat. Res. 2015, 17, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Abouzgia, M.B.; James, D.F. Measurements of shaft speed while drilling through bone. J. Oral Maxillofac. Surg. 1995, 53, 1308–1315; discussion 1315–1306. [Google Scholar] [CrossRef]
- Aghvami, M.; Brunski, J.B.; Serdar Tulu, U.; Chen, C.H.; Helms, J.A. A Thermal and Biological Analysis of Bone Drilling. J. Biomech. Eng. 2018, 140, 101010. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.C. Effect of drilling into bone. J. Oral Surg. 1958, 16, 22–30. [Google Scholar]
- Borges, G.A.; Costa, R.C.; Nagay, B.E.; Magno, M.B.; Maia, L.C.; Barão, V.A.R.; Mesquita, M.F. Long-term outcomes of different loading protocols for implant-supported mandibular overdentures: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 125, 732–745. [Google Scholar] [CrossRef]
- Karl, M.; Albrektsson, T. Clinical Performance of Dental Implants with a Moderately Rough (TiUnite) Surface: A Meta-Analysis of Prospective Clinical Studies. Int. J. Oral Maxillofac. Implant. 2017, 32, 717–734. [Google Scholar] [CrossRef] [Green Version]
- Gulati, M.; Govila, V.; Verma, S.; Rajkumar, B.; Anand, V.; Aggarwal, A.; Jain, N. In Vivo Evaluation of Two-Piece Implants Placed Following One-Stage and Two-Stage Surgical Protocol in Posterior Mandibular Region. Assessment of Alterations in Crestal Bone Level. Clin. Implant Dent. Relat. Res. 2015, 17, 854–861. [Google Scholar] [CrossRef]
- Hof, M.; Tepper, G.; Semo, B.; Arnhart, C.; Watzek, G.; Pommer, B. Patients’ perspectives on dental implant and bone graft surgery: Questionnaire-based interview survey. Clin. Oral Implant. Res. 2014, 25, 42–45. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental implants inserted in fresh extraction sockets versus healed sites: A systematic review and meta-analysis. J. Dent. 2015, 43, 16–41. [Google Scholar] [CrossRef]
- Seyssens, L.; De Lat, L.; Cosyn, J. Immediate implant placement with or without connective tissue graft: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 284–301. [Google Scholar] [CrossRef]
- Giro, G.; Marin, C.; Granato, R.; Bonfante, E.A.; Suzuki, M.; Janal, M.N.; Coelho, P.G. Effect of drilling technique on the early integration of plateau root form endosteal implants: An experimental study in dogs. J. Oral Maxillofac. Surg. 2011, 69, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Yeniyol, S.; Jimbo, R.; Marin, C.; Tovar, N.; Janal, M.N.; Coelho, P.G. The effect of drilling speed on early bone healing to oral implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Pereira, M.D.; Smith, A.A.; Houschyar, K.S.; Yin, X.; Mouraret, S.; Brunski, J.B.; Helms, J.A. Multiscale Analyses of the Bone-implant Interface. J. Dent. Res. 2015, 94, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyac, B.R.; Salvi, G.; Leahy, B.; Li, Z.; Salmon, B.; Hoffmann, W.; Helms, J.A. A novel system exploits bone debris for implant osseointegration. J. Periodontol. 2021, 92, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.R.; James, D.F. Drilling in bone: Modeling heat generation and temperature distribution. J. Biomech. Eng. 2003, 125, 305–314. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Matthews, L.S.; Hirsch, C. Temperatures measured in human cortical bone when drilling. J. Bone Jt. Surg. Am. Vol. 1972, 54, 297–308. [Google Scholar] [CrossRef]
- Wang, L.; Aghvami, M.; Brunski, J.; Helms, J. Biophysical regulation of osteotomy healing: An animal study. Clin. Implant Dent. Relat. Res. 2017, 19, 590–599. [Google Scholar] [CrossRef]
- Laursen, M.; Christensen, F.B.; Bünger, C.; Lind, M. Optimal handling of fresh cancellous bone graft: Different peroperative storing techniques evaluated by in vitro osteoblast-like cell metabolism. Acta Orthop. Scand. 2003, 74, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Maus, U.; Andereya, S.; Gravius, S.; Siebert, C.H.; Schippmann, T.; Ohnsorge, J.A.; Niedhart, C. How to store autologous bone graft perioperatively: An in vitro study. Arch. Orthop. Trauma Surg. 2008, 128, 1007–1011. [Google Scholar] [CrossRef]
- Miron, R.J.; Gruber, R.; Hedbom, E.; Saulacic, N.; Zhang, Y.; Sculean, A.; Bosshardt, D.D.; Buser, D. Impact of bone harvesting techniques on cell viability and the release of growth factors of autografts. Clin. Implant Dent. Relat. Res. 2013, 15, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Isler, S.C.; Cansiz, E.; Tanyel, C.; Soluk, M.; Selvi, F.; Cebi, Z. The effect of irrigation temperature on bone healing. Int. J. Med. Sci. 2011, 8, 704–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawy, G.F.; Rowe, P.J.; Riches, P.E. Thermal Damage Done to Bone by Burring and Sawing With and Without Irrigation in Knee Arthroplasty. J. Arthroplast. 2016, 31, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etcheson, A.W.; Miley, D.D.; Gillespie, M.J. Osseous coagulum collected in bone traps: Potential for bacterial contamination and methods for decontamination. J. Oral Implantol. 2007, 33, 109–115. [Google Scholar] [CrossRef]
- Chen, C.H.; Coyac, B.R.; Arioka, M.; Leahy, B.; Tulu, U.S.; Aghvami, M.; Holst, S.; Hoffmann, W.; Quarry, A.; Bahat, O.; et al. A Novel Osteotomy Preparation Technique to Preserve Implant Site Viability and Enhance Osteogenesis. J. Clin. Med. 2019, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Coyac, B.R.; Sun, Q.; Leahy, B.; Salvi, G.; Yuan, X.; Brunski, J.B.; Helms, J.A. Optimizing autologous bone contribution to implant osseointegration. J. Periodontol. 2020, 91, 1632–1644. [Google Scholar] [CrossRef]
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J. Am. Acad. Orthop. Surg. 1995, 3, 1–8. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Naji, B.M.; Abdelsameaa, S.S.; Alqutaibi, A.Y.; Said Ahmed, W.M. Immediate dental implant placement with a horizontal gap more than two millimetres: A randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2021, 50, 683–690. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Liu, B.; Yuan, X.; Guo, S.; Helms, J.A. Improving intraoperative storage conditions for autologous bone grafts: An experimental investigation in mice. J. Tissue Eng. Regen. Med. 2019, 13, 2169–2180. [Google Scholar] [CrossRef]
- Meljon, A.; Geisendorf, M.; Kamber, S.; Fuchs, F.; Irastorza-Landa, A. Evaluation of heat generated by a new site preparation using low-speed tools without irrigation. In Proceedings of the EAO Digital Days, Milan, Italy, 12–14 October 2021. [Google Scholar]
- Grisar, K.; Sinha, D.; Schoenaers, J.; Dormaar, T.; Politis, C. Retrospective Analysis of Dental Implants Placed Between 2012 and 2014: Indications, Risk Factors, and Early Survival. Int. J. Oral Maxillofac. Implant. 2017, 32, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimmel, M.; Müller, F.; Suter, V.; Buser, D. Implants for elderly patients. Periodontol. 2000 2017, 73, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Melton, L.J., 3rd; Harris, T.; Borrud, L.; Shepherd, J.; McGowan, J. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos. Int. 2009, 20, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekholm, U.; Zarb, G.A. Patient Selection and Preparation. In Tissue-Integrated Prosthesis. Osseointegration in Clinical Dentistry; Branemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence Publishing Co., Inc.: Chicago, IL, USA; London, UK; Berlin, Germany; Rio de Janeiro, Brazil; Tokyo, Japan, 1985; Volume 1, pp. 199–208. [Google Scholar]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Minear, S.; Leucht, P.; Jiang, J.; Liu, B.; Zeng, A.; Fuerer, C.; Nusse, R.; Helms, J.A. Wnt proteins promote bone regeneration. Sci. Transl. Med. 2010, 2, 29ra30. [Google Scholar] [CrossRef] [Green Version]
- Velikov, S.; Susin, C.; Heuberger, P.; Irastorza-Landa, A. A New Site Preparation Protocol That Supports Bone Quality Evaluation and Provides Predictable Implant Insertion Torque. J. Clin. Med. 2020, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Zemp, J.; Velikov, S.; Weißbrot, S.; Fuchs, F. New low-speed site preparation protocol significantly reduces noise. In Proceedings of the IADR General Session, Washington, DC, USA, 18–21 March 2020. [Google Scholar]
- Staas, T.; Urban, I.; Fabbri, G. Safety and effectiveness of the N1 concept system: Real-world data with 1-year follow-up. In Proceedings of the EAO Digital Days, Milan, Italy, 12–14 October 2021. [Google Scholar]

| Experimental Group | Sample Size | Tool | Rotational Velocity (rpm) | Irrigation | Time Points for Data Analysis | Analyses Performed |
|---|---|---|---|---|---|---|
| Conventional osteotomy site preparation | 18 | Final OsteoMed drill | 1350 | yes | 0.5 days 7 days | µCT, histology, IHC |
| N1 site preparation | 18 | Osseo-Shaper | 50 | no | 0.5 days 7 days | µCT, histology, IHC |
| Company | Osteotomy Instrument | External Diameter (mm) | Revolutions per Minute (rpm) | Irrigation |
|---|---|---|---|---|
| OsteoMed | Pilot drill | 0.8 | 2000 | yes |
| 2nd drill | 1.0 | 1600 | yes | |
| Final drill | 1.2 | 1350 | yes | |
| Nobel Biocare | Pilot drill | 0.8 | 2000 | yes |
| Miniaturized version of osseoshaping tool | crestal = 1.33 apical = 0.525 | 50 | no |
| Patient Number | Patient Characteristics | Implant Recipient Site Characteristics | Surgical Details | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | FDI Position | Bone Quality and Quantity | Additional Notes on Bone Conditions | Site Type | Insertion Torque (Ncm) of the Osseoshaping Tool | Implant Insertion Torque (Ncm) | Simultaneous Bone Augmentation | |
| 1 | 38 | female | 11 | 3, B | buccal concavity | healed | Not reported | 45 | yes |
| 2 | 47 | female | 15 | 4, C | Sinus lift | healed | Not reported | 45 | no |
| 16 | 4, C | Sinus lift | healed | Not reported | 45 | no | |||
| 3 | 58 | female | 12 | 4, B | buccal concavity | recent extraction (12 weeks) | 40 * | 61 | yes |
| 14 | 4, B | - | healed | 10 | 25 | no | |||
| 22 | 4, B | large concavity | healed | 40 * | 39 | yes | |||
| 37 | 4, B | - | healed | 19 | 55 | no | |||
| 38 | 4, B | - | extraction | 5 | 50 | yes | |||
| 4 | 64 | female | 46 | 2, C | narrow ridge | healed | 34/21 ** | 60 | no |
| 5 | 60 | female | 46 | 2, C | - | healed | 23 | 50 | yes |
| 47 | 3, D | - | healed | 21 | 50 | yes | |||
| 36 | 3, B | - | healed | 16 | 37 | no | |||
| 37 | 3, B | - | healed | 16 | 31 | no | |||
| 6 | 18 | male | 21 | 3, B | large concavity, 6 mm lingual defect | healed | 35 | 55 | yes |
| 7 | 26 | male | 36 | 3, B | buccal concavity | healed | 38 | 55 | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahat, O.; Yin, X.; Holst, S.; Zabalegui, I.; Berroeta, E.; Pérez, J.; Wöhrle, P.; Sörgel, N.; Brunski, J.; Helms, J.A. An Osteotomy Tool That Preserves Bone Viability: Evaluation in Preclinical and Clinical Settings. J. Clin. Med. 2022, 11, 2536. https://doi.org/10.3390/jcm11092536
Bahat O, Yin X, Holst S, Zabalegui I, Berroeta E, Pérez J, Wöhrle P, Sörgel N, Brunski J, Helms JA. An Osteotomy Tool That Preserves Bone Viability: Evaluation in Preclinical and Clinical Settings. Journal of Clinical Medicine. 2022; 11(9):2536. https://doi.org/10.3390/jcm11092536
Chicago/Turabian StyleBahat, Oded, Xing Yin, Stefan Holst, Ion Zabalegui, Eva Berroeta, Javier Pérez, Peter Wöhrle, Norbert Sörgel, John Brunski, and Jill A. Helms. 2022. "An Osteotomy Tool That Preserves Bone Viability: Evaluation in Preclinical and Clinical Settings" Journal of Clinical Medicine 11, no. 9: 2536. https://doi.org/10.3390/jcm11092536
APA StyleBahat, O., Yin, X., Holst, S., Zabalegui, I., Berroeta, E., Pérez, J., Wöhrle, P., Sörgel, N., Brunski, J., & Helms, J. A. (2022). An Osteotomy Tool That Preserves Bone Viability: Evaluation in Preclinical and Clinical Settings. Journal of Clinical Medicine, 11(9), 2536. https://doi.org/10.3390/jcm11092536






