The Influence of New Bioactive Materials on Pulp–Dentin Complex Regeneration in the Assessment of Cone Bone Computed Tomography (CBCT) and Computed Micro-Tomography (Micro-CT) from a Present and Future Perspective—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Review Questions
- To what extent is tertiary dentin visualised using CBCT and micro-CT?
- What parameters of tertiary dentin can be measured using CBCT and micro-CT?
- What bioactive materials are most often used for vital pulp therapy?
2.3. Information Sources and Search Strategy
- In vitro and in vivo studies that evaluated tertiary dentin in CBCT or micro-CT imaging.
- Publications concerning the properties of the dentin bridge in CBCT or micro-CT imaging.
2.4. Eligibility Criteria
2.5. Risk of Bias in the Studies Included
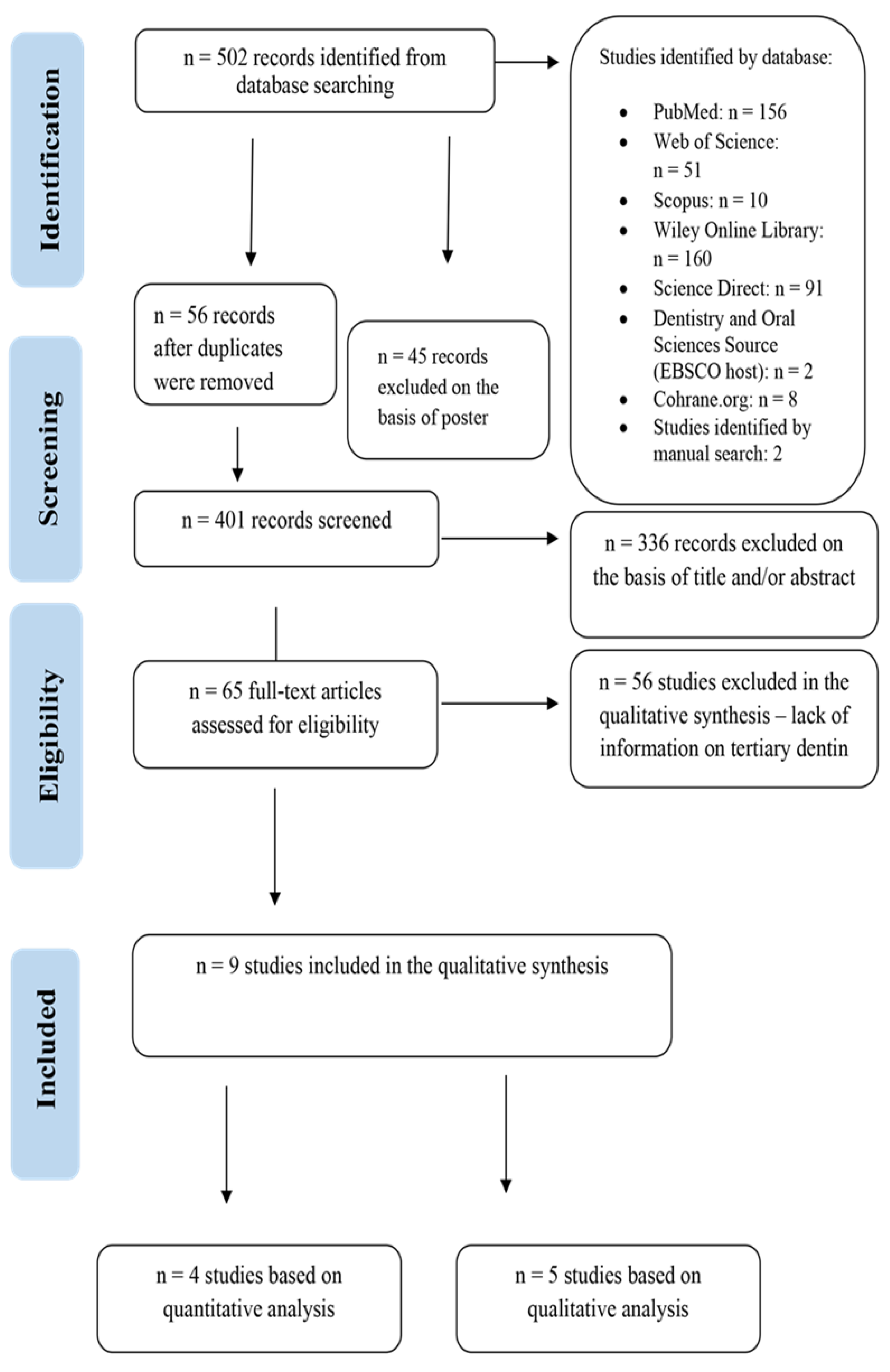
3. Results
3.1. Assessment of Studies
3.2. Results concerning Studies on Tertiary Dentin Visualisation Using CBCT
3.3. Results concerning Studies on Tertiary Dentin Using Visualisation with Micro-CT
4. Discussion
4.1. Visualization of Tertiary Dentin
4.2. Areas of Newly Formed Tertiary Dentin
4.3. Thickness of Tertiary Dentin
4.4. Tertiary Dentin Volume
4.5. Radiodensity of Tertiary Dentin
4.6. General Future Aspects of Using X-ray Techniques in Tertiary Dentin Imaging
5. Conclusions
- CBCT and micro-CT analyses can be useful in the assessment of tertiary dentin formed beneath the bioactive material applied during vital pulp treatment. The limitations of micro-CT studies that can only be carried out in vivo indirectly, i.e., on extracted teeth, should be taken into account.
- In CBCT and micro-CT analyses, the formed tertiary dentin is sufficient for determining whether the biomaterial applied to the pulp is effective in producing new hard tissue. The quality and quantity of tertiary dentine depends on the type of: pulp capping, bioactive materials applied to the pulp, observation time, tooth type and species. The conducted research shows that bioactive calcium silicate cement contributes to the formation of tertiary dentin, which is superior compared to calcium hydroxide.
- Most studies employ a qualitative analysis which does not require calibration phantoms and is less time-consuming by far, more often than the quantitative analysis. However, unlike the quantitative technique, this technique is subjective.
- The fundamental limitation of micro-CT is the exposure time and the amount of radiation dose which prevents the visualisation of human teeth in the patient’s oral cavity and allows for it only following the extraction of teeth.
Funding
Conflicts of Interest
References
- Tran, X.V.; Salehi, H.; Truong, M.T.; Sandra, M.; Sadoine, J.; Jacquot, B.; Cuisinier, F.; Chaussain, C.; Boukpessi, T. Reparative mineralized tissue characterization after direct pulp capping with calcium-silicate-based cements. Materials 2019, 12, 2102. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, L.; Fransson, H.; Bruun, G.; Markvart, M.; Kjældgaard, M.; Näsman, P.; Hedenbjörk-Lager, A.; Dige, I.; Thordrup, M. Randomized clinical trials on deep carious lesions: 5-Year Follow-up. J. Dent. Res. 2017, 96, 747–753. [Google Scholar] [CrossRef]
- Bertassoni, L.E. Dentin on the nanoscale: Hierarchical organization, mechanical behavior and bioinspired engineering. Dent. Mater. 2017, 33, 637–649. [Google Scholar] [CrossRef]
- Galler, K.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef]
- Ten Cate, A. Oral Histology: Development, Structure and Function, 5th ed.; Mosby Inc.: St. Louis, MO, USA, 1998; p. 497. [Google Scholar]
- Kinney, J.; Habelitz, S.; Marshall, S.; Marshall, G. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J. Dent. Res. 2003, 82, 957–961. [Google Scholar] [CrossRef] [Green Version]
- Nudel, I.; Pokhojaev, A.; Bitterman, Y.; Shpack, N.; Fiorenza, L.; Benazzi, S.; Sarig, R. Secondary Dentin Formation Mechanism: The Effect of Attrition. Int. J. Environ. Res. Public Health 2021, 18, 9961. [Google Scholar] [CrossRef]
- Tronstad, L.; Langeland, K. Effect of Attrition on Subjacent Dentin and Pulp. J. Dent. Res. 1971, 50, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Review Inflammation-regeneration interplay in the dentine-pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef]
- Olsson, H.; Petersson, K.; Rohlin, M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int. Endod. J. 2006, 39, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Fransson, H.; Wolf, E.; Petersson, K. Formation of a hard tissue barrier after experimental pulp capping or partial pulpotomy in humans: An updated systematic review. Int. Endod. J. 2016, 49, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.-H.; Choi, S.-H.; Koh, J.-T.; Lee, B.-N.; Chang, H.-S.; Hwang, I.-N.; Oh, W.-M.; Hwang, Y.-C. Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo. Restor. Dent. Endod. 2021, 46, e17. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Nowicka, A.; Lipski, M.; Ricucci, D. Histological evaluation of hard tissue formation after direct pulp capping with a fast-setting mineral trioxide aggregate (RetroMTA) in humans. Clin. Oral Investig. 2019, 23, 4289–4299. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2000; p. 749. [Google Scholar]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Kunert, G.G.; Kunert, I.R.; Filho, L.C.D.C.; de Figueiredo, J.A.P. Permanent teeth pulpotomy survival analysis: Retrospective follow-up. J. Dent. 2015, 43, 1125–1131. [Google Scholar] [CrossRef]
- Alqaderi, H.; Lee, C.-T.; Borzangy, S.; Pagonis, T.C. Coronal pulpotomy for cariously exposed permanent posterior teeth with closed apices: A systematic review and meta-analysis. J. Dent. 2016, 44, 1–7. [Google Scholar] [CrossRef]
- Taha, N.A.; Ahmad, M.B.; Ghanim, A. Assessment of Mineral Trioxide Aggregate pulpotomy in mature permanent teeth with carious exposures. Int. Endod. J. 2017, 50, 117–125. [Google Scholar] [CrossRef]
- Scarfe, W.C.; Levin, M.D.; Gane, D.; Farman, A.G. Use of Cone Beam Computed Tomography in Endodontics. Int. J. Dent. 2009, 2009, 634567. [Google Scholar] [CrossRef]
- Kapshe, N.; Pujar, M.; Jaiswal, S. Cone beam computed tomography: A review. Int. J. Oral Health Dent. 2020, 6, 71–77. [Google Scholar] [CrossRef]
- Maszybrocka, J.; Stwora, A.; Gapiński, B.; Skrabalak, G.; Karolus, M. Morphology and surface topography of Ti6Al4V lattice structure fabricated by selective laser sintering. Bull. Pol. Acad. Sci. Tech. Sci. 2017, 65, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Djomehri, S.I.; Candell, S.; Case, T.; Browning, A.; Marshall, G.W.; Yun, W.; Lau, S.H.; Webb, S.; Ho, S.P. Mineral Density Volume Gradients in Normal and Diseased Human Tissues. PLoS ONE 2015, 10, e0121611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guldberg, R.E.; Lin, A.S.; Coleman, R.; Robertson, G.; Duvall, C. Microcomputed tomography imaging of skeletal development and growth. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, A.J.; Grine, F.E. High-resolution measurement of Neandertal tooth enamel thickness by micro-focal computed tomography. S. Afr. J. Sci. 2005, 101, 219–220. [Google Scholar]
- Olejniczak, A.; Grine, F.E. Assessment of the accuracy of dental enamel thickness measurements using microfocal X-ray computed tomography. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288, 263–275. [Google Scholar] [CrossRef]
- Butz, F.; Ogawa, T.; Chang, T.-L.; Nishimura, I. Three-dimensional bone-implant integration profiling using micro-computed tomography. Int. J. Oral Maxillofac. Implant. 2006, 21, 687–695. [Google Scholar]
- Min, Y.; Fan, B.; Cheung, G.S.; Gutmann, J.L.; Fan, M. C-shaped Canal System in Mandibular Second Molars Part III: The Morphology of the Pulp Chamber Floor. J. Endod. 2006, 32, 1155–1159. [Google Scholar] [CrossRef]
- Huang, T.T.; Jones, A.S.; He, L.H.; Darendeliler, M.A.; Swain, M. Characterisation of enamel white spot lesions using X-ray micro-tomography. J. Dent. 2007, 35, 737–743. [Google Scholar] [CrossRef]
- Cheung, L.H.; Cheung, G.S. Evaluation of a Rotary Instrumentation Method for C-shaped Canals with Micro-computed Tomography. J. Endod. 2008, 34, 1233–1238. [Google Scholar] [CrossRef]
- Morinaga, K.; Kido, H.; Sato, A.; Watazu, A.; Matsuura, M. Chronological Changes in the Ultrastructure of Titanium-Bone Interfaces: Analysis by Light Microscopy, Transmission Electron Microscopy, and Micro-Computed Tomography. Clin. Implant Dent. Relat. Res. 2008, 11, 59–68. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2012, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Elkhadem, A.; Mickan, S.; Richards, D. Adverse events of surgical extrusion in treatment for crown-root and cervical root fractures: A systematic review of case series/reports. Dent. Traumatol. 2013, 30, 1–14. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sackett, D.L.; Cook, D.J. Users’ guides to the medical literature: II. how to use an article about therapy or prevention A. Are the results of the study valid? Evidence-Based Medicine Working Group. J. Assoc. Am. Med. Coll. 1993, 270, 2598–2601. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sackett, D.L.; Cook, D.J. Users’ guides to the medical literature: II. How to use an article about therapy or prevention: B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA J. Assoc. Am. Med. Coll. 1994, 271, 59–63. [Google Scholar] [CrossRef]
- Bui, A.H.; Pham, K.V. Evaluation of Reparative Dentine Bridge Formation after Direct Pulp Capping with Biodentine. J. Int. Soc. Prev. Community Dent. 2021, 11, 77–82. [Google Scholar]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: A 2-year randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 4621–4634. [Google Scholar] [CrossRef]
- Muruganandhan, J.; Sujatha, G.; Poorni, S.; Srinivasan, M.R.; Boreak, N.; Al-Kahtani, A.; Mashyakhy, M.; Chohan, H.; Bhandi, S.; Thirumal Raj, A.; et al. Comparison of four dental pulp-capping agents by cone-beam computed tomography and histological techniques—A split-mouth design ex vivo study. Appl. Sci. 2021, 11, 3045. [Google Scholar] [CrossRef]
- Yaemkleebbua, K.; Osathanon, T.; Nowwarote, N.; Limjeerajarus, C.N.; Sukarawan, W. Analysis of hard tissue regeneration and Wnt signalling in dental pulp tissues after direct pulp capping with different materials. Int. Endod. J. 2019, 52, 1605–1616. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Ali, M.; Yoneda, N.; Ishimoto, T.; Nakano, T.; Hayashi, M. Novel evaluation method of dentin repair by direct pulp capping using high-resolution micro-computed tomography. Clin. Oral Investig. 2018, 22, 2879–2887. [Google Scholar] [CrossRef]
- Okamoto, M.; Ali, M.; Komichi, S.; Watanabe, M.; Huang, H.; Ito, Y.; Miura, J.; Hirose, Y.; Mizuhira, M.; Takahashi, Y.; et al. Surface Pre-Reacted Glass Filler Contributes to Tertiary Dentin Formation through a Mechanism Different Than That of Hydraulic Calcium-Silicate Cement. J. Clin. Med. 2019, 8, 1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Song, Y.-S.; Min, K.-S.; Kim, S.-H.; Koh, J.-T.; Lee, B.-N.; Chang, H.-S.; Hwang, I.-N.; Oh, W.-M.; Hwang, Y.-C. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor. Dent. Endod. 2016, 41, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, K.; Hayano, S.; Yanagita, T.; Kurosaka, H.; Kawanabe, N.; Itoh, S.; Ono, M.; Kuboki, T.; Kamioka, H.; Yamashiro, T. Topical Application of Lithium Chloride on the Pulp Induces Dentin Regeneration. PLoS ONE 2015, 10, e0121938. [Google Scholar] [CrossRef]
- Hara, M.; Horibe, K.; Mori, H.; Nakamura, H. The role of canonical Wnt signaling in dentin bridge formation. J. Oral Biosci. 2021, 63, 199–209. [Google Scholar] [CrossRef]
- Al-Hezaimi, K.; Salameh, Z.; Al-Fouzan, K.; Al Rejaie, M.; Tay, F.R. Histomorphometric and Micro-computed Tomography Analysis of Pulpal Response to Three Different Pulp Capping Materials. J. Endod. 2011, 37, 507–512. [Google Scholar] [CrossRef]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kołecki, J.; Buczkowska-Radlińska, J. Tomographic Evaluation of Reparative Dentin Formation after Direct Pulp Capping with Ca(OH)2, MTA, Biodentine, and Dentin Bonding System in Human Teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef]
- AlShwaimi, E.; Majeed, A.; Ali, A.A. Pulpal Responses to Direct Capping with Betamethasone/Gentamicin Cream and Mineral Trioxide Aggregate: Histologic and Micro-Computed Tomography Assessments. J. Endod. 2016, 42, 30–35. [Google Scholar] [CrossRef]
- Oenning, A.C.; Jacobs, R.; Pauwels, R.; Stratis, A.; Hedesiu, M.; Salmon, B. Cone-beam CT in paediatric dentistry: DIMITRA project position statement. Pediatric Radiol. 2018, 48, 308–316. [Google Scholar] [CrossRef]
- De Grauwe, A.; Ayaz, I.; Shujaat, S.; Dimitrov, S.; Gbadegbegnon, L.; Vande Vannet, B.; Jacobs, R. CBCT in orthodontics: A systematic review on justification of CBCT in a paediatric population prior to orthodontic treatment. Eur. J. Orthod. 2019, 41, 381–389. [Google Scholar] [CrossRef]
- Patel, S.; Brown, J.; Semper, M.; Abella, F.; Mannocci, F. European Society of Endodontology position statement: Use of cone beam computed tomography in Endodontics: European Society of Endodontology (ESE) developed by. Int. Endod. J. 2019, 52, 1675–1678. [Google Scholar] [CrossRef] [Green Version]
- Kaasalainen, T.; Ekholm, M.; Siiskonen, T.; Kortesniemi, M. Dental cone beam CT: An updated review. Phys. Med. 2021, 88, 193–217. [Google Scholar] [CrossRef]
- Brüllmann, D.; Schulze, R.K. Spatial resolution in CBCT machines for dental/maxillofacial applications—What do we know today? Dentomaxillofac. Radiol. 2015, 44, 20140204. [Google Scholar] [CrossRef] [Green Version]
- Mathur, V.P.; Dhillon, J.K.; Logani, A.; Kalra, G. Evaluation of indirect pulp capping using three different materials: A randomized control trial using cone-beam computed tomography. Indian J. Dent. Res. 2016, 27, 623. [Google Scholar] [CrossRef]
- Palomo, J.M.; Kau, C.H.; Palomo, L.B. Three dimensional cone beam computerized tomography. Dent. Today 2006, 25, 130. [Google Scholar]
- Shetty, D.C.; Manchanda, A.; Urs, A.B.; Sirohi, Y. A color contrast aided density imaging technique to differentiate between dental hard tissues and its relevance. Indian J. Dent. Res. 2011, 22, 266–269. [Google Scholar] [CrossRef]
- Marshall, G.W., Jr. Dentin: Microstructure and characterization. Quintessence Int. 1993, 24, 606–617. [Google Scholar]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. 2011, 1, 711–735. [Google Scholar] [CrossRef]
- Ma, S.; Imazato, S.; Chen, J.H.; Mayanagi, G.; Takahashi, N.; Ishimoto, T.; Nakano, T. Efects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent. Mater. J. 2012, 31, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Kaga, M.; Kakuda, S.; Ida, Y.; Toshima, H.; Endo, K.; Sano, H. Inhibition of enamel demineralization by buffering efect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 2014, 122, 78–83. [Google Scholar]
- Ali, M.; Okamoto, M.; Komichi, S.; Watanabe, M.; Huang, H.; Takahashi, Y.; Hayashi, M. Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/β-catenin signaling. Acta Biomater. 2019, 96, 594–604. [Google Scholar] [CrossRef]
- Bansal, K.; Neha, A.J.; Jain, A.A. Biodentine VS MTA: A comparitive analysis. Int. J. Oral Health Dent. 2020, 6, 201–208. [Google Scholar] [CrossRef]
- Peyrin, F.; Dong, P.; Pacureanu, A.; Langer, M. Micro- and nano-CT for the study of bone ultrastructure. Curr. Osteoporos. Rep. 2014, 12, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, I.A.K.; Schmidt, F.N.; Wölfel, E.M.; Plumeyer, C.; Milovanovic, P.; Gioia, R.; Tonelli, F.; Bale, H.A.; Jähn, K.; Besio, R.; et al. Severely impaired bone material quality in chihuahua zebrafish resembles classical dominant human osteogenesis imperfect. J. Bone Miner. Res. 2018, 33, 1489–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Database | Filters | Search Phrases | |
|---|---|---|---|
| Medline (PubMed) (156) | Free full text Full text Clinical Trial Journal Article Randomized Controlled Trial Meta-Analysis Review Systematic Review Last 10 years | 1 | (cone bone tomography) OR (CBCT) |
| 2 | ((Dentin) OR (Dental bridge)) OR (Dental pulp) | ||
| 3 | Formation | ||
| ALL | (cone bone computed tomography OR CBCT) AND (Dentin OR Dental bridge OR dental pulp) AND (Formation) | ||
| Web of Science (25) | Web of Science Categories: DENTISTRY ORAL SURGERY MEDICINE Document Types: ARTICLE Last 10 years | 1 | TOPIC: TS= cone bone computed tomography OR TS = CBCT |
| 2 | (TS = (dentin) OR TS = (dental bridge) OR TS = (dental pulp)) | ||
| 3 | (TS = (formation)) | ||
| ALL | TS = (cone bone computed tomography) OR TS = (CBCT) AND ((TS = (dentin) OR TS = (dental bridge) OR TS = (dental pulp)) AND TS = (formation) | ||
| Science Direct (91) Scopus (49) Wiley Online Library (271) Dentistry and Oral Sciences Source (19) | Subject areas: Medicine and Dentistry Article type: Research articles Review Last 10 years Open access Language: English | 1 2 3 ALL | cone bone computed tomography OR CBCT Dentin OR Dental bridge OR Dental pulp Formation (cone bone computed tomography OR CBCT) AND (Dentin OR Dental bridge OR dental pulp) AND (Formation) |
| Cochrane.org (8) | Subject areas: Dentistry and oral health Article type: Cochrane reviews Cochrane Central Register of Controlled Trials Jan 2012 and Jul 2021, in Cochrane Reviews, Trials | 1 2 3 ALL | cone bone computed tomography OR CBCT “Dentin” “Dentin bridge” Formation (cone bone computed tomography OR CBCT) AND (Dentin OR Dental bridge) AND (Formation) |
| Database | Filters | No. | Search Phrases |
|---|---|---|---|
| Medline (PubMed) (156) | Free full text Full text Clinical Trial Journal Article Randomized Controlled Trial Meta-Analysis Review Systematic Review Last 10 years | 1 | (micro computed tomography) OR (micro CT) |
| 2 | ((Dentin) OR (Dental bridge)) OR (Dental pulp) | ||
| 3 | Formation | ||
| ALL | (Micro computed tomography OR Micro CT) AND (Dentin OR Dental bridge) AND (Formation) | ||
| Web of Science (51) | Web of Science Categories: DENTISTRY ORAL SURGERY MEDICINE Document Types: ARTICLE Last 10 years | 1 2 3 ALL | TOPIC: (TS = (micro computed tomography) OR TS = (micro CT)) (TS = (dentin) OR TS = (dental bridge) OR TS = (dental pulp)) (TS = (formation)) (TS = (micro computed tomography) OR TS = (micro CT)) AND (TS = (dentin) OR TS = (dental bridge) OR TS = (dental pulp)) AND (TS = (formation)) |
| Science Direct (91) Scopus (10) Wiley Online Library (160) Dentistry and Oral Sciences Source (26) | Subject areas: Medicine and Dentistry Article type: Research articles Review Last 10 years Open access Language: English | 1 2 3 ALL | micro computed tomography OR micro CT Dentin OR Dental bridge OR Dental pulp Formation (Micro computed tomography OR Micro CT) AND (Dentin OR Dental bridge) AND (Formation) |
| Cochrane.org (11) | Subject areas: Dentistry and oral health Article type: Cochrane reviews Cochrane Central Register of Controlled Trials Jan 2012 and Jul 2021, in Cochrane Reviews, Trials | 1 2 3 ALL | micro computed tomography OR micro CT “Dentin” “Dentin bridge” Formation (micro computed tomography OR CBCT) AND (Dentin OR Dental bridge) AND (Formation) |
| Criteria | Included | Excluded |
|---|---|---|
| Full text | Available | Unavailable |
| Publication language | English | Other |
| Type of publication | Journal article | Books, documents |
| Type of research | Clinical trail Randomized controlled Research articles Review Meta-analysis Case Report | - |
| Subject area | Dentistry and oral surgery medicine | Other |
| Publication stage | Final, in press | Other |
| Clear Aim | Clear Protocol | Inclusion of Consecutive Patients, Animals | Collection of Data | Justification of Sample Size | Follow-Up Period Appropriate to the Aim of the Study | Endpoints Appropriate to the Aim of the Study | Blinded Analysis | Study Quality | |
|---|---|---|---|---|---|---|---|---|---|
| CBCT | |||||||||
| Bui et al., 2021 [38] | 2 | 2 | 2 | 1 | 0 | 2 | 2 | 0 | good |
| Holiel et al., 2021 [39] | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | good |
| Muruganandhan et al., 2021 [40] | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | good |
| Micro-CT | |||||||||
| Yoon et al., 2021 [14] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | good |
| Yaemkleebbua et al., 2019 [41] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | good |
| Okamoto et al., 2019 [42] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | good |
| Okamoto et al., 2018 [43] | 2 | 1 | 2 | 2 | 0 | 1 | 2 | 2 | good |
| Kim et al., 2016 [44] | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | good |
| Ishimoto et al., 2015 [45] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | good |
| Hara et al., 2021 [46] | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | good |
| Al-Hezaimi et al., 2011 [47] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | good |
| High Level of Evidence: |
|---|
| The study was judged to have a high level of evidence if it fulfilled all of the criteria below: There was a sufficiently large sample of patients and teeth to detect a treatment effect, preferably calculated by a power analysis. The study was a true prospective experiment in which investigators randomly assigned the sample of patients and teeth to one or more intervention groups and a control group. The sample was described such that the pulp status was clear. The study personnel were blinded regarding the intervention. The procedure for performing the pulp capping was described in sufficient detail regarding the size, type and site of exposure as well as materials used, to permit replication. There was a proper account of the patients and teeth that entered the trial and attributed to its conclusion. The analysis of the hard tissue formation and status of the pulp were adequate; that is, the criteria were specified in the text or with a reference. The results were well documented and presented in terms of relevant data. |
| Moderate Level of Evidence: |
| A study was judged to have a moderate level of evidence if any of the above criteria was not met. Conversely, the study was judged not to have deficits that are described for studies with a low level of evidence. |
| Low Level of Evidence: |
| A study was judged to have a low level of evidence if it met any of the following criteria: There was not a sufficiently large sample of patients and teeth. There was not a randomization process. The sample and procedure were not described in sufficient detail to permit replication. |
| Authors | Type of Research | Methods | Species | Pulp Exposure | The Material Used for the Pulp | Examined Teeth Sample Size (n) | Experiment Time |
|---|---|---|---|---|---|---|---|
| Analysis CBCT | |||||||
| Mathur et al., 2016 (India) [50] | Randomized Controlled Trial | CBCT, Clinical examination | Human (children) | IPC | CH (setting type), GIC (Type VII), MTA | Primary second molars, Permanent first molar n = 95 | 2 y |
| Bui et al., 2021 (Vietnam) [38] | Research Study | CBCT Intraoral radiograph, | Human | DPC | BD | Premolars n = 11 | 9–12 w |
| Holiel et al., 2021 (Egypt) [39] | Comparative Study | CBCT Clinical examinations, | Human | DPC | TDMH, BD, MTA | Posterior teeth n = 45 | 2 y |
| Nowicka et al., 2015 (Poland) [48] | Randomized Controlled Trial | CBCT Clinical examination, CBCT Intraoral radiograph, Histological sections | Human | DPC | CH, MTA, BD SBU | Maxillary and mandibular third molars n = 44 | 6 w |
| Muruganandhan et al., 2021 (India) [40] | Comparative Study | CBCT, Histological sections | Human | DPC | CH, MTA, BD ERRM | Premolars n = 60 | 8 w |
| Analysis micro-CT | |||||||
| Yoon et al., 2021 (Korea) [14] | Comparative Study | Micro-CT, Histological analysis, immunofluorescence staining | Sprague– Dawley Rats | DPC | ProRoot MTA, OST 100 μM + ProRoot MTA, OST 10 mM + ProRoot MTA | Maxillary molars n = 32 | 4 w |
| Yaemkleebbua et al., 2019 (Thailand) [41] | Comparative Study | Micro-CT, Histological analysis, immunofluorescence staining | Rats | DPC | CH, MTA, BD | Maxillary molars n = 32 | 4 w |
| Okamoto et al., 2019 (Japan) [42] | Research Study | Micro-CT, scanning electron microscopy (SEM), micro-X-ray fluorescence (μXRF) | Rats | DPC | S-PRG, MTA | First molars n = 32 | 4 w |
| Okamoto et al., 2018 (Japan) [43] | Comparative Study | Micro-CT, histologic analyses | Wistar Rats | DPC | ProRoot MTA, iRoot BP Plus | First molars n = 9 | 4 w |
| Kim et al., 2016 (Korea) [44] | Research Study | Micro-CT, histologic analyses immunohistochemical analysis using dentin sialoprotein (DSP) | Sprague- Dawley Rats | DPC | ProRoot MTA, BD, BA | First molars n = 10 | 4 w |
| Ishimoto et al., 2015 (Japan) [45] | Research Study | Micro-CT, histologic analyses, In situ hybridization, Cell culture and real-time RT-PCR analysis | Sprague- Dawley Rats | TPA | LiCl | First premolars n = 45 | 6 w |
| Hara et al., 2021 (Japan) [46] | Research Study | Micro-CT, Histological analysis, Immunohistochemical staining, Double fluorescent staining | Old male mice | DPC | MTA | First molars | 28 d |
| Al-Hezaimi et al., 2011 (Saudi Arabia) [47] | Comparative Study | Micro-CT Histomorphometric analysis | Old baboons | DPC | CH, (PC) | Premolars n = 30 | 4 m |
| Alshwaimi et al., 2016 (Saudi Arabia) [49] | Randomized Controlled Trial | Micro-CT scanning and histologic analyses | Human | DPC | BG MTA | First molars n = 18 | 8 w |
| Analysis CBCT | |||||||
|---|---|---|---|---|---|---|---|
| Source | Apparatus | Apparatus Parameters | |||||
| Voltage (kV) | Current (µA) | Field of View (cm) | Voxel Size (mm3) | Slice Dimensions (Pixels) | Thickness of the Cut Layer (mm) | ||
| Bui et al., 2021 (Vietnam) [38] | ProMax® 3DX-ray units Planmeca, Helsinki, Finland | 90 | 140 | 5 × 5 | 0.10 | 1024 × 1024 | |
| Holiel et al., 2021 (Egypt) [39] | CBCT imaging D-CBCT i-CAT FLEX, KaVo, Germany | 90 | 90 | 5 × 5 | 0.12 | - | 20 |
| Mathur et al., 2016 (India) [50] | CBCT i-CAT; Imaging Sciences International, Hatfield, PA, USA | - | - | 13 × 16 | 0.5 | - | 12 |
| Muruganandhan et al., 2021 (India) [40] | No data | - | - | - | - | - | - |
| Nowicka et al., 2015 (Poland) [48] | Cranex 3D, No. SE 1100155, Software Version Scanora 5.1.0.9; Soredex, Tuusula, Finland | Exposure parameters were standardized for each patient | |||||
| Analysis micro-CT | |||||||
| Source | Apparatus | Apparatus parameters | |||||
| Voltage (kV) | Current (mA) | Rotation (°) | Voxel size (mm3) | Filter (mm) | Exposure time (min) | ||
| Yoon et al., 2021 (Korea) [14] | Micro-CT system SkyScan 1172, Brucker, Aartselaar, Belgium | 70 | 141 | 180 | - | 0.5 Aluminium | - |
| Yaemkleebbua et al., 2019 (Thailand) [41] | Micro-CT 35, Scanco Medical, Brüttisellen, Switzerland | 70 | 114 | - | 0.10 | - | - |
| Okamoto et al., 2019 (Japan) [42] | Micro-CT scanner R-mCT2 Rigaku, Tokyo, Japan | 90 | 160 | - | 0.10 | - | 3 |
| Okamoto et al., 2018 (Japan) [43] | Micro-CT scanner SMX100CT Shimadzu, Kyoto, Japan | 50 | 150 | - | 0.71 | - | 20 |
| Kim et al., 2016 (Korea) [44] | Micro-CT system SkyScan 1172, Brucker, Aartselaar, Belgium | 70 | 141 | 180 | - | 0.5 Aluminium | - |
| Ishimoto et al., 2015 (Japan) [45] | Micro-CT system InspeXio SMX-90CT Shimadzu, Kyoto, Japan | 90 | 119 | - | 0.17 | - | - |
| Hara et al., 2021 (Japan) [46] | No data | - | - | - | - | - | - |
| Al-Hezaimi et al., 2011 (Saudi Arabia) [47] | Micro-CT system SkyScan1172; Brucker, Kontich, Belgium | 110 | 96 | - | 0.37 | 1 Aluminium | - |
| Alshwaimi et al., 2016 (Saudi Arabia) [49] | Micro-CT system SkyScan1172; Brucker, Kontich, Belgium | 70 | 139 | 360 | 0.89 | 0.5 Aluminium | - |
| Tertiary Dentin | |||||
|---|---|---|---|---|---|
| Source | Quantitative Assessment | Qualitative Assessment | Conclusion | ||
| Radiodensity (Mean Percentage Gain of Radiodensity at Site as Compared to Healthy Dentin %) | Thickness (mm) | Volume (mm3) | |||
| Analysis CBCT | |||||
| Bui et al., 2021 [38] | - | - | - | BD Extracted teeth 1.1009 BD Real patient teeth 0.6186 | BD could induce the formation of reparative dentin in direct pulp capping. The CBCT scan was the reliable modality for evaluation of dentin bridge formation. |
| Mathur et al., 2016 [50] | - | CH 78 | CH 0.490 | - | All three dental materials tested, i.e., CH(setting), GIC Type VII, and MTA, were found to be equally suitable for VPT, following clinical and radiographic criteria. The success rate with CH (setting) was found to be 93.5%; with GIC (Type VII), it was 97%, and with MTA, it was 100%, respectively. |
| GIC VII 79 | GIC VII 0.510 | ||||
| MTA 84 | MTA 0.540 | ||||
| Holiel et al., 2021 [39] | - | TDMH 98 | TDMH 0.235 | - | TDMH has a greater potential to induce dentin bridge formation than BD and MTA under standardized conditions, suggesting its suitability as a direct pulp capping material in future clinical applications. |
| BD 81 | BD 0.147 | ||||
| MTA 78 | MTA 0.930 | ||||
| Murugan and han et al., 2021 [40] | Radiopaque structure between the pulp-capping agent and the pulpal space indicates the formation of a dentinal bridge. | - | - | - | MTA exhibited superior performance in dentinal bridge formation when compared to the CH, BD and EERM. |
| CH 9 islands of calcified material 6 complete dentinal bridge | |||||
| MTA 6 islands of calcified material 9 complete dentinal bridge | |||||
| BD 8 islands of calcified material 7 complete dentinal bridge | |||||
| ERRM 6 islands of calcified material 9 complete dentinal bridge | |||||
| Nowicka et al., 2015 [48] | - | - | CH 0.182 ProRoot MTA 0.230 | CH 0.30 n = 7 Pro Root MTA 0.45 n = 8 | In conclusion, the volume of formed reparative dentin bridges depends on the material used for direct pulp capping. BD and MTA induced the formation of bridges with a significantly higher average volume compared with SBU. |
| BD 0.212 | BD 0.47 n = 8 | ||||
| SBU 0.030 | SBU 0.07 n = 2 | ||||
| Analysis micro-CT | |||||
| Yoon et al., 2021 [14] | OST + ProRoot MTA showed more mineralized bridge than the ProRoot MTA. | - | - | - | OST can be a supplementary pulp-capping material when used with MTA to make a synergistic effect in hard tissue formation. |
| Yaemkleebbua et al., 2019 [41] | The MTA and Biodentine exhibited significantly higher BV/TV ratio compared with the CH. | - | - | - | All test materials promoted dentine bridge formation. |
| Okamoto et al., 2019 [42] | - | - | - | S-PRG and MTA ˜0.05 | S-PRG cement is a bioactive material that may be useful in direct pulp capping. |
| Okamoto et al., 2018 [43] | - | - | - | Pro Root MTA ˜0.045 iRoot BP Plus 0.02 | Micro-CT analysis was confirmed as an accurate, objective, and inclusive approach for evaluating quality and quantity of dentin repair. |
| Kim et al., 2016 [44] | ProRoot MTA homogeneous hard tissue and complete hard tissue bridge. BD calcification tissue exhibiting different degrees of saturation. BA hard tissue, unevenly distributed observed at the pulpal floor and lateral wall of the pulp chamber. | - | - | - | BD and BA could be considered as alternatives to ProRoot MTA. |
| Ischimoto et al., 2015 [45] | The dentin bridges were uniform in radiopacity. | - | - | - | LiCl has great potential as a bioactive mediating organ-specific trans differentiation. |
| Hara et al., 2021 [46] | Radiopaque regions covered the exposed pulp surface. | - | - | - | Material that activates canonical Wnt (regulator of the Dentin sialophosphoprotein expression) signalling promotes healing of the pulp–dentin complex. |
| Al-Hezaimi et al., 2011 [47] | - | - | CH 0.22 | - | Tertiary dentin varies in thickness but not in quality, depending on the pulp material used. |
| ProRoot 0.43 | |||||
| Alshwaimi et al., 2016 [49] | - | - | PC 0.4 | - | Confirmed the efficacy of MTA for direct pulp capping. |
| BG 0.22 | |||||
| MTA 0.78 | |||||
| Source | Nowicka et al., 2015 [48] | Okamoto et al., 2019 [43] | Okamoto et al., 2018 [42] |
|---|---|---|---|
| Imaging technique | CBCT | Micro-CT | Micro-CT |
| Species | Human | Adult rats | Adult rats |
| Young Dentin | 1600 | ||
| Mature Dentin | 2300–2500 | ||
| Tertiary dentin | 1000–1300 | 1400–1500 | 1100–1400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palczewska-Komsa, M.P.; Gapiński, B.; Nowicka, A. The Influence of New Bioactive Materials on Pulp–Dentin Complex Regeneration in the Assessment of Cone Bone Computed Tomography (CBCT) and Computed Micro-Tomography (Micro-CT) from a Present and Future Perspective—A Systematic Review. J. Clin. Med. 2022, 11, 3091. https://doi.org/10.3390/jcm11113091
Palczewska-Komsa MP, Gapiński B, Nowicka A. The Influence of New Bioactive Materials on Pulp–Dentin Complex Regeneration in the Assessment of Cone Bone Computed Tomography (CBCT) and Computed Micro-Tomography (Micro-CT) from a Present and Future Perspective—A Systematic Review. Journal of Clinical Medicine. 2022; 11(11):3091. https://doi.org/10.3390/jcm11113091
Chicago/Turabian StylePalczewska-Komsa, Mirona Paula, Bartosz Gapiński, and Alicja Nowicka. 2022. "The Influence of New Bioactive Materials on Pulp–Dentin Complex Regeneration in the Assessment of Cone Bone Computed Tomography (CBCT) and Computed Micro-Tomography (Micro-CT) from a Present and Future Perspective—A Systematic Review" Journal of Clinical Medicine 11, no. 11: 3091. https://doi.org/10.3390/jcm11113091
APA StylePalczewska-Komsa, M. P., Gapiński, B., & Nowicka, A. (2022). The Influence of New Bioactive Materials on Pulp–Dentin Complex Regeneration in the Assessment of Cone Bone Computed Tomography (CBCT) and Computed Micro-Tomography (Micro-CT) from a Present and Future Perspective—A Systematic Review. Journal of Clinical Medicine, 11(11), 3091. https://doi.org/10.3390/jcm11113091






