A New Approach for Evaluation of Cardiovascular Fitness and Cardiac Responses to Maximal Exercise Test in Master Runners: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Approach to the Problem
2.2. Participants
2.3. Material and Testing
2.3.1. Anthropometric Variables
2.3.2. Cardiorespiratory Fitness
2.3.3. HR and HRV Measures
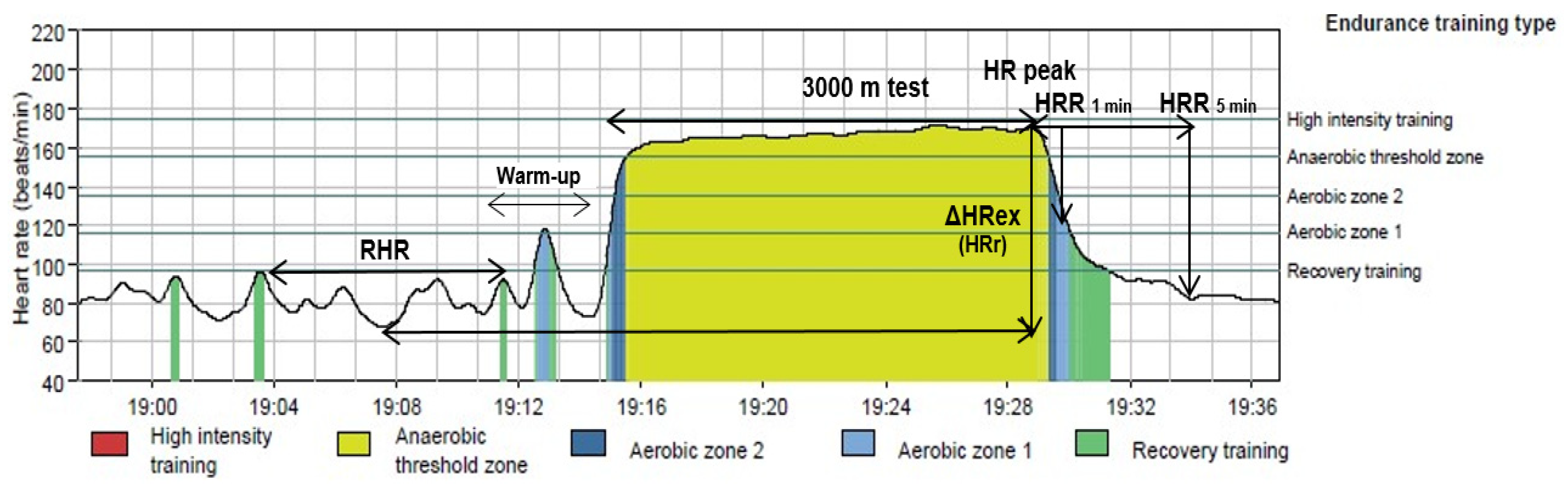
2.3.4. Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Cardiac Autonomic Function at Rest
4.2. Cardiac Autonomic Function during Maximal Exercise
4.3. Cardiac Autonomic Function at Recovery after Exercise
4.4. Heart Rate Variability
4.5. Relationship between the Different HR Behaviors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Walker, A.; Maltby, T. Active ageing: A strategic policy solution to demographic ageing in the European Union. Int. J. Soc. Welf. 2012, 21, S117–S130. [Google Scholar] [CrossRef]
- Harridge, S.D.R.; Lazarus, N.R. Physical activity, aging, and physiological function. Physiology 2017, 32, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geard, D.; Reaburn, P.; Rebar, A.; Dionigi, R. Masters athletes: Exemplars of successful aging? J. Aging Phys. Act. 2017, 25, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.A.; Wiswell, R.A.; Marcell, T.J. Exercise and the Master Athlete—A Model of Successful Aging? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M1009–M1011. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.; Nosaka, K.; Brisswalter, J. L’athlète master d’endurance, un modèle de vieillissement réussi. Sci. Sports 2012, 27, 63–76. [Google Scholar] [CrossRef]
- Churchill, T.W.; Baggish, A.L. Cardiovascular Care of Masters Athletes. J. Cardiovasc. Transl. Res. 2020, 13, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lepers, R.; Stapley, P.J. Master Athletes Are Extending the Limits of Human Endurance. Front. Physiol. 2016, 7, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganse, B.; Ganse, U.; Dahl, J.; Degens, H. Linear Decrease in Athletic Performance during the Human Life Span. Front. Physiol. 2018, 9, 1100. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-Time Running Reduces All-Cause and Cardiovascular Mortality Risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Fien, S.; Climstein, M.; Quilter, C.; Buckley, G.; Henwood, T.; Grigg, J.; Keogh, J.W.L. Anthropometric, physical function and general health markers of Masters athletes: A cross-sectional study. PeerJ 2017, 5, e3768. [Google Scholar] [CrossRef] [Green Version]
- Deus, L.A.; Sousa, C.V.; Rosa, T.S.; Filho, J.M.S.; Santos, P.A.; Barbosa, L.D.; Silva Aguiar, S.; Souza, L.H.R.; Simões, H.G. Heart rate variability in middle-aged sprint and endurance athletes. Physiol. Behav. 2019, 205, 39–43. [Google Scholar] [CrossRef]
- Lakatta, E.G. Changes in cardiovascular function with aging. Eur. Heart J. 1990, 11, 22–29. [Google Scholar] [CrossRef]
- Fluckiger, L.; Boivin, J.-M.; Quilliot, D.; Jeandel, C.; Zannad, F. Differential Effects of Aging on Heart Rate Variability and Blood Pressure Variability. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1999, 54, B219–B224. [Google Scholar] [CrossRef] [Green Version]
- Buchheit, M.; Chivot, A.; Parouty, J.; Mercier, D.; Al Haddad, H.; Laursen, P.B.; Ahmaidi, S. Monitoring endurance running performance using cardiac parasympathetic function. Eur. J. Appl. Physiol. 2010, 108, 1153–1167. [Google Scholar] [CrossRef]
- van de Vegte, Y.J.; Tegegne, B.S.; Verweij, N.; Snieder, H.; van der Harst, P. Genetics and the heart rate response to exercise. Cell. Mol. Life Sci. 2019, 76, 2391–2409. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, A.; Cerasola, D.; Zangla, D.; Proia, P.; Russo, G.; Lo Presti, R.; Traina, M. Heart rate recovery after exercise and maximal oxygen uptake in sedentary patients with type 2 diabetes. J. Biol. Res. 2015, 88, 7–8. [Google Scholar]
- Dimkpa, U. Post-exercise heart rate recovery: An index of cardiovascular fitness. J. Exerc. Physiol. Online 2009, 12, 10–22. [Google Scholar]
- Yataco, A.R.; Fleisher, L.A.; Katzel, L.I. Heart Rate Variability and Cardiovascular Fitness in Senior Athletes. Am. J. Cardiol. 1997, 80, 1389–1391. [Google Scholar] [CrossRef]
- Brown, S.J.; Brown, J.A. Resting and postexercise cardiac autonomic control in trained masters athletes. J. Physiol. Sci. 2007, 57, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, S.A.; Marcell, T.J.; Victoria Jaque, S.; Wiswell, R.A. A longitudinal assessment of change in VO2max and maximal heart rate in master athletes. Med. Sci. Sports Exerc. 2001, 33, 1744–1750. [Google Scholar] [CrossRef]
- Wiswell, R.A.; Hawkins, S.A.; Jaque, S.V.; Hyslop, D.; Constantino, N.; Tarpenning, K.; Marcell, T.; Schroeder, E.T. Relationship Between Physiological Loss, Performance Decrement, and Age in Master Athletes. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M618–M626. [Google Scholar] [CrossRef] [Green Version]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart Rate Recovery after Submaximal Exercise Testing as a Predictor of Mortality in a Cardiovascularly Healthy Cohort. Ann. Intern. Med. 2000, 132, 552. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- WHO. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–146. [Google Scholar]
- Esfarjani, F.; Laursen, P.B. Manipulating high-intensity interval training: Effects on, the lactate threshold and 3000m running performance in moderately trained males. J. Sci. Med. Sport 2007, 10, 27–35. [Google Scholar] [CrossRef]
- Nuuttila, O.-P.; Nikander, A.; Polomoshnov, D.; Laukkanen, J.; Häkkinen, K. Effects of HRV-Guided vs. Predetermined Block Training on Performance, HRV and Serum Hormones. Int. J. Sports Med. 2017, 38, 909–920. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Parak, J.; Tarniceriu, A.; Renevey, P.; Bertschi, M.; Delgado-Gonzalo, R.; Korhonen, I. Evaluation of the beat-to-beat detection accuracy of PulseOn wearable optical heart rate monitor. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 8099–8102. [Google Scholar]
- Parak, J.; Korhonen, I. Accuracy of Firstbeat Bodyguard 2 Beat-to-Beat Heart Rate Monitor; White Paper; Firstbeat Technol Ltd.: Jyväskylä, Finland, 2013; pp. 1–3. [Google Scholar]
- Latorre-Román, P.A.; Floody, P.D.; Martínez-Redondo, M.; Salas-Sánchez, J.; Consuegra-González, P.J.; Aragón-Vela, J.; Robles-Fuentes, A.; Sarabia-Cachadiña, E.; Párraga-Montilla, J.A. Comprehensive cardiac evaluation to maximal exercise in a contemporary population of prepubertal children. Pediatr. Res. 2021, 1–10. [Google Scholar] [CrossRef]
- Bobkowski, W.; Stefaniak, M.E.; Krauze, T.; Gendera, K.; Wykretowicz, A.; Piskorski, J.; Guzik, P. Measures of Heart Rate Variability in 24-h ECGs Depend on Age but Not Gender of Healthy Children. Front. Physiol. 2017, 8, 311. [Google Scholar] [CrossRef] [Green Version]
- Cachadiña, E.S.; De la Cruz Torres, B.; Sixto, A.S.; Martín, P.F.; Berral de la Rosa, F.J.; Orellana, J.N. Heart rate variability is lower in patients with intermittent claudication: A preliminary study. Arch. Med. Deporte Rev. Fed. Española Med. Deporte Confed. Iberoam. Med. Deporte 2018, 35, 218–221. [Google Scholar]
- European Society of Cardiology Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [CrossRef] [Green Version]
- von Scheidt, F.; Meier, S.; Krämer, J.; Apitz, A.; Siaplaouras, J.; Bride, P.; Kaestner, M.; Apitz, C. Heart Rate Response During Treadmill Exercise Test in Children and Adolescents with Congenital Heart Disease. Front. Pediatr. 2019, 7, 65. [Google Scholar] [CrossRef]
- Peçanha, T.; Silva-Júnior, N.D.; Forjaz, C.L. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014, 34, 327–339. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-Rate Profile during Exercise as a Predictor of Sudden Death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Lauer, M.S.; Earnest, C.P.; Church, T.S.; Kampert, J.B.; Gibbons, L.W.; Blair, S.N. Heart Rate Recovery Following Maximal Exercise Testing as a Predictor of Cardiovascular Disease and All-Cause Mortality in Men With Diabetes. Diabetes Care 2003, 26, 2052–2057. [Google Scholar] [CrossRef] [Green Version]
- Young, F.L.S.; Leicht, A.S. Short-term stability of resting heart rate variability: Influence of position and gender. Appl. Physiol. Nutr. Metab. 2011, 36, 210–218. [Google Scholar] [CrossRef]
- Sinnreich, R.; Kark, J.D.; Friedlander, Y.; Sapoznikov, D.; Luria, M.H. Five minute recordings of heart rate variability for population studies: Repeatability and age-sex characteristics. Heart 1998, 80, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Boyett, M.R.; D’Souza, A.; Zhang, H.; Morris, G.M.; Dobrzynski, H.; Monfredi, O. Viewpoint: Is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J. Appl. Physiol. 2013, 114, 1351–1355. [Google Scholar] [CrossRef]
- D’Souza, A.; Sharma, S.; Boyett, M.R. CrossTalk opposing view: Bradycardia in the trained athlete is attributable to a downregulation of a pacemaker channel in the sinus node. J. Physiol. 2015, 593, 1749–1751. [Google Scholar] [CrossRef]
- Jensen-Urstad, K.; Saltin, B.; Ericson, M.; Storck, N.; Jensen-Urstad, M. Pronounced resting bradycardia in male elite runners is associated with high heart rate variability. Scand. J. Med. Sci. Sports 2007, 7, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Wadiat, F.; Aman, R.; Bashir, M. Impact of high-intensity aerobic exercises upon resting heart rate & body mass index of the girl- athletes (case study of gomal university, Dera Ismail Khan). Spark 2020, 5, 149–162. [Google Scholar]
- Goorakani, Y.; Sedigh Rahimabadi, M.; Dehghan, A.; Kazemi, M.; Chijan, M.R.; Bijani, M.; Shahraki, H.R.; Davoodi, A.; Farjam, M.; Homayounfar, R. Correlation of resting heart rate with anthropometric factors and serum biomarkers in a population-based study: Fasa PERSIAN cohort study. BMC Cardiovasc. Disord. 2020, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, A.; Liu, D.M.; Kaholokula, J.K. Relationship of resting heart rate and physical activity with insulin sensitivity in a population-based survey. J. Diabetes Metab. Disord. 2015, 14, 41. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, A.; Bianco, A.; Paoli, A.; Cerasola, D.; Alagna, S.; Messina, G.; Zangla, D.; Traina, M. Resting sympatho-vagal balance is related to 10 km running performance in master endurance athletes. Eur. J. Transl. Myol. 2018, 28, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.; Park, S.; Kim, Y.-J.; Min, S.-Y.; Kim, Y.R.; Nam, G.-B.; Choi, K.-J.; Kim, Y.-H. The exercise heart rate profile in master athletes compared to healthy controls. Clin. Physiol. Funct. Imaging 2016, 36, 286–292. [Google Scholar] [CrossRef]
- Wilhelm, M.; Roten, L.; Tanner, H.; Wilhelm, I.; Schmid, J.P.; Saner, H. Gender differences of atrial and ventricular remodeling and autonomic tone in nonelite athletes. Am. J. Cardiol. 2011, 108, 1489–1495. [Google Scholar] [CrossRef]
- Nes, B.M.; Janszky, I.; Wisløff, U.; Støylen, A.; Karlsen, T. Age-predicted maximal heart rate in healthy subjects: The HUNT Fitness Study. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic incompetence: Causes, consequences, and management. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J.V.; Dewey, F.E.; Hadley, D.M.; Myers, J.; Froelicher, V.F. Autonomic Nervous System Interaction With the Cardiovascular System During Exercise. Prog. Cardiovasc. Dis. 2006, 48, 342–362. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac Parasympathetic Reactivation Following Exercise: Implications for Training Prescription. Sport. Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Mendoza, A. Dissociation of heart rate variability and heart rate recovery in well-trained athletes. Eur. J. Appl. Physiol. 2012, 112, 2757–2766. [Google Scholar] [CrossRef]
- Bentley, R.F.; Vecchiarelli, E.; Banks, L.; Gonçalves, P.E.O.; Thomas, S.G.; Goodman, J.M. Heart rate variability and recovery following maximal exercise in endurance athletes and physically active individuals. Appl. Physiol. Nutr. Metab. 2020, 45, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Shetler, K.; Marcus, R.; Froelicher, V.F.; Vora, S.; Kalisetti, D.; Prakash, M.; Do, D.; Myers, J. Heart rate recovery: Validation and methodologic issues. J. Am. Coll. Cardiol. 2001, 38, 1980–1987. [Google Scholar] [CrossRef] [Green Version]
- Suzic Lazic, J.; Dekleva, M.; Soldatovic, I.; Leischik, R.; Suzic, S.; Radovanovic, D.; Djuric, B.; Nesic, D.; Lazic, M.; Mazic, S. Heart rate recovery in elite athletes: The impact of age and exercise capacity. Clin. Physiol. Funct. Imaging 2017, 37, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.B.; Banister, E.W.; Blaber, A.P. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003, 33, 33–46. [Google Scholar] [CrossRef]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef]
- Emery, C.F.; Stoney, C.M.; Thayer, J.F.; Williams, D.; Bodine, A. Sex and family history of cardiovascular disease influence heart rate variability during stress among healthy adults. J. Psychosom. Res. 2018, 110, 54–60. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Würth, L.; Schneider, E.; Thayer, J.F.; Ditzen, B.; Jarczok, M.N. A Systematic Review and Meta-Analysis of Within-Person Changes in Cardiac Vagal Activity across the Menstrual Cycle: Implications for Female Health and Future Studies. J. Clin. Med. 2019, 8, 1946. [Google Scholar] [CrossRef] [Green Version]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-Term Heart Rate Variability—Influence of Gender and Age in Healthy Subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchheit, M.; Gindre, C. Cardiac parasympathetic regulation: Respective associations with cardiorespiratory fitness and training load. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H451–H458. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Zmora, R.; Duval, S.; Chow, L.S.; Lloyd-Jones, D.M.; Schreiner, P.J. Cardiorespiratory Fitness, Adiposity, and Heart Rate Variability: The Coronary Artery Risk Development in Young Adults Study. Med. Sci. Sports Exerc. 2019, 51, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Jae, S.Y.; Kurl, S.; Laukkanen, J.A.; Zaccardi, F.; Choi, Y.-H.; Fernhall, B.; Carnethon, M.; Franklin, B.A. Exercise Heart Rate Reserve and Recovery as Predictors of Incident Type 2 Diabetes. Am. J. Med. 2016, 129, 536.e7–536.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.; Tan, S.Y.; Abella, J.; Aleti, V.; Froelicher, V.F. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur. J. Prev. Cardiol. 2007, 14, 215–221. [Google Scholar] [CrossRef]
- Georgoulias, P. Abnormal heart rate recovery immediately after treadmill testing: Correlation with clinical, exercise testing, and myocardial perfusion parameters. J. Nucl. Cardiol. 2003, 10, 498–505. [Google Scholar] [CrossRef]
- Raffin, J.; Barthélémy, J.-C.; Dupré, C.; Pichot, V.; Berger, M.; Féasson, L.; Busso, T.; Da Costa, A.; Colvez, A.; Montuy-Coquard, C.; et al. Exercise Frequency Determines Heart Rate Variability Gains in Older People: A Meta-Analysis and Meta-Regression. Sports Med. 2019, 49, 719–729. [Google Scholar] [CrossRef]
- Herzig, D.; Asatryan, B.; Brugger, N.; Eser, P.; Wilhelm, M. The Association between Endurance Training and Heart Rate Variability: The Confounding Role of Heart Rate. Front. Physiol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Poirier, P. Exercise, Heart Rate Variability, and Longevity. Circulation 2014, 129, 2085–2087. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T. Similitude in the cardiovascular system of mammals. J. Exp. Biol. 2001, 204, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vicente, A.; Hernando, D.; Santos-Lozano, A.; Rodríguez-Romo, G.; Vicente-Rodríguez, G.; Pueyo, E.; Bailón, R.; Garatachea, N. Heart Rate Variability and Exceptional Longevity. Front. Physiol. 2020, 11, 1164. [Google Scholar] [CrossRef] [PubMed]

| All (n = 50) Mean (SD) | Men (n = 29) Mean (SD) | Women (n = 21) Mean (SD) | p-Values | |
|---|---|---|---|---|
| Age (years) | 43.28 (5.25) | 43.86 (5.35) | 42.47 (5.26) | 0.367 |
| Body mass (kg) | 65.33 (9.55) | 71.01 (7.29) | 57.49 (6.15) | <0.001 |
| Body height (cm) | 168.36 (8.25) | 173.1(6.85) | 161.8 (4.83) | <0.001 |
| BMI (kg/m2) | 22.66 (2.16) | 23.63 (1.95) | 21.31 (1.71) | <0.001 |
| Body fat (%) | 14.62 (6.96) | 9.21 (2.51) | 21.84 (3.50) | <0.001 |
| WC (cm) | 79.98 (6.79) | 83.86 (5.28) | 74.61 (4.68) | <0.001 |
| WtHR | 0.47 (0.03) | 0.48 (0.04) | 0.46 (0.02) | 0.015 |
| 3-km race (s) | 732.26 (97.71) | 661.89 (44.12) | 829.42 (62.93) | <0.001 |
| RPE (6–20) | 14.60 (1.46) | 14.96 (1.31) | 14.10 (1.55) | 0.043 |
| All (n = 50) Mean (SD) | Men (n = 29) Mean (SD) | Women (n = 21) Mean (SD) | p-Values | |
|---|---|---|---|---|
| RHR (bpm) | 59.50 (12.21) | 57.32 (13.06) | 62.55 (10.49) | 0.127 |
| Average HR exercise (bpm) | 169.77 (9.27) | 169.60 (9.84) | 170.00 (8.69) | 0.738 |
| HR-peak (bpm) | 178.10 (10.62) | 177.78 (11.05) | 178.52 (10.26) | 0.968 |
| HRr (bmp) | 117.68 (15.64) | 118.57 (17.47) | 116.45 (12.99) | 0.594 |
| Chronotropic index | 1.01 (0.10) | 1.00 (0.12) | 1.01 (0.07) | 0.908 |
| HRR1min (bpm) | 48.29 (10.51) | 49.00 (10.13) | 47.42 (11.14) | 0.319 |
| HRR5min (bpm) | 83.31 (8.57) | 85.46 (7.61) | 80.66 (9.13) | 0.097 |
| RMSSD (ms), at rest | 51.44 (25.87) | 57.59 (29.35) | 43.15 (17.76) | 0.085 |
| SDNN (ms), at rest | 100.25 (35.87) | 111.59 (37.45) | 84.95 (27.70) | 0.012 |
| HF (ms2), at rest | 4946.74 (3512.39) | 5938.70 (4059.21) | 3607.59 (2008.59) | 0.156 |
| LF (ms2), at rest | 2555.31 (2150.96) | 3056.90 (2451.63) | 1878.15 (1461.59) | 0.045 |
| LF/HF, at rest | 2.63 (1.7) | 2.77 (1.91) | 2.44 (1.43) | 0.747 |
| Variables | r | p-Values |
|---|---|---|
| RHR vs. BMI | 0.352 | 0.016 |
| HR-peak vs. age | −0.369 | 0.009 |
| HR-peak vs. HRR5min | 0.476 | 0.001 |
| HRR5min vs. HRr | 0.542 | <0.001 |
| HRR5min vs. CI | 0.495 | 0.001 |
| RMSSD vs. RHR | −0.741 | <0.001 |
| SDNN vs. RHR | −0.646 | <0.001 |
| HF vs. RHR | −0.566 | <0.001 |
| LF vs. RHR | −0.447 | 0.002 |
| LF/HF vs. RHR | 0.486 | 0.001 |
| HRr vs. RMSSD | 0.505 | 0.001 |
| HRr vs. SDNN | 0.484 | 0.001 |
| HRr vs. HF | 0.374 | 0.015 |
| HRr vs. LF | 0.455 | 0.002 |
| 3 km time vs. BMI | 0.314 | 0.028 |
| 3 km time vs. WtHR | 0.288 | 0.045 |
| 3 km time vs. body fat | 0.374 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latorre-Román, P.Á.; García-Pinillos, F.; Salas Sánchez, J.; Jiménez, M.M.; Serrano Huete, V.; Martínez Redondo, M.; Vela, J.A.; Párraga-Montilla, J.A. A New Approach for Evaluation of Cardiovascular Fitness and Cardiac Responses to Maximal Exercise Test in Master Runners: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 1648. https://doi.org/10.3390/jcm11061648
Latorre-Román PÁ, García-Pinillos F, Salas Sánchez J, Jiménez MM, Serrano Huete V, Martínez Redondo M, Vela JA, Párraga-Montilla JA. A New Approach for Evaluation of Cardiovascular Fitness and Cardiac Responses to Maximal Exercise Test in Master Runners: A Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(6):1648. https://doi.org/10.3390/jcm11061648
Chicago/Turabian StyleLatorre-Román, Pedro Á., Felipe García-Pinillos, Jesús Salas Sánchez, Marcos Muñoz Jiménez, Víctor Serrano Huete, Melchor Martínez Redondo, Jerónimo Aragón Vela, and Juan A. Párraga-Montilla. 2022. "A New Approach for Evaluation of Cardiovascular Fitness and Cardiac Responses to Maximal Exercise Test in Master Runners: A Cross-Sectional Study" Journal of Clinical Medicine 11, no. 6: 1648. https://doi.org/10.3390/jcm11061648
APA StyleLatorre-Román, P. Á., García-Pinillos, F., Salas Sánchez, J., Jiménez, M. M., Serrano Huete, V., Martínez Redondo, M., Vela, J. A., & Párraga-Montilla, J. A. (2022). A New Approach for Evaluation of Cardiovascular Fitness and Cardiac Responses to Maximal Exercise Test in Master Runners: A Cross-Sectional Study. Journal of Clinical Medicine, 11(6), 1648. https://doi.org/10.3390/jcm11061648








