Changes in Fat Oxidation and Body Composition after Combined Exercise Intervention in Sedentary Obese Chinese Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Anthropometrical and Body Composition Assessments
2.4. Lipid Profile (LP)
2.5. Exercise Test
2.5.1. Maximal Graded Exercise Test (GXT)
2.5.2. Rowing Test
2.6. CAEH Intervention
2.7. Dietary Recommendation
2.8. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Effect of Training on the Body Compositions and Lipid Profiels
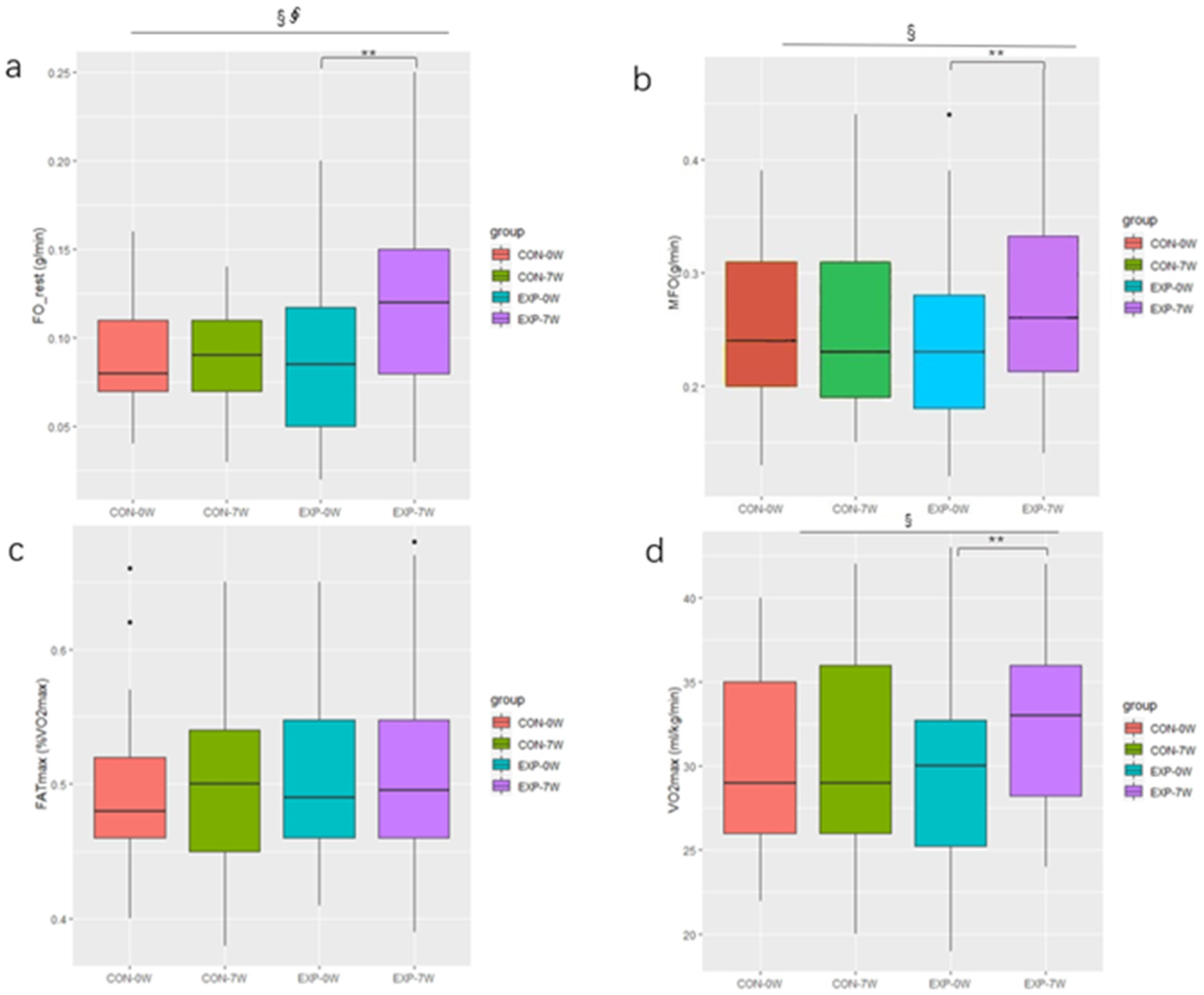
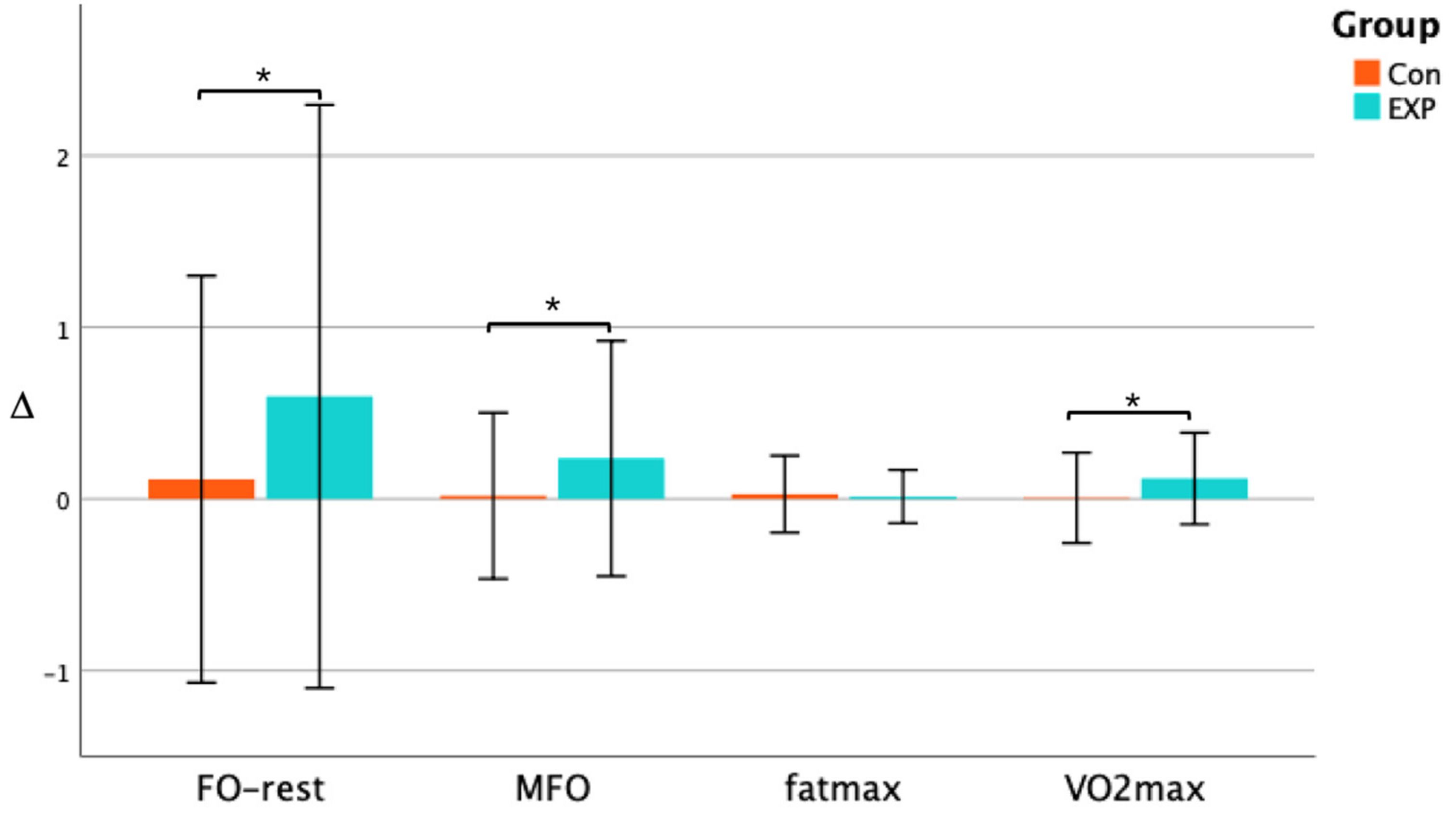
3.3. Effect of Training on Fat Oxidation and VO2max
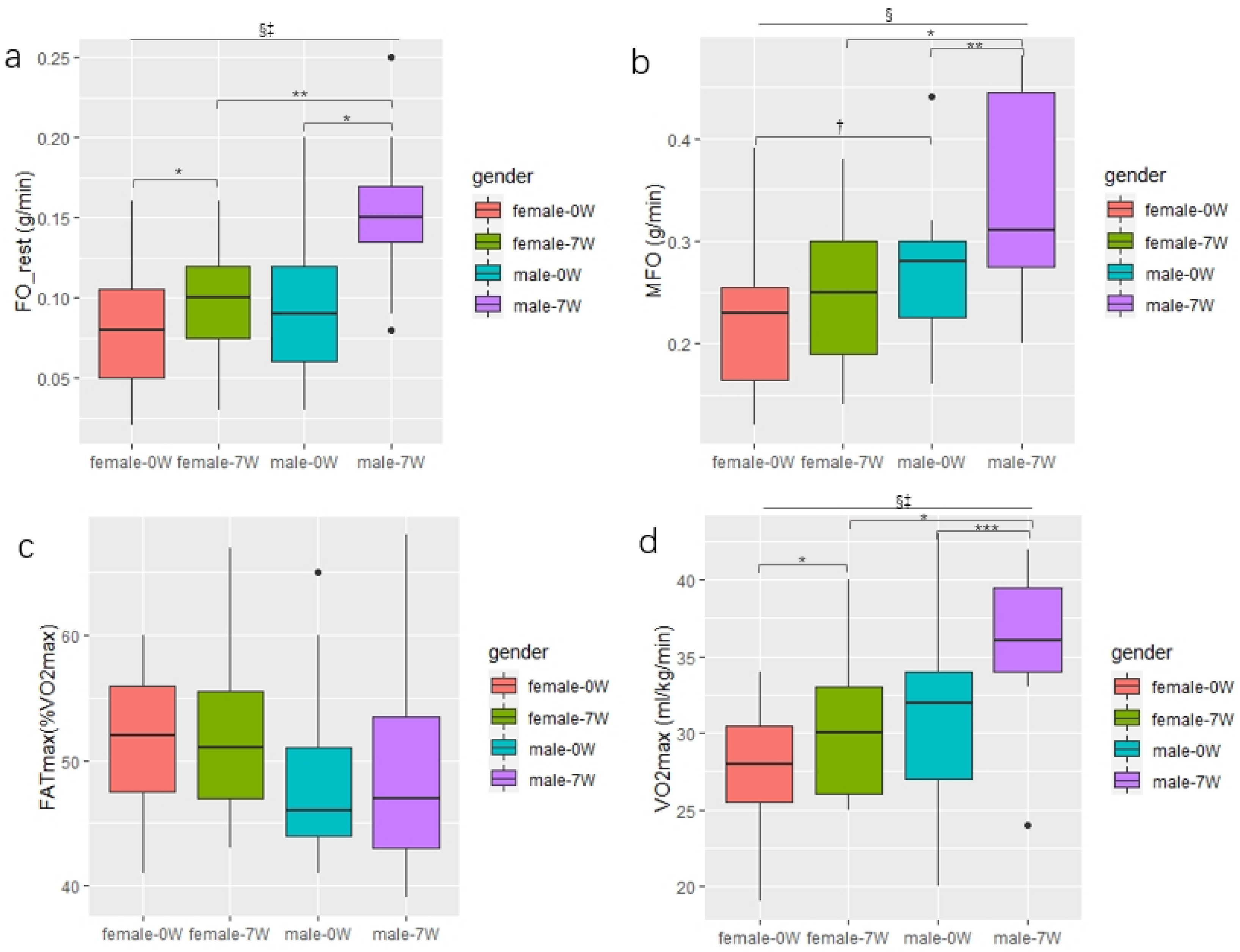
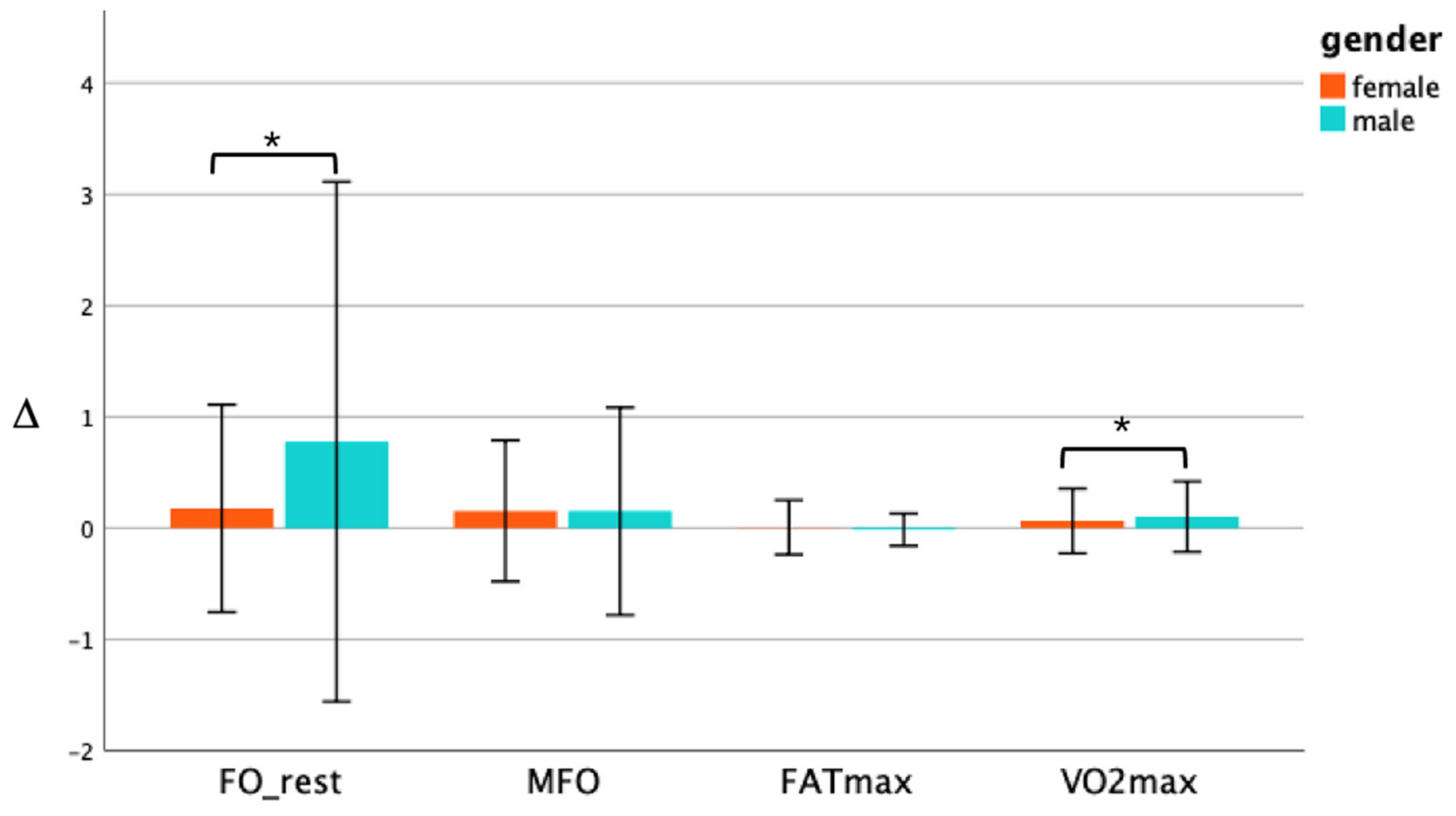
3.4. Changes in the Fat Oxidation and VO2max between genders in the EXP Group
4. Discussion
4.1. Body Composition
4.2. Lipid Profile
4.3. MFO and FATmax
4.4. VO2max
4.5. Gender Differences
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Chin, S.-H.; Kahathuduwa, C.; Binks, M. Physical activity and obesity: What we know and what we need to know. Obes. Rev. 2016, 17, 1226–1244. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Kartsonaki, C.; Du, H.; Millwood, I.Y.; Guo, Y.; Chen, Y.; Bian, Z.; Yang, L.; Walters, R.; Bragg, F.; et al. Physical Activity, Sedentary Leisure Time, Circulating Metabolic Markers, and Risk of Major Vascular Diseases. Circ. Genom. Precis. Med. 2019, 12, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Liang, Q.; Zhou, W.; Huang, X.; Hu, L.; You, C.; Li, J.; Wu, Y.; Li, P.; Wu, Q.; et al. Sed-entary behavior and the risk of cardiac-cerebral vascular diseases in southern China. Medicine 2018, 97, e12838. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhou, Q.; Zhang, D.; Qin, P.; Li, Q.; Tian, G.; Liu, D.; Chen, X.; Liu, L.; Liu, F.; et al. Association of total sedentary behaviour and television viewing with risk of overweight/obesity, type 2 diabetes and hypertension: A dose–response meta-analysis. Diabetes Obes. Metab. 2019, 22, 79–90. [Google Scholar] [CrossRef]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Obesity Medicine Association. Definition of Obesity. 2017. Available online: https://obesitymedicine.org/definition-of-obesity/ (accessed on 27 January 2022).
- Cao, L.; Jiang, Y.; Li, Q.; Wang, J.; Tan, S. Exercise Training at Maximal Fat Oxidation Intensity for Overweight or Obese Older Women: A Randomized Study. J. Sports Sci. Med. 2019, 18, 413–418. [Google Scholar]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; Merchan-Ramirez, E.; Labayen, I.; Ruiz, J.R. Relationships between diet and basal fat oxidation and maximal fat oxidation during exercise in sedentary adults. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.; Hansen, I.M.D.; Wismann, J.F.; Olsen, M.H.; Brage-Andersen, M.R.; Sahl, R.E.; Hansen, M.; Ingersen, A.; Modvig, J.L.; Schmücker, M.; et al. Maximal Fat Oxidation Rate Is Higher in Fit Women and Unfit Women with Obesity, Compared to Normal-weight Unfit Women. J. Clin. Endocrinol. Metab. 2021, 106, e4389–e4399. [Google Scholar] [CrossRef]
- Dong, X.H.; Chen, Y.T.; Han, J. The Characteristics of Substrate Metabolism and Energy Consumption in Obese and Normal Weight Student during Different Exercise Modes. Chin. J. Sports Med. 2016, 35, 411–416, 422. [Google Scholar]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [Green Version]
- Barwell, N.D.; Malkova, D.; Leggate, M.; Gill, J.M. Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metabolism 2009, 58, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Optimizing fat oxidation through exercise and diet. Nutrition 2004, 20, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Relation Between Plasma Lactate Concentration and Fat Oxidation Rates Over a Wide Range of Exercise Intensities. Laryngo-Rhino-Otologie 2004, 25, 32–37. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Metab. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, Y.; Jain, S.S.; McFarlan, J.T.; Snook, L.A.; Chabowski, A.; Bonen, A. Exercise- and training-induced upregula-tion of skeletal muscle fatty acid oxidation are not solely dependent on mitochondrial machinery and biogenesis. J. Physiol. 2013, 591, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Randell, R.K.; Rollo, I.; Roberts, T.J.; Dalrymple, K.J.; Jeukendrup, A.E.; Carter, J.M. Maximal Fat Oxidation Rates in an Athletic Population. Med. Sci. Sports Exerc. 2017, 49, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lazzer, S.; Tringali, G.; Caccavale, M.; De Micheli, R.; Abbruzzese, L.; Sartorio, A. Effects of high-intensity interval training on physical capacities and substrate oxidation rate in obese adolescents. J. Endocrinol. Investig. 2016, 40, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Guevara, I.; Urquidez-Romero, R.; Pérez-León, J.; González-Rodríguez, E.; Moreno-Brito, V.; Ramos-Jiménez, A. Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2020, 17, 7888. [Google Scholar] [CrossRef] [PubMed]
- Numao, S.; Hayashi, Y.; Katayama, Y.; Matsuo, T.; Tanaka, K. Sex differences in substrate oxidation during aerobic ex-ercise in obese men and postmenopausal obese women. Metabolism 2009, 58, 1312–1319. [Google Scholar] [CrossRef]
- Knechtle, B.; Müller, G.; Willmann, F.; Kotteck, K.; Eser, P.; Knecht, H. Fat Oxidation in Men and Women Endurance Athletes in Running and Cycling. Endoscopy 2004, 25, 38–44. [Google Scholar] [CrossRef]
- Verheggen, R.J.H.M.; Maessen, M.; Green, D.J.; Hermus, A.R.M.M.; Hopman, M.T.E.; Thijssen, D.H.T. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: Distinct effects on body weight and visceral adipose tissue. Obes. Rev. 2016, 17, 664–690. [Google Scholar] [CrossRef] [PubMed]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2018, 92, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2017, 48, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, E.W. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. In WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020.
- Slentz, C.A.; Duscha, B.D.; Johnson, J.L.; Ketchum, K.; Aiken, L.B.; Samsa, G.P.; Houmard, J.A.; Bales, C.W.; Kraus, W.E. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—A ran-domized controlled study. Arch. Intern. Med. 2004, 164, 31–39. [Google Scholar] [CrossRef] [PubMed]
- van Aggel-Leijssen, D.P.; Saris, W.H.; Hul, G.B.; van Baak, M.A. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am. J. Clin. Nutr. 2001, 73, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Lyytikäinen, A.; Lamberg-Allardt, C.; Kannas, L.; Cheng, S. Food consumption and nutrient intakes with a special focus on milk product consumption in early pubertal girls in Central Finland. Public Health Nutr. 2005, 8, 284–289. [Google Scholar] [CrossRef]
- Caudwell, P.; Gibbons, C.; Finlayson, G.; Näslund, E.; Blundell, J. Exercise and weight loss: No sex differences in body weight response to exercise. Exerc. Sport Sci. Rev. 2014, 42, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. Int. Rev. J. 2017, 8, 511–519. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes. Rev. 2020, 22, e13137. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2014, 36, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.C.; Chen, W.H.; Tian, Y. Research Progress on Maximum Fat Oxidation Exercise Intensity. J. Wuhan Inst. Phys. Educ. 2019, 53, 95–100. [Google Scholar]
- Thorogood, A.; Mottillo, S.; Shimony, A.; Filion, K.; Joseph, L.; Genest, J.; Pilote, L.; Poirier, P.; Schiffrin, E.; Eisenberg, M.J. Isolated Aerobic Exercise and Weight Loss: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Med. 2011, 124, 747–755. [Google Scholar] [CrossRef]
- Clark, J.E. Diet, exercise or diet with exercise: Comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18-65 years old) who are overfat, or obese; systematic review and meta-analysis. J. Diabetes Metab. Disord. 2015, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.J. Study on the Influence Mechanism of Exercise Weight Loss on FATmax in Obese Middle-Aged Women. Master’s Thesis, Tianjin Institute of Physical Education, Tianjin, China, 2019. [Google Scholar]
- Schubert, M.M.; Clarke, H.; Seay, R.F.; Spain, K.K. Impact of 4 weeks of interval training on resting metabolic rate, fitness, and health-related outcomes. Appl. Physiol. Nutr. Metab. 2017, 42, 1073–1081. [Google Scholar] [CrossRef]
- Jeppesen, J.; Kiens, B. Regulation and limitations to fatty acid oxidation during exercise. J. Physiol. 2012, 590, 1059–1068. [Google Scholar] [CrossRef]
- Nordby, P.; Saltin, B.; Helge, J.W. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: A role for muscle oxidative capacity? Scand. J. Med. Sci. Sports 2006, 16, 209–214. [Google Scholar] [CrossRef]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. Content 1997, 273, E268–E275. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maunder, E.; Plews, D.J.; Kilding, A.E. Contextualising Maximal Fat Oxidation During Exercise: Determinants and Norma-tive Values. Front. Physiol. 2018, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spriet, L.L. New Insights into the Interaction of Carbohydrate and Fat Metabolism During Exercise. Sports Med. 2014, 44, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima-Silva, A.E.; Bertuzzi, R.C.; Pires, F.O.; Gagliardi, J.F.; Barros, R.V.; Hammond, J.; Kiss, M.A. Relationship be-tween training status and maximal fat oxidation rate. J. Sports Sci. Med. 2010, 9, 31–35. [Google Scholar] [PubMed]
- Bagley, L.; Slevin, M.; Bradburn, S.; Liu, D.; Murgatroyd, C.; Morrissey, G.; Carroll, M.; Piasecki, M.; Gilmore, W.S.; McPhee, J.S. Sex differences in the effects of 12 weeks sprint interval training on body fat mass and the rates of fatty acid oxidation and VO2max during exercise. BMJ Open Sport Exerc. Med. 2016, 2, e000056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharhag-Rosenberger, F.; Meyer, T.; Walitzek, S.; Kindermann, W. Effects of One Year Aerobic Endurance Training on Resting Metabolic Rate and Exercise Fat Oxidation in Previously Untrained Men and Women. Laryngo-Rhino-Otologie 2010, 31, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Bircher, S.; Knechtle, B. Relationship between Fat Oxidation and Lactate Threshold in Athletes and Obese Women and Men. J. Sports Sci. Med. 2004, 3, 174–181. [Google Scholar] [PubMed]
- Boone, J.; Bouckaert, J.; Barstow, T.J.; Bourgois, J. Influence of priming exercise on muscle deoxy [Hb + Mb] during ramp cycle exercise. Eur. J. Appl. Physiol. 2011, 112, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Lijauco, C.C.; Hearon, C.M.; Angadi, S.S.; Nelson, M.D.; Sarma, S.; Nanayakkara, S.; La Gerche, A.; Haykowsky, M.J. Mechanisms of the Improvement in Peak VO2 with Exercise Training in Heart Failure with Reduced or Preserved Ejection Fraction. Heart Lung Circ. 2018, 27, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Goran, M.; Fields, D.; Hunter, G.; Herd, S.; Weinsier, R. Total body fat does not influence maximal aerobic capacity. Int. J. Obes. 2000, 24, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- Weiss, E.P.; Jordan, R.C.; Frese, E.M.; Albert, S.G.; Villareal, D.T. Effects of Weight Loss on Lean Mass, Strength, Bone, and Aerobic Capacity. Med. Sci. Sports Exerc. 2017, 49, 206–217. [Google Scholar] [CrossRef]
- Sidossis, L.S.; Gastaldelli, A.; Klein, S.; Wolfe, R.R. Regulation of plasma fatty acid oxidation during low- and high-intensity exercise. Am. J. Physiol. Content 1997, 272, E1065–E1070. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Gardner, A.W.; Arciero, P.J.; Calles-Escandon, J.; Poehlman, E.T. Gender differences in fat oxidation and sym-pathetic nervous system activity at rest and during submaximal exercise in older individuals. Clin. Sci. 1998, 95, 59–66. [Google Scholar] [CrossRef]
- Fletcher, G.; Eves, F.F.; Glover, E.I.; Robinson, S.L.; Vernooij, C.A.; Thompson, J.L.; Wallis, G.A. Dietary intake is inde-pendently associated with the maximal capacity for fat oxidation during exercise. Am. J. Clin. Nutr. 2017, 105, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, D.; Schutz, Y.; Montani, J.-P.; Dulloo, A.G.; Miles-Chan, J.L. Sex difference in substrate oxidation during low-intensity isometric exercise in young adults. Appl. Physiol. Nutr. Metab. 2016, 41, 977–984. [Google Scholar] [CrossRef]
- Venables, M.C.; Achten, J.; Jeukendrup, A.E. Determinants of fat oxidation during exercise in healthy men and women: A cross-sectional study. J. Appl. Physiol. 2005, 98, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Maher, A.C.; Akhtar, M.; Vockley, J.; Tarnopolsky, M.A. Women have higher protein content of beta-oxidation enzymes in skeletal muscle than men. PLoS ONE 2010, 5, e12025. [Google Scholar] [CrossRef] [Green Version]

| Variables | CON Group (n = 25) | EXP Group (n = 31) |
|---|---|---|
| Basic information | ||
| Age (years) | 40.1 ± 6.9 | 41.2 ± 8.9 |
| Male, n (%) | 11 (44%) | 13 (42%) |
| Height (cm) | 166 ± 8.0 | 166 ± 9.2 |
| Fat oxidation capacity | ||
| FO_rest (g/min) | 0.09 ± 0.03 | 0.09 ± 0.04 |
| MFO (g/min) | 0.25 ± 0.08 | 0.24 ± 0.08 |
| FATmax (%VO2 max) | 49 ± 7 | 50 ± 6 |
| Aerobic capacity | ||
| VO2max (ml/kg/min) | 30.3 ± 5.5 | 29.1 ± 5.1 |
| Body composition | ||
| BMI (kg/m2) | 25.2 ± 2.0 | 26.4 ± 4.1 |
| Weight (kg) | 69.2 ± 10.2 | 72.2 ± 16.4 |
| Body fat percent (%) | 32.3 ± 5.1 | 32.8 ± 4.8 |
| VFA (cm2) | 97.5 ± 22.7 | 103.2 ± 35.3 |
| Skeletal muscle (kg) | 27.3 ± 6.3 | 27.0 ± 7.3 |
| Lipid profile | ||
| TC (mmol/L) | 4.8 ± 0.9 | 5.1 ± 0.8 |
| TG (mmol/L) | 1.6 ± 1.0 | 1.5 ± 1.0 |
| HDL-C (mmol/L) | 1.5 ± 0.3 | 1.6 ± 0.4 |
| LDL-C (mmol/L) | 3.0 ± 0.9 | 3.5 ± 0.7 |
| CON Group (n = 25) | EXP Group (n = 31) | |||||
|---|---|---|---|---|---|---|
| Pre | Post | ∆ | Pre | Post | ∆ | |
| BMI (kg/m2) ∮ | 25.2 ± 2.0 | 25.1 ± 2.4 | −0.01 ± 0.22 | 26.4 ± 4.1 | 25.2 ± 3.7 ** | −0.06 ± 0.18 b |
| Weight (kg) | 69.2 ± 10.2 | 67.9 ± 17.1 | −0.09 ± 0.30 | 72.2 ± 16.4 | 68.0 ± 19.8 | −0.09 ± 0.23 |
| Body fat percent (%) § | 32.3 ± 5.1 | 31.3 ± 5.4 | −0.07 ± 0.23 | 32.8 ± 4.8 | 29.6 ± 5.4 *** | −0.13 ± 0.18 a |
| VFA (cm2) § | 97.5 ± 22.7 | 95.3 ± 26.3 | −0.04 ± 0.30 | 103.2 ± 35.3 | 90.4 ± 31.7 *** | −0.14 ± 0.20 a |
| Skeletal muscle (kg) | 27.3 ± 6.3 | 27.2 ± 6.3 | −0.02 ± 0.10 | 27.0 ± 7.3 | 27.7 ± 7.4 | 0.01 ± 0.20 |
| TC (mmol/L) § | 4.8 ± 0.9 | 4.7 ± 0.9 | −0.01 ± 0.09 | 5.1 ± 0.8 | 4.7 ± 0.8 * | −0.05 ± 0.16 a |
| TG (mmol/L) | 1.6 ± 1.0 | 1.6 ± 1.5 | 0.01 ± 0.34 | 1.5 ± 1.0 | 1.4 ± 0.8 | −0.02 ± 0.28 |
| HDL-C (mmol/L) | 1.5 ± 0.3 | 1.5 ± 0.3 | −0.01 ± 0.09 | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.02 ± 0.14 |
| LDL-C (mmol/L) | 3.0 ± 0.9 | 2.9 ± 0.9 | −0.02 ± 0.17 | 3.5 ± 0.7 | 3.2 ± 0.8 | −0.03 ± 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Lei, S.; Zhao, T.; Xie, Y.; Zhou, Z.; Cheng, S.; Wang, X. Changes in Fat Oxidation and Body Composition after Combined Exercise Intervention in Sedentary Obese Chinese Adults. J. Clin. Med. 2022, 11, 1086. https://doi.org/10.3390/jcm11041086
Cao J, Lei S, Zhao T, Xie Y, Zhou Z, Cheng S, Wang X. Changes in Fat Oxidation and Body Composition after Combined Exercise Intervention in Sedentary Obese Chinese Adults. Journal of Clinical Medicine. 2022; 11(4):1086. https://doi.org/10.3390/jcm11041086
Chicago/Turabian StyleCao, Jingguo, Siman Lei, Tong Zhao, Yuting Xie, Zunqiang Zhou, Sulin Cheng, and Xiuqiang Wang. 2022. "Changes in Fat Oxidation and Body Composition after Combined Exercise Intervention in Sedentary Obese Chinese Adults" Journal of Clinical Medicine 11, no. 4: 1086. https://doi.org/10.3390/jcm11041086
APA StyleCao, J., Lei, S., Zhao, T., Xie, Y., Zhou, Z., Cheng, S., & Wang, X. (2022). Changes in Fat Oxidation and Body Composition after Combined Exercise Intervention in Sedentary Obese Chinese Adults. Journal of Clinical Medicine, 11(4), 1086. https://doi.org/10.3390/jcm11041086






