Abstract
Sepsis is a life-threatening syndrome characterized by a dysregulated host response to an infection that may evolve rapidly into septic shock and multiple organ failure. Management of sepsis relies on the early recognition and diagnosis of infection and the providing of adequate and prompt antibiotic therapy and organ support. A novel protein biomarker, the pancreatic stone protein (PSP), has recently been studied as a biomarker of sepsis and the available evidence suggests that it has a higher diagnostic performance for the identification of infection than the most used available biomarkers and adds prognostic value. This review summarizes the clinical evidence available for PSP in the diagnosis and prognosis of sepsis.
1. Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Although sepsis incidence and mortality seems to be decreasing worldwide, it still represents a total of 19.7% (18.2–21.4) of all global deaths [2], and is the leading cause of in-hospital death and hospital readmission, as well the most expensive hospital condition to treat [3,4]. Despite considerable improvements in the management of sepsis, including early administration of adequate antibiotic therapy and support of organ dysfunction, mortality rates still remain high and early recognition of sepsis is essential and a major determinant of the disease’s outcome [5,6,7].
The lack of a gold standard test to diagnose infection as well as the overly sensitive and nonspecific features of signs and symptoms of sepsis led medical societies to endorse the use of biomarkers (“inflammatory variables”) as surrogate markers of infections to help clinicians in its diagnosis [8]. However, in clinical practice, the diagnosis of infection still relies on the intersection of three vectors: systemic manifestations, organ dysfunction and microbiological documentation [9], and no single biomarker or diagnostic test, per se, has been validated to diagnose infection.
The weaknesses of the current framework used to diagnose infection and sepsis are illustrated by several examples: the disagreement between sepsis diagnosis at the intensive care unit or emergency department admission and posthoc assessment [10,11], leading to erroneous treatment of >40% of patients as septic with an unlikely infection [12], or the inadequate antibiotic prescription for patients admitted with viral diseases (e.g., influenza) [13]. There is a growing need for fast and adequate infectious disease diagnostic procedures [14], although special attention should be focused on the features of an ideal diagnostic test—ASSURED—affordable, sensitive, specific, user-friendly, rapid, equipment-free, and delivered to those in need [15,16].
The majority of biomarkers used in sepsis assess prognosis [17]. A good predictive biomarker of infection, however, should be absent if the patient is not infected, should appear concomitantly with and ideally preceding the clinical manifestations of infection, and disappear with successful therapy or remain elevated if infection is refractory to treatment [18,19]. In the context of septic shock, the association between delay in antibiotic administration and death seems stronger than in septic patients without shock [20] supporting the recommendation to administer antimicrobials within one hour in all patients with septic shock. Therefore, point-of-care testing is appealing, as it might provide clinicians with a rapid and readily available diagnosis.
2. Materials and Methods
The selection of studies to describe pancreatic stone protein function, diagnostic and prognostic ability was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidance [21]—Figure 1.
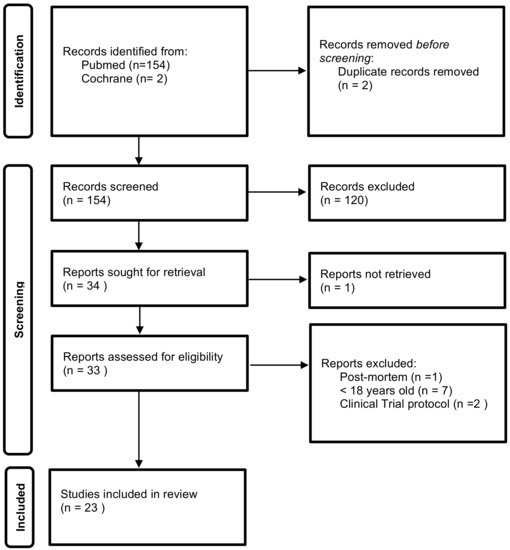
Figure 1.
Identification of studies flow diagram.
Relevant studies up to October 2021 were searched in Pubmed and Cochrane Library databases with the terms “pancreatic stone protein”, “sepsis biomarker(s)” and their combination. Moreover, references of the retrieved manuscripts were also manually cross-searched for further relevant publications. The inclusion criteria were as follows: (1) studies including adult (>18 years old) patients, and (2) studies published with full-text. The exclusion criteria were as follows: (1) studies using data retrieved post mortem. (2) clinical trial protocol.
3. Pancreatic Stone Protein (PSP): Structure, Function and Kinetics
Lithostathine and regenerating protein 1 (Reg I) were described by different groups working on pancreatitis and diabetes during the decade of the 1980s [22]. Later on, both proteins were found to be structurally identical, synthesized in the pancreatic acinar cells as a single polypeptide and secreted into the duodenum along the same secretory pathway as the exocrine enzymes. Therefore, they were renamed as pancreatic stone protein, since its first attributed function was (inaccurately) thought to be the inhibition of calcium carbonate crystals precipitation in the pancreatic juice [23,24]. Later on, the discovery of PSP in other organs besides the pancreas (e.g., brain) [25,26] and the discovery of its functional antibacterial activity [27] led investigators to explore whether it could be involved in other processes besides solubilization of the pancreatic content.
Nowadays it is established that PSP is a 14 kDa insoluble polypeptide encoded by a single transcript of the reg gene, resulting in a 144-amino acid length glycoprotein, structurally similar to C-type lectin-like proteins, [28] which are calcium-dependent glycan-binding proteins involved in the process of cell to cell and host-cell interaction, including adhesion and signaling receptors in homeostasis and innate immunity as well leukocyte and platelet trafficking in inflammatory responses [29]. PSP levels were shown to be slightly higher in patients with Type-2 diabetes mellitus compared with healthy individuals [30], being significantly higher in the subset of patients with diabetic kidney disease [31], probably due to a filtration effect suggesting renal dysfunction [32].
To determine its biological and functional role, a pivotal observation was accidently made in rat experiments by the group of Rolf Graf in which PSP was found to be an indicator of systemic stress [33]. This observation was then clinically confirmed by the demonstration in humans that the pancreas senses remote organ damage and systemic stress and responds by secreting PSP in the absence of pancreatic tissue damage [34]. As an acute-phase protein, PSP might be involved in promoting cell proliferation during regenerative processes [35], through regulation by IL-6 and other cytokines that are released after tissue injury [36,37], rendering to the pancreas what Reding et al. [38] call “the role of an acute phase organ”.
The role of PSP in the immune and inflammatory response to infection prompted its identification as a potential biomarker of infection and sepsis.
The evaluation of PSP has evolved from conventional laboratory methods, such as the isoform-specific enzyme-linked immunosorbent assay (ELISA) using the sandwich technique [34], to point of care methods at the patient’s bedside [39]. The latter underwent analytical validation and maintained a reliable performance [39,40] with faster results, which is particularly appealing in the diagnosis of septic patients, for whom speed of intervention is crucial for the prognosis [1].
4. PSP Performance for the Diagnosis of Infection and Sepsis
The performance of PSP as a biomarker of infection and sepsis has been evaluated in several patient populations and clinical settings [34,41,42,43,44,45,46,47]—Table 1.

Table 1.
Characteristics of studies evaluating PSP diagnostic performance for infection and/or sepsis. ED—Emergency department; ICU- Intensive Care Unit; SIRS—Systemic Inflammatory Response Syndrome; PSP—pancreatic stone protein; sCD25—soluble CD25; PCT—procalcitonin; HBP—heparin binding protein; CRP—C-reactive protein; IL6—interleukin-6; WBC—White blood count; AUC ROC—areas under receiver operating characteristic curves; IQR—interquartile range.
Although the majority of available studies used the 2001 definitions of sepsis and infection [8], overall the performance of PSP discriminating infection/sepsis vs. no infection/sepsis is at least comparable to other canonical biomarkers of infection and might even be better in some particular situations. Gukasjan et al. [48] found significantly higher PSP levels at ICU admission [15.2 (11.2–23.2) ng/mL vs. 125.0 (25.0–419.0) ng/mL] in patients with secondary peritonitis, compared to a control group of 43 patients admitted for elective surgery. After cardiac surgery, PSP performed better than CRP and white blood cell count for the diagnosis of infection [43].
Klein et al. [49] showed that in a cohort of burn patients admitted to the ICU without sepsis, the serum levels of PSP remained unchanged over time not only after the initial burn injury but also after secondary debridement procedures in contrast to CRP and PCT both of which significantly increased after inflammatory and/or surgical insults, suggesting that PSP might be a more robust biomarker of sepsis in this particular setting. In another cohort of burn patients admitted to the ICU, PSP demonstrated a 3.3–5.5-fold increase for up to 72 h before the diagnosis of sepsis [50] and among those with inhalation injury and ARDS, PSP was the strongest marker to identify sepsis when compared to CRP and PCT both by its higher values and steeper increase over time [51].
Scherr et al. [52] showed that among patients admitted to the emergency department with exacerbation of chronic obstructive pulmonary disease, PSP levels were significantly higher among those with positive sputum cultures compared to those with negative sputum cultures at exacerbation and those with stable disease.
Finally, Prazac et al. [53] have recently conducted an individual patient level meta-analysis and found PSP to perform better than CRP or PCT for the diagnosis of community-acquired infections in the emergency department and surgical infections after cardiac surgery.
PSP demonstrates a significant interaction between time and presence of sepsis [46,47], suggesting that besides a fixed cut-off value (as in standard ROC curve analysis) the time-related kinetics of PSP has a crucial role in the identification of sepsis when considering the time-dependency of the infectious/septic event. CRP had also shown usefulness in the timely stratification of the risk of infection in critically ill patients (patients presenting maximum daily CRP variation >4.1 mg/dL plus a CRP level >8.7 mg/dL had an 88% risk of ICU-acquired infection [54]), in prediction of VAP in the first six days of mechanical ventilation (rate of CRP change per day, highest level and maximum amplitude of variation were all significantly associated with VAP development [55]), and in anticipation of community-acquired bloodstream infection (CRP concentrations began to increase 3.1 days before diagnosis [56]). Such an approach of time-profiling a biomarker may be more helpful, informative and accurate [47]. According to Pugin et al. [47] PSP outperformed the other classic biomarkers by its relative increase even five days before clinical diagnosis of sepsis compared to three days for PCT and two days for CRP.
5. PSP Performance for the Prognosis of Septic Patients
In addition to its usefulness in the diagnosis of infection, PSP has shown a good performance in the prognosis of septic patients. Table 2 describes the main characteristics of studies on the prognostic value of PSP.

Table 2.
Characteristics of studies evaluating PSP prognostic value in patients with infection and/or sepsis. VAP—ventilator-associated pneumonia; PSP—pancreatic stone protein; SOFA—Sequential organ failure assessment; ICU- Intensive Care Unit; PCT—procalcitonin; CRP—C-reactive protein; IL6—interleukin-6; IL-8—interleuki-8; TNF-α—tumor necrosis factor alpha; IL-1ß—interleukin-1beta WBC—White blood count; AUC ROC—areas under receiver operating characteristic curves; IQR—interquartile range; OR—odds ratio; CI—confidence interval; ELISA—Enzyme-Linked Immunosorbent Assay; APACHEII—Acute Physiology and Chronic Health Evaluation II; SAPSII—Simplified Acute Physiology Score; SE—sensitivity; SP—specificity.
Boeck et al. [57] retrospectively evaluated PSP in a cohort of 101 patients with VAP, and found significantly higher values in non-survivors both on the day of diagnosis and on day 7, with different predictive mortality thresholds at each time point.
Que et al. [58] prospectively analyzed the serum value of a set of biomarkers (PSP, CRP, PCT, IL-6, IL-8, IL-10, TNF-α and IL-1 ß) in 107 patients with severe sepsis and septic shock (according to the Sepsis 2 criteria) in the first 24 h after ICU admission. PSP was the only analysed biomarker significantly increased in non-survivors.
Guadiana-Romualdo et al. [59] evaluated the prognostic ability of PSP in septic patients. PSP was measured in the first 6 h after diagnosis (baseline) and on the second day of admission to the ICU in 122 patients. It was found not only that PSP was significantly higher in non-survivors at both measurement times but also that there was a decreasing trend of PCT between measurements in the group of survivors.
In patients admitted to the ICU in the immediate postoperative period of abdominal surgery for secondary peritonitis (n = 91), PSP assessed at admission was the only biomarker (compared to CRP, PCT, IL-6 and WBC) with the ability to discriminate between clinical severity and predict mortality [48].
Que et al. [60] analysed two cohorts of ICU septic patients (total n = 249) to assess the prognostic value of PSP and to validate a mortality predictive model using severity scores and biomarkers. Higher PSP values were associated with clinical severity (significantly in both cohorts) and non-survivors (reaching statistical significance in only one cohort). Models with the addition of biomarkers (PSP, CRP and PCT) with severity indexes showed a better predictive capacity for in-hospital mortality than each parameter individually.
More recently, in a population of SARS-CoV2-infected patients admitted to the emergency department, PSP was higher in non-survivors but was not accurate to discriminate patients with organ dysfunctions that required admission to the ICU [61].
6. Clinical Application
6.1. Emergency Department
In the reality of the emergency department, PSP can be useful for the early diagnosis of infection and for the triage of patients based on the risk of mortality. The diagnostic ability of PSP may be relevant not only through its sensitivity for timely diagnosis, but also through its negative predictive value, which can lead to a reduction in inappropriate antibiotic prescriptions, which in compliance with antibiotic stewardship strategies. The importance of a triage based on analytical clinical data (such as a biomarker) has been well demonstrated in recent years, in which due to the context of the SARS-CoV2 pandemic, it has become more fundamental than ever to manage resources and ensure that existing resources are best adapted to patients’ needs.
6.2. Intensive Care Unit
In intensive care units, single assessments would predominantly have a prognostic value at admission. This data would make it possible to optimize the allocation of resources and can serve as a quality assessment and benchmarking tool (as is already the case with some severity indices, such as APACHE IV and SAPS 3 scores [62]). The possibility of performing serial assessments of PSP in ICUs would allow for a sentinel effect of infection in patients hospitalized for non-infectious causes and/or monitoring infection response to antibiotic therapy.
7. Data Analysis, Limitations and Questions to Be Clarified
It is not yet clear whether PSP assessment is more useful when performed at specific times (at admission or when there is any clinical suspicion of infection), or serially. It is interesting that, in published trials, quite different thresholds for both diagnosis and prognosis were identified. Without well-defined thresholds, the interpretation of the PSP value in single measurements becomes more complex when compared to the analysis of the PSP trend during hospitalization. The association of PSP value with other biomarkers or with clinical severity indices (eventually included in decision and clinical intervention algorithms) represents a new and interesting strategy to overcome the limited prognostic performance of single parameters, but it is also very conditioned when a well-defined risk threshold does not exist.
The relationship of PSP with the presence and severity of organ dysfunctions is another factor to be clarified and which may determine the potential of PSP to stratify patients early according to disease severity, alone or in combination with other scores or indicators. Furthermore, it is equally important to know the changes in PSP kinetics in patients with invasive organ support (such as dialysis and other extracorporeal techniques), and to understand to what extent such changes modify its diagnostic and prognostic usefulness.
It is expected that in the short term the feasibility, clinical utility, and economic benefit of real-time measurements of PSP using point-of-care technology (versus conventional laboratory measurement) will be confirmed. This will facilitate further studies leading to a better and more complete understanding of PSP kinetics in different patients and settings (for example, gram-positive and gram-negative bacteria, viral, fungal and/or parasitic infections). Hopefully, such data will translate into an optimized approach to septic patients (by selecting the intervention that most suits each patient’s condition) and thus contribute to a better outcome.
The applicability and value of PSP in non-infectious circumstances remains to be investigated. As a positive acute-phase protein and systemic stress marker, it will be important to understand its behavior and potential usefulness (diagnostic, prognostic, severity marker) in non-infectious inflammatory circumstances such as trauma and pancreatitis or more generally in other pancreatic diseases.
8. Conclusions
PSP accuracy for the diagnosis of infection and sepsis among a wide spectrum of clinical settings seems to be, at least, comparable to the other classical biomarkers currently used in clinical practice. Furthermore, it seems to outperform those biomarkers in the prediction of sepsis, accounting for its earlier relative increase before clinical diagnosis, and it adds prognostic value.
Key Messages
- Pancreatic stone protein is secreted by the pancreas and rises in response to stress induced by systemic infection and sepsis
- Pancreatic stone protein levels start to increase before the development of clinical signs and symptoms of sepsis
- Pancreatic stone protein could be useful in the identification of patients with worse outcomes
- Pancreatic stone protein performance in the diagnosis of sepsis is, at least, comparable to other biomarkers
- The role of pancreatic stone protein in clinical practice is still to be determined
Author Contributions
Conceptualization, P.F. and D.N.; methodology, P.F.; software, P.F.; validation, D.N., L.C. and P.P.; formal analysis, P.F. and D.N.; investigation, P.F. and D.N.; resources, P.F. and D.N.; data curation, P.F.; writing—original draft preparation, P.F. and D.N.; writing—review and editing, P.F.; visualization, P.F.; supervision, P.P.; project administration, P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276–288. [Google Scholar] [CrossRef]
- Mayr, F.B.; Talisa, V.B.; Balakumar, V.; Chang, C.-C.H.; Fine, M.; Yende, S. Proportion and Cost of Unplanned 30-Day Readmissions after Sepsis Compared with Other Medical Conditions. J. Am. Med. Assoc. 2017, 317, 530–531. [Google Scholar] [CrossRef]
- Levy, M.M.; Macias, W.L.; Vincent, J.-L.; Russell, J.A.; Silva, E.; Trzaskoma, B.; Williams, M.D. Early changes in organ function predict eventual survival in severe sepsis. Crit. Care Med. 2005, 33, 2194–2201. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority —A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsat, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Póvoa, P.; Coelho, L. Which Biomarkers Can Be Used as Diagnostic Tools for Infection in Suspected Sepsis? Semin Respir. Crit. Care Med. 2021, 42, 662–671. [Google Scholar] [CrossRef]
- Heffner, A.C.; Horton, J.M.; Marchick, M.R.; Jones, A.E. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin. Infect. Dis. 2010, 50, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Tidswell, R.; Parker, T.; Brealey, D.; Singer, M. Sepsis—The broken code how accurately is sepsis being diagnosed? J. Infect. 2020, 81, e31–e32. [Google Scholar] [CrossRef]
- Klein Klouwenberg, P.M.; Cremer, O.L.; van Vught, L.A.; Ong, D.S.; Frencken, J.F.; Schultz, M.J.; Bonten, M.J.; van der Poll, T. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: A cohort study. Crit. Care 2015, 19, 319. [Google Scholar] [CrossRef] [Green Version]
- McGeer, A.; Green, K.A.; Plevneshi, A.; Shigayeva, A.; Siddiqi, N.; Raboud, J.; Low, D.E.; Toronto Invasive Bacterial Diseases Network. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin. Infect. Dis. 2007, 45, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Bissonnette, L.; Bergeron, M.G. Diagnosing infections-current and anticipated technologies for point-of-care diagnostics and home-based testing. Clin. Microbiol. Infect. 2010, 16, 1044–1053. [Google Scholar] [CrossRef] [Green Version]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef]
- Urdea, M.; Penny, L.A.; Olmsted, S.S.; Giovanni, M.Y.; Kaspar, P.; Shepherd, A.; Wilson, P.; Dahl, C.A.; Buchsbaum, S.; Moeller, G.; et al. Requirements for high impact diagnostics in the developing world. Nature 2006, 444, 73–79. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.; Vincent, J.-L. Biomarkers of Sepsis: Time for a Reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Marshall, J.C.; Vincent, J.L.; Fink, M.P.; Cook, D.J.; Rubenfeld, G.; Foster, D.; Fisher, C.J., Jr.; Faist, E.; Reinhart, K. Measures, markers, and mediators: Toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, 25–26 October 2000. Crit. Care Med. 2003, 31, 1560–1567. [Google Scholar] [CrossRef]
- Povoa, P. Serum markers in community-acquired pneumonia and ventilator-associated pneumonia. Curr. Opin. Infect. Dis. 2008, 21, 157–162. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Academia and Clinic Annals of Internal Medicine Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Graf, R. Pancreatic stone protein—Sepsis and the riddles of the exocrine pancreas. Pancreatology 2020, 20, 301–304. [Google Scholar] [CrossRef]
- De Caro, A.; Lohse, J.; Sarles, H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem. Biophys. Res. Commun. 1979, 87, 1176–1182. [Google Scholar] [CrossRef]
- Terazono, K.; Yamamoto, H.; Takasawa, S.; Shiga, K.; Yonemura, Y.; Tochino, Y.; Okamoto, H. A novel gene activated in regenerating islets. J. Biol. Chem. 1988, 263, 2111–2114. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Ozturk, M.; Wands, J.R. Enhanced expression of an exocrine pancreatic protein in Alzheimer’s disease and the developing human brain. J. Clin. Investig. 1990, 86, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, M.; de la Monte, S.M.; Gross, J.; Wands, J.R. Elevated levels of an exocrine pancreatic secretory protein in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1989, 86, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Iovanna, J.; Frigerio, J.M.; Dusetti, N.; Ramare, F.; Raibaud, P.; Dagorn, J.C. Lithostathine, an inhibitor of CaCO3 crystal growth in pancreatic juice, induces bacterial aggregation. Pancreas 1993, 8, 597–601. [Google Scholar] [CrossRef]
- Jin, C.X.; Hayakawa, T.; Ko, S.B.H.; Ishiguro, H.; Kitagawa, M. Pancreatic stone protein/regenerating protein family in pancreatic and gastrointestinal diseases. Intern. Med. 2011, 50, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Cummings, R.; McEver, R. C-Type Lectins. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Yang, J.; Li, L.; Raptis, D.; Li, X.; Li, F.; Chen, B.; He, J.; Graf, R.; Sun, Z. Pancreatic stone protein/regenerating protein (PSP/reg): A novel secreted protein up-regulated in type 2 diabetes mellitus. Endocrine 2015, 48, 856–862. [Google Scholar] [CrossRef]
- Li, L.; Jia, D.; Graf, R.; Yang, J. Elevated serum level of pancreatic stone protein/regenerating protein (PSP/reg) is observed in diabetic kidney disease. Oncotarget 2017, 8, 38145–38151. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Dong, B.; Reding, T.; Peng, Y.; Lin, H.; Zhi, M.; Han, M.; Graf, R.; Li, L. Association of Serum PSP/REG Iα with Renal Function in Pregnant Women. Biomed. Res. Int. 2019, 2019, 6970890. [Google Scholar] [CrossRef] [Green Version]
- Graf, R.; Schiesser, M.; Lüssi, A.; Went, P.; Scheele, G.A.; Bimmler, D. Coordinate regulation of secretory stress proteins (PSP/reg, PAP I, PAP II, and PAP III) in the rat exocrine pancreas during experimental acute pancreatitis. J. Surg. Res. 2002, 105, 136–144. [Google Scholar] [CrossRef]
- Keel, M.; Härter, L.; Reding, T.; Sun, L.-K.; Hersberger, M.; Seifert, B.; Bimmler, D.; Graf, R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit. Care Med. 2009, 37, 1642–1648. [Google Scholar] [CrossRef]
- Unno, M.; Nata, K.; Noguchi, N.; Narushima, Y.; Akiyama, T.; Ikeda, T.; Nakagawa, K.; Takasawa, S.; Okamoto, H. Production and characterization of Reg knockout mice: Reduced proliferation of pancreatic beta-cells in Reg knockout mice. Diabetes 2002, 51, S478–S483. [Google Scholar] [CrossRef] [Green Version]
- Dusetti, N.J.; Ortiz, E.M.; Mallo, G.V.; Dagorn, J.C.; Iovanna, J.L. Pancreatitis-associated protein I (PAP I), an acute phase protein induced by cytokines. Identification of two functional interleukin-6 response elements in the rat PAP I promoter region. J. Biol. Chem. 1995, 270, 22417–22421. [Google Scholar] [CrossRef] [Green Version]
- Dusetti, N.J.; Mallo, G.V.; Ortiz, E.M.; Keim, V.; Dagorn, J.C.; Iovanna, J.L. Induction of lithostathine/reg mRNA expression by serum from rats with acute pancreatitis and cytokines in pancreatic acinar AR-42J cells. Arch. Biochem. Biophys. 1996, 330, 129–132. [Google Scholar] [CrossRef]
- Reding, T.; Palmiere, C.; Pazhepurackel, C.; Schiesser, M.; Bimmler, D.; Schlegel, A.; Süss, U.; Steiner, S.; Mancina, L.; Seleznik, G.; et al. The pancreas responds to remote damage and systemic stress by secretion of the pancreatic secretory proteins PSP/regI and PAP/regIII. Oncotarget 2017, 8, 30162–30174. [Google Scholar] [CrossRef] [Green Version]
- Putallaz, L.; van den Bogaard, P.; Laub, P.; Rebeaud, F. Nanofluidics Drives Point-of-care Technology for on the Spot Protein Marker Analysis with Rapid Actionable Results. J. Nanomed. Nanotechnol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Eggimann, P.; Que, Y.A.; Rebeaud, F. Measurement of pancreatic stone protein in the identification and management of sepsis. Biomark. Med. 2019, 13, 135–145. [Google Scholar] [CrossRef] [Green Version]
- García de Guadiana-Romualdo, L.; Berger, M.; Jiménez-Santos, E.; Rebollo-Acebes, S.; Jiménez-Sánchez, R.; Esteban-Torrella, P.; Hernando-Holgado, A.; Ortín-Freire, A.; Albaladejo-Otón, M.D. Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department. Eur. J. Clin. Investig. 2017, 47, 297–304. [Google Scholar] [CrossRef]
- Llewelyn, M.J.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, H.J.; Csordas, A.; Falk, V.; Slankamenac, K.; Rudiger, A.; Schönrath, F.; Rodriguez Cetina Biefer, H.; Starck, C.T.; Graf, R. Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique. PLoS ONE 2015, 10, e0120276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlato, M.; Philippart, F.; Rouquette, A.; Moucadel, V.; Puchois, V.; Blein, S.; Bedos, J.P.; Diehl, J.L.; Hamzaoui, O.; Annane, D.; et al. Circulating biomarkers may be unable to detect infection at the early phase of sepsis in ICU patients: The CAPTAIN prospective multicenter cohort study. Intensive Care Med. 2018, 44, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- García De Guadiana-Romualdo, L.; Jiménez-Santos, E.; Cerezuela-Fuentes, P.; Español-Morales, I.; Berger, M.; Esteban-Torrella, P.; Hernando-Holgado, A.; Albaladejo-Otón, M.D. Analyzing the capability of PSP, PCT and sCD25 to support the diagnosis of infection in cancer patients with febrile neutropenia. Clin. Chem. Lab. Med. 2019, 57, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Niggemann, P.; Buehler, P.K.; Lehner, F.; Schweizer, R.; Rittirsch, D.; Fuchs, N.; Waldner, M.; Steiger, P.; Giovanoli, P.; et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity. Ann. Surg. 2021, 274, e1179–e1186. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.; Daix, T.; Pagani, J.L.; Morri, D.; Giacomucci, A.; Dequin, P.F.; Guitton, C.; Que, Y.A.; Zani, G.; Brealey, D.; et al. Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: A prospective multicentric study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Gukasjan, R.; Raptis, D.A.; Schulz, H.U.; Halangk, W.; Graf, R. Pancreatic Stone protein predicts outcome in patients with peritonitis in the ICU. Crit. Care Med. 2013, 41, 1027–1036. [Google Scholar] [CrossRef]
- Klein, H.J.; Buehler, P.K.; Niggemann, P.; Rittirsch, D.; Schweizer, R.; Waldner, M.; Giovanoli, P.; Cinelli, P.; Reding, T.; Graf, R.; et al. Expression of Pancreatic Stone Protein is Unaffected by Trauma and Subsequent Surgery in Burn Patients. World J. Surg. 2020, 44, 3000–3009. [Google Scholar] [CrossRef]
- Niggemann, P.; Rittirsch, D.; Karl Buehler, P.; Schweizer, R.; Giovanoli, P.; Reding, T.; Graf, R.; Plock, J.A.; Klein, H.J. Incidence and time point of sepsisdetection as related to different sepsis definitions in severely burned patients and their accompanying time course of pro-inflammatory biomarkers. J. Pers. Med. 2021, 11, 701. [Google Scholar] [CrossRef]
- Klein, H.J.; Rittirsch, D.; Buehler, P.K.; Schweizer, R.; Giovanoli, P.; Cinelli, P.; Plock, J.A.; Reding, T.; Graf, R. Response of routine inflammatory biomarkers and novel Pancreatic Stone Protein to inhalation injury and its interference with sepsis detection in severely burned patients. Burns 2021, 47, 338–348. [Google Scholar] [CrossRef]
- Scherr, A.; Graf, R.; Bain, M.; Christ-Crain, M.; Müller, B.; Tamm, M.; Stolz, D. Pancreatic stone protein predicts positive sputum bacteriology in exacerbations of copd. Chest 2013, 143, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Prazak, J.; Irincheeva, I.; Llewelyn, M.J.; Stolz, D.; García de Guadiana Romualdo, L.; Graf, R.; Reding, T.; Klein, H.J.; Eggimann, P.; Que, Y.A. Accuracy of pancreatic stone protein for the diagnosis of infection in hospitalized adults: A systematic review and individual patient level meta-analysis. Crit. Care 2021, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P.; Coelho, L.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. Early identification of intensive care unit-acquired infections with daily monitoring of C-reactive protein: A prospective observational study. Crit. Care 2006, 10, R63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Póvoa, P.; Martin-Loeches, I.; Ramirez, P.; Bos, L.D.; Esperatti, M.; Silvestre, J.; Gili, G.; Goma, G.; Berlanga, E.; Espassa, M.; et al. Biomarker kinetics in the prediction of VAP diagnosis: Results from the BioVAP study. Ann. Intensive Care 2016, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvik, O.S.; Póvoa, P.; Magnussen, B.; Vinholt, P.J.; Pedersen, C.; Jensen, T.G.; Kolmos, H.J.; Lassen, A.T.; Gradel, K.O. C-reactive protein and albumin kinetics before community-acquired bloodstream infections—A Danish population-based cohort study. Epidemiol. Infect. 2020, 148, e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeck, L.; Graf, R.; Eggimann, P.; Pargger, H.; Raptis, D.A.; Smyrnios, N.; Thakkar, N.; Siegemund, M.; Rakic, J.; Tamm, M.; et al. Pancreatic stone protein: A marker of organ failure and outcome in ventilator-associated pneumonia. Chest 2011, 140, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Delodder, F.; Guessous, I.; Graf, R.; Bain, M.; Calandra, T.; Liaudet, L.; Eggimann, P. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Crit. Care 2012, 16, R114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García De Guadiana-Romualdo, L.; Albaladejo-Otón, M.D.; Berger, M.; Jiménez-Santos, E.; Jiménez-Sánchez, R.; Esteban-Torrella, P.; Rebollo-Acebes, S.; Hernando-Holgado, A.; Ortín-Freire, A.; Trujillo-Santos, J. Prognostic performance of pancreatic stone protein in critically ill patients with sepsis. Biomark. Med. 2019, 13, 1469–1480. [Google Scholar] [CrossRef]
- Que, Y.A.; Guessous, I.; Dupuis-Lozeron, E.; De Oliveira, C.R.A.; Oliveira, C.F.; Graf, R.; Seematter, G.; Revelly, J.P.; Pagani, J.L.; Liaudet, L.; et al. Prognostication of mortality in critically Ill patients with severe infections. Chest 2015, 148, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Van Singer, M.; Brahier, T.; Brochu Vez, M.J.; Gerhard Donnet, H.; Hugli, O.; Boillat-Blanco, N. Pancreatic stone protein for early mortality prediction in COVID-19 patients. Crit. Care 2021, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R. Clinical review: Scoring systems in the critically ill. Crit. Care 2010, 14, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).