Acute Kidney Injury in Patients with Severe ARDS Requiring Extracorporeal Membrane Oxygenation: Incidence, Prognostic Impact and Risk Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethics Principles
2.3. Initiation and Management of ECMO
2.4. Definition of Endpoint and Outcomes
- AKI 1: Increase of serum creatinine by ≥0.3 mg/dL (≥26.4 µmol/L) or increase to ≥150–200% from baseline or urine output < 0.5 mL/kg/h for >6 h;
- AKI 2: increase of serum creatinine to >200–300% from baseline and/or urine output < 0.5 mL/kg/h for >12 h;
- AKI 3: increase of serum creatinine to >300% from baseline or serum creatinine ≥ 4.0 mg/dL (≥354 µmol/L) after a rise of at least 44 µmol/L or treatment with renal replacement therapy and/or urine output < 0.3 mL/kg/h for > 24 h or anuria for 12 h.
2.5. Statistical Analysis
3. Results
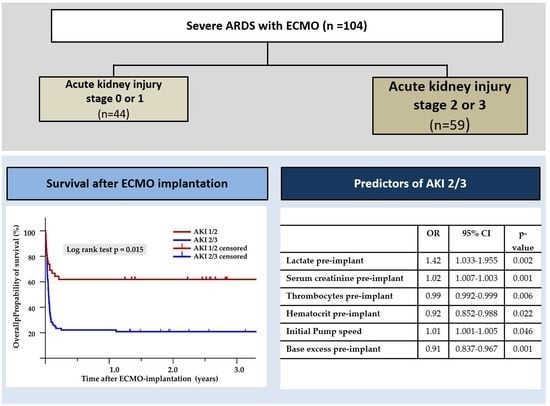
3.1. Patients’ Characteristics
3.2. Comparison of Pre-ECMO Characteristics between AKI 2/3 and AKI 0/1
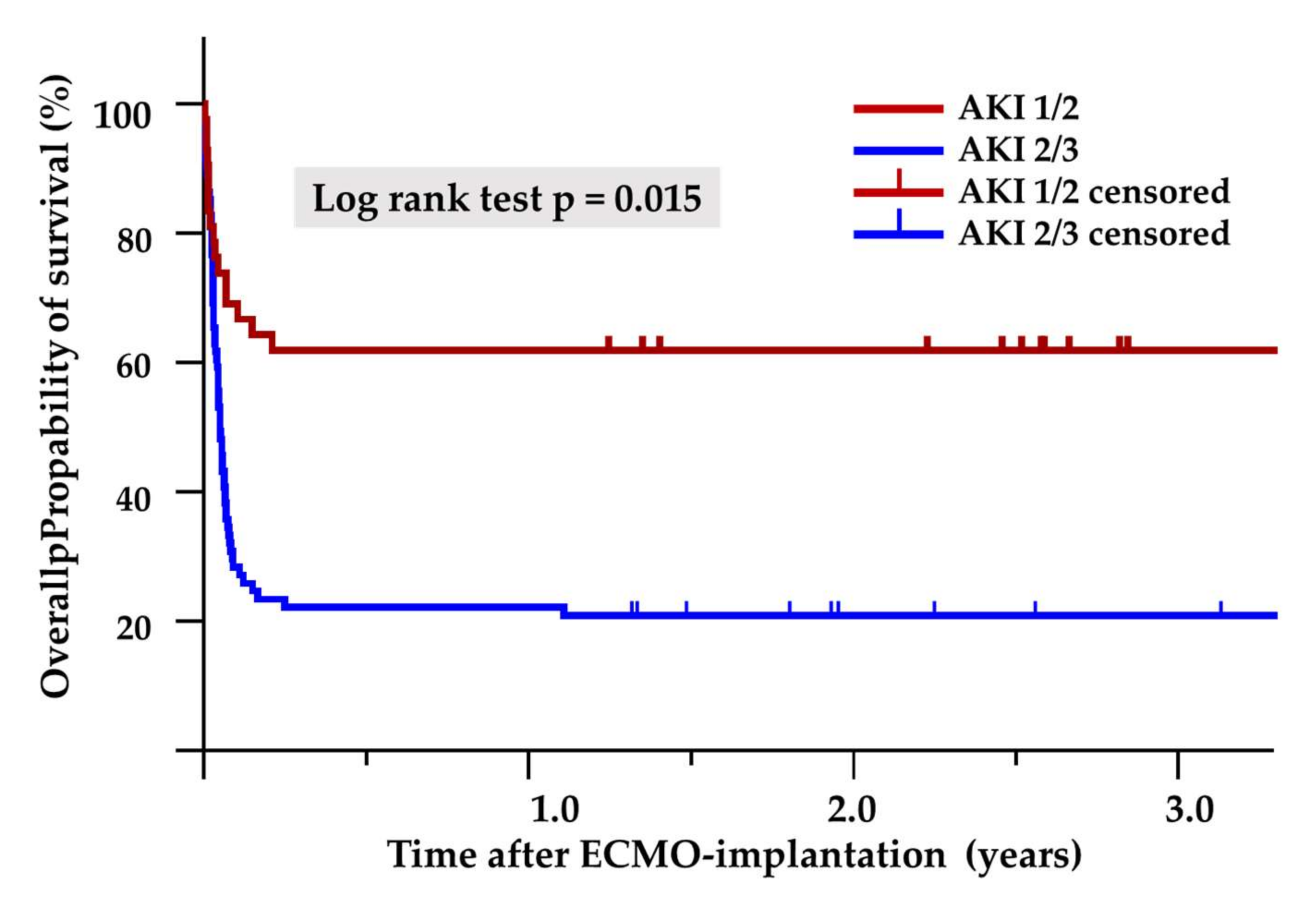
3.3. Impact on Outcome
3.4. Predictors of AKI 2/3
3.5. Comparison of Pre-ECMO Characteristics between AKI Survivors and AKI Non-Survivors
3.6. Complications after ECMO Implantation in AKI Survivors Compared to AKI Non-Survivors
4. Discussion
4.1. Introduction
4.2. Incidence of AKI
4.3. Impact of AKI
4.4. Risk Factors for AKI
4.5. Serum Creatinine
4.6. Thrombocytopenia
4.7. Initial Pump Speed
4.8. Hematocrit
4.9. Lactate
4.10. Base Excess
4.11. Comparison between AKI Survivors and Non-Survivors
4.12. Limitations
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cherian, S.V.; Kumar, A.; Akasapu, K.; Ashton, R.W.; Aparnath, M.; Malhotra, A. Salvage therapies for refractory hypoxemia in ARDS. Respir. Med. 2018, 141, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Zangrillo, A.; Landoni, G.; Biondi-Zoccai, G.; Greco, M.; Greco, T.; Frati, G.; Patroniti, N.; Antonelli, M.; Pesenti, A.; Pappalardo, F. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit. Care Resusc. 2013, 15, 172–178. [Google Scholar] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Lertjitbanjong, P.; Aeddula, N.R.; Bathini, T.; Watthanasuntorn, K.; Srivali, N.; Mao, M.A.; Kashani, K. Incidence and Impact of Acute Kidney Injury in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. J. Clin. Med. 2019, 8, 981. [Google Scholar] [CrossRef] [Green Version]
- Kilburn, D.J.; Shekar, K.; Fraser, J.F. The Complex Relationship of Extracorporeal Membrane Oxygenation and Acute Kidney Injury: Causation or Association? Biomed. Res. Int. 2016, 2016, 1094296. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Lameire, N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care. 2013, 17, 204. [Google Scholar] [CrossRef] [Green Version]
- Devasagayaraj, R.; Cavarocchi, N.C.; Hirose, H. Does acute kidney injury affect survival in adults with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation? Perfusion 2018, 33, 375–382. [Google Scholar] [CrossRef]
- Chen, S.W.; Lu, Y.A.; Lee, C.C.; Chou, A.H.; Wu, V.C.; Chang, S.W.; Fan, P.C.; Tian, Y.C.; Tsai, F.C.; Chang, C.H. Long-term outcomes after extracorporeal membrane oxygenation in patients with dialysis-requiring acute kidney injury: A cohort study. PLoS ONE 2019, 14, e0212352. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, E.; Lamanna, I.; Fagnoul, D.; Vincent, J.L.; de Backer, D.; Silvio Taccone, F. The Impact of Renal Failure and Renal Replacement Therapy on Outcome During Extracorporeal Membrane Oxygenation Therapy. Artif. Organs 2016, 40, 746–754. [Google Scholar] [CrossRef]
- Lee, S.W.; Yu, M.Y.; Lee, H.; Ahn, S.Y.; Kim, S.; Chin, H.J.; Na, K.Y. Risk Factors for Acute Kidney Injury and In-Hospital Mortality in Patients Receiving Extracorporeal Membrane Oxygenation. PLoS ONE. 2015, 10, e0140674. [Google Scholar] [CrossRef] [Green Version]
- Fleming, G.M.; Askenazi, D.J.; Bridges, B.C.; Cooper, D.S.; Paden, M.L.; Selewski, D.T.; Zappitelli, M. A multicenter international survey of renal supportive therapy during ECMO: The Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J. 2012, 58, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joannidis, M.; Druml, W.; Forni, L.; Groeneveld, A.; Honore, P.; Hoste, E.; Ostermann, M.; Oudemans-van Straaten, H.; Schetz, M. Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017. Intensive Care Med. 2017, 43, 730–749. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Cheng, Z.; Wang, L.; Li, B. Analysis of the risk factors of acute kidney injury in patients receiving extracorporeal membrane oxygenation. Clin. Nephrol. 2018, 90, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Bembea, M.M. Extracorporeal Membrane Oxygenation: Insult to Acute Kidney Injury. Pediatr. Crit. Care Med. 2016, 17, 1186–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.L.; Aguilera, C.; Knopf, K.B.; Chen, T.M.; Maslove, D.M.; Kuschner, W.G. Thrombocytopenia in the intensive care unit. J. Intensive Care Med. 2013, 28, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Hockings, L.E.; Andrews, R.K.; Aubron, C.; Gardiner, E.E.; Pellegrino, V.A.; Davis, A.K. Extracorporeal membrane oxygenation—hemostatic complications. Transfus. Med. Rev. 2015, 29, 90–101. [Google Scholar] [CrossRef]
- Lou, S.; MacLaren, G.; Best, D.; Delzoppo, C.; Butt, W. Hemolysis in pediatric patients receiving centrifugal-pump extracorporeal membrane oxygenation: Prevalence, risk factors, and outcomes. Crit. Care Med. 2014, 42, 1213–1220. [Google Scholar] [CrossRef]
- Borasino, S.; Kalra, Y.; Elam, A.R.; O’Meara, L.C.; Timpa, J.G.; Goldberg, K.G.; Gaddis, J.L.; Alten, J.A. Impact of Hemolysis on Acute Kidney Injury and Mortality in Children Supported with Cardiac Extracorporeal Membrane Oxygenation. J. Extra Corpor. Technol. 2018, 50, 217–224. [Google Scholar]
- Pourafkari, L.; Arora, P.; Porhomayon, J.; Dosluoglu, H.H.; Arora, P.; Nader, N.D. Acute kidney injury after non-cardiovascular surgery: Risk factors and impact on development of chronic kidney disease and long-term mortality. Curr. Med. Res. Opin. 2018, 34, 1829–1837. [Google Scholar] [CrossRef]
- Sukmark, T.; Lumlertgul, N.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Srisawat, N. SEA-MAKE score as a tool for predicting major adverse kidney events in critically ill patients with acute kidney injury: Results from the SEA-AKI study. Ann. Intensive Care 2020, 10, 42. [Google Scholar] [CrossRef]
- Hilder, M.; Herbstreit, F.; Adamzik, M.; Beiderlinden, M.; Bürschen, M.; Peters, J.; Frey, U.H. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: The PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Crit. Care 2017, 21, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerent, A.; Almeida, J.; Almeida, E.; Lousada, A.; Park, C.; Ribeiro, J.; Fukushima, J.; Leme, A.; Osawa, E.; Rezende, A. Base deficit and SOFA score are predictive factors of early acute kidney injury in oncologic surgical patients. Crit. Care 2015, 19, 1–201. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Huang, C.C.; Wu, T.I.; Chang, Y.S.; Wang, C.L.; Lin, P.J. Is there a preinterventional mechanical ventilation time limit for candidates of adult respiratory extracorporeal membrane oxygenation. ASAIO J. 2017, 63, 650–658. [Google Scholar] [CrossRef]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Zogheib, E.; Rozé, H.; Repesse, X.; Lebreton, G.; Luyt, C.E.; Trouillet, J.L.; Bréchot, N.; Nieszkowska, A.; Dupont, H.; et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013, 39, 1704–1713. [Google Scholar] [CrossRef]
- Fang, J.; Li, R.; Chen, Y.; Qin, J.J.; Hu, M.; Huang, C.L.; Cheng, L.; He, Y.; Li, Y.; Zhou, Q.; et al. Extracorporeal Membrane Oxygenation Therapy for Critically Ill Coronavirus Disease 2019 Patients in Wuhan, China: A Retrospective Multicenter Cohort Study. Curr. Med Sci. 2021, 41, 1–13. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Munshi, L.; del Sorbo, L.; Fan, E. The early change in PaCO2 after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am. J. Respir. Crit. Care Med. 2020, 201, 1525–1535. [Google Scholar] [CrossRef]
- Mehta, R.L.; Bouchard, J.; Soroko, S.B.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M.; Himmelfarb, J. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011, 37, 241–248. [Google Scholar] [CrossRef] [Green Version]
| AKI 0/1 (n = 44) | AKI 2/3 (n = 59) | p-Value | |
|---|---|---|---|
| Age (years) | 53.35 ± 16.56 | 55.19 ± 14.87 | n.s. |
| Height (cm) | 172.47 ± 7.12 | 171.47 ± 10.23 | n.s. |
| Weight (kg) | 83.08 ± 23.63 | 84.15 ± 20.36 | n.s. |
| BMI (kg/m2) | 24.17 ± 6.45 | 24.45 ± 5.29 | n.s. |
| Female gender | 16 (36.4) | 22 (37.3) | n.s. |
| Reason for ARDS (n, %) | |||
| Pneumonia | 27 (61.4) | 42 (71.2) | n.s. |
| Exacerbation of COPD | 5 (11.4) | 3 (5.8) | n.s. |
| H1N1 Pneumonia | 4 (9.1) | 6 (10.2) | n.s. |
| Other | 8 (18.2) | 7 (11.9) | n.s. |
| Primary oxygenation failure (n, %) | 33 (75.0) | 47 (79.7) | n.s. |
| Primary hypercapnic acidosis (n, %) | 11 (25.0) | 12 (20.3) | n.s. |
| aHTN (n, %) | 24 (54.5) | 32 (54.2) | n.s. |
| AFib (n, %) | 5 (11.4) | 8 (13.6) | n.s. |
| PHT (n, %) | 4 (9.1) | 8 (13.6) | n.s. |
| Diabetes (n, %) | 4 (9.1) | 15 (25.9) | 0.043 |
| CAD (n, %) | 12 (27.3) | 14 (23.7) | n.s. |
| s/p CPR (n, %) | 6 (13.6) | 6 (10.2) | n.s. |
| HIT (n, %) | 3 (6.8) | 0 (0) | n.s. |
| Liver failure (n, %) | 1 (2.3) | 5 (8.5) | n.s. |
| CKD (n, %) | 1 (2.3) | 15 (25.4) | <0.001 |
| Duration of mechanical ventilation (h) | 85.12 ±134.70 | 87.54 ± 100.55 | n.s. |
| FiO2 | 91.00 ± 17.87 | 93.53 ± 13.97 | n.s. |
| Pinsp (mbar) | 33.77 ± 5.72 | 33.16 ± 5.43 | n.s. |
| PEEP (mbar) | 14.56 ± 4.40 | 14.33 ± 4.24 | n.s. |
| PaO2/FiO2 (mmHg) | 10.55 ± 72.82 | 92.01 ± 58.92 | n.s. |
| Tidal volume (mL) | 414.07 ± 131.92 | 438.88 ± 136.57 | n.s. |
| Compliance (mL/mbar) | 23.45 ± 10.02 | 24.93 ± 11.29 | n.s. |
| LISS | 3.52 ± 0.55 | 3.51 ± 0.50 | n.s. |
| paO2 (mmHg) | 91.31 ± 53.27 | 78.63 ± 29.33 | n.s. |
| pCO2 (mmHg) | 79.50 ± 31.33 | 72.01 ± 24.92 | n.s. |
| pH | 7.23 ± 0.13 | 7.20 ± 0.13 | n.s. |
| Base excess (mmol/L) | 2.36 ± 5.43 | −1.70 ± 7.56 | 0.007 |
| HCO3 (mmol/L) | 31.37 ± 7.22 | 26.74 ± 7.70 | 0.006 |
| SaO2 (%) | 92.49 ± 5.77 | 90.64 ± 7.14 | n.s. |
| Lactate (mmol/L) | 1.58 ± 1.29 | 2.73 ± 2.40 | 0.008 |
| Hemoglobin (g/dL) | 11.49 ± 2.61 | 10.69 ± 1.79 | n.s. |
| Hematocrit (%) | 35.39 ± 7.06 | 32.35 ± 5.10 | 0.017 |
| Urea (mmol/L) | 7.85 ± 5.20 | 13.08 ± 8.43 | 0.001 |
| Serum creatinine (mmol/L) | 83.19 ± 30.11 | 144.94 ± 95.95 | 0.001 |
| WBC (×109/L) | 15.33 ± 7.94 | 14.76 ± 9.03 | n.s. |
| Procalcitonin (µg/L) | 4.31 ± 7.71 | 13.69 ± 28.51 | n.s. |
| CRP (mg/L) | 202.37 ± 136.33 | 199.89 ± 127.97 | n.s. |
| INR | 1.17 ± 0.23 | 2.01 ± 0.19 | n.s. |
| aPTT (s) | 41.21 ± 21.18 | 48.12 ± 20.99 | n.s. |
| D-dimers (mg/L) | 8.92 ± 12.06 | 7.54 ±10.49 | n.s. |
| Initial blood flow ECMO (L/min) | 3.38 ± 0.89 | 3.69 ± 0.822 | n.s. |
| Initial revolution (min−1) | 2970.55 ± 348.33 | 3370.53 ± 472.59 | 0.035 |
| Initial airflow (L/min) | 3.50 ± 1.56 | 3.64 ± 1.55 | n.s. |
| Platelets (×109/L) | 288.57 ± 186.39 | 185.21 ± 137.92 | 0.002 |
| CK (U/L) | 423.47 ± 938.25 | 560.46 ± 1008.46 | n.s. |
| Total bilirubin (µmmol/L) | 16.89 ± 22.71 | 28.14 ± 48.86 | n.s. |
| AST (U/L) | 83.53 ± 126.49 | 86.07 ± 88.13 | n.s. |
| ALT/(U/L) | 53.74 ± 65.53 | 51.72 ± 72.95 | n.s. |
| LDH (U/min) | 393.12 ±197.70 | 438.11 ± 254.44 | n.s. |
| Albumin (g/dL) | 24.45 ± 7.97 | 23.01 ± 7.31 | n.s. |
| AKI 0/1 (n = 44) | AKI 2/3 (n = 59) | p-Value | |
|---|---|---|---|
| GI bleeding (n, %) | 5 (11.4) | 12 (20.3) | n.s. |
| ENT bleeding (n, %) | 22 (50.0) | 47 (79.7) | 0.008 |
| ECMO bleeding (n, %) | 16 (36.4) | 31 (52.5) | n.s. |
| Pulmonary bleeding (n, %) | 5 (11.4) | 17 (28.8) | 0.033 |
| Hemolysis (n, %) | 1 (2.3) | 5 (8.5) | n.s. |
| Pneumothorax (n, %) | 7 (15.9) | 6 (10.2) | n.s. |
| Cardiac complications (n, %) | 17 (38.6) | 19 (32.2) | n.s. |
| Neurological complications (n, %) | 11 (25) | 19 (32.2) | n.s. |
| Infectious complications (n, %) | 35 (79.5) | 56 (94.9) | <0.001 |
| Blood stream infection (n, %) | 12 (27.2) | 33 (55.9) | 0.005 |
| CPR (n, %) | 4 (9.1) | 3 (5.1) | n.s. |
| Weaning failure from ECMO (n, %) | 15 (34.1) | 30 (50.9) | 0.08 |
| 30-day mortality (n, %) | 17 (38.6) | 35 (59.3) | n.s. |
| Hospital mortality (n, %) | 17 (38.6) | 37 (62.7) | 0.021 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Lactate pre-implant | 1.42 | 1.033–1.955 | 0.002 |
| Serum creatinine pre-implant | 1.018 | 1.007–1.003 | 0.001 |
| Thrombocytes pre-implant | 0.995 | 0.992–0.999 | 0.006 |
| Hematocrit pre-implant | 0.917 | 0.852–0.988 | 0.022 |
| Initial Pump speed | 1.009 | 1.001–1.005 | 0.046 |
| Base excess pre-implant | 0.913 | 0.837–0.967 | 0.001 |
| AKI Survivors (n = 22) | AKI Non-Survivors (n = 37) | p-Value | |
|---|---|---|---|
| Age (years) | 52.38 ± 15.80 | 56.79 ± 14.29 | n.s. |
| Height (cm) | 173.47 ± 8.20 | 170.20 ± 11.28 | n.s. |
| Weight (kg) | 81.18 ± 16.89 | 86.03 ± 22.36 | n.s. |
| BMI (kg/m2) | 23.44 ± 16.89 | 86.03 ± 22.36 | n.s. |
| Female gender | 5 (22.7) | 17 (45.9) | n.s. |
| Reason for ARDS (n, %) | |||
| Pneumonia | 14 (63.6) | 28 (75.7) | n.s. |
| Exacerbation of COPD | 2 (9.1) | 1 (2.7) | n.s. |
| H1N1 Pneumonia | 4 (18.2) | 2 (5.4) | n.s. |
| Others | 2 (9.1) | 5 (13.5) | n.s. |
| Primary hypercapnic acidosis (n, %) | 0 (0.0) | 12 (32.4) | 0.003 |
| Primary oxygenation failure (n, %) | 22 (0.0) | 25 (67.6) | 0.003 |
| aHTN (n, %) | 10 (45.5) | 22 (59.5) | n.s. |
| AFib (n, %) | 3 (13.6) | 5 (13.5) | n.s. |
| PHT (n, %) | 1 (4.5) | 7 (18.9) | n.s. |
| Diabetes (n, %) | 8 (36.4) | 7 (18.9) | n.s. |
| CAD (n, %) | 5 (22.7) | 9 (24.3) | n.s. |
| s/p CPR (n, %) | 3 (13.6) | 3 (8.1) | n.s. |
| HIT (n,%) | 0 (0) | 0 (0) | n.s. |
| Liver failure (n, %) | 1 (4.5) | 4 (10.8) | n.s. |
| CKD (n, %) | 4 (18.2) | 11 (50.0) | n.s. |
| Duration of mechanical ventilation (h) | 75.90 ± 86.81 | 91.14 ± 108.16 | 0.078 |
| FiO2 | 98.05 ± 4.58 | 91.06 ± 16.6 | n.s. |
| Pinsp (mbar) | 33.61 ± 4.99 | 32.92 ± 5.70 | n.s. |
| PEEP (mbar) | 14.77 ± 3.78 | 14.11 ± 4.50 | n.s. |
| PaO2/FiO2 (mmHg) | 77.16 ± 29.24 | 99.42 ± 68.43 | n.s. |
| Tidal volume (mL) | 444.92 ± 95.33 | 434.95 ± 160.10 | n.s. |
| Compliance (mL/mbar) | 23.69± 7.27 | 21.73 ± 7.37 | n.s. |
| LISS | 3,61 0.39 | 3.44 ± 0.55 | n.s. |
| paO2 (mmHg) | 75.77 ± 28.70 | 80.13 ± 27.9 | n.s. |
| pCO2 (mmHg) | 64.44 ± 44.31 | 76.12 ± 27.90 | 0.08 |
| pH | 7.20 0.12 | 7.19 ± 0.15 | n.s |
| Base excess (mmol/L) | −4.23 ± 9.8 | 0.52 ±8.3 | 0.1 |
| HCO3 (mmol/L) | 23.36 ± 4.19 | 28.22 ± 8.44 | 0.04 |
| SaO2 (%) | 89.38 ± 0.37 | 91.28 ± 6.61 | n.s. |
| Lactate (mmol/L) | 3.48 ± 2.35 | 2.31 ± 2.36 | n.s. |
| Hemoglobin (g/dL) | 10.92 ± 2.15 | 10.57 ± 1.57 | n.s. |
| Hematocrit (%) | 32.63 ± 6.54 | 32.19 ± 4.19 | n.s. |
| Urea (mmol/L) | 13.63 ± 6.54 | 13.08 ± 9.19 | n.s. |
| Serum creatinine (mmol/L) | 124.95 ± 77.77 | 180.92 ± 115.72 | 0.03 |
| WBC (×109/L) | 15.33 ± 9.10 | 14.44 ± 9.11 | n.s. |
| Procalcitonin (µg/L) | 22.95 ± 36.13 | 8.32 ± 22.3 | n.s. |
| CRP (mg/L) | 212.98 ± 130.90 | 192.41 ± 127.57 | n.s. |
| INR | 1.25 ± 0.19 | 1.23 ±0.30 | n.s. |
| aPTT (s) | 47.55 ± 18.49 | 48.44 ± 22.51 | n.s. |
| D-dimers (mg/L) | 4.39 ± 5.56 | 9.48 ± 12.4 | n.s. |
| Initial blood flow ECMO (L/min) | 3.43 ± 0.72 | 3.83 ± 0.54 | n.s. |
| Initial revolution (min−1) | 3193.75 ± 379.89 | 3424.92 ± 498.08 | n.s. |
| Initial airflow (L/min) | 3.58 ± 1.23 | 3.66 ± 1.73 | n.s. |
| Platelets (×109/L) | 168.75 ±170.84 | 194.36 ± 117.51 | n.s. |
| CK (U/L) | 380.21 ± 586.14 | 678.55 ± 1204.05 | n.s. |
| Total bilirubin (µmmol/L) | 23.97 ± 24.7 | 30.48 ± 58.30 | n.s. |
| AST (U/L) | 67.59 ± 55.57 | 97.99 ± 103.3 | n.s. |
| ALT/(U/L) | 68.45 ± 209.67 | 43.09 ± 43.67 | n.s. |
| LDH (U/min) | 501.88 ± 361.69 | 402.68 ± 167.33 | n.s. |
| Albumin (g/dL) | 21.65 ± 7.67 | 23.77 ± 7.16 | n.s. |
| AKI-Survivors (n = 22) | AKI-Non-Survivors (n = 37) | p-Value | |
|---|---|---|---|
| GI bleeding (n, %) | 5 (22.7) | 7 (18.9) | n.s. |
| ENT bleeding (n, %) | 16 (72.7) | 31 (83.8) | n.s. |
| ECMO bleeding (n, %) | 10 (45.5) | 22 (59.5) | n.s. |
| Pulmonary bleeding (n, %) | 4 (18.2) | 13 (35.1) | n.s. |
| Hemolysis (n, %) | 2 (9.1) | 4 (10.8) | n.s. |
| Pneumothorax (n, %) | 3 (18.2) | 3 (8.2) | n.s. |
| Cardiac complications (n, %) | 3 (13.6) | 16 (43.2) | 0.0186 |
| Neurological complications (n, %) | 11 (50.0) | 8 (21.6) | 0.024 |
| Infectious complications (n, %) | 19 (86.4) | 37 (100.0) | 0.047 |
| Blood stream infection (n, %) | 12 (54.5) | 21 (56.8) | n.s. |
| CPR (n, %) | 0 (0) | 3 (8.0) | n.s. |
| Weaning failure from ECMO (n, %) | 0 (0) | 30 (81.1) | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarczyk, K.; Huenges, K.; Bewig, B.; Balke, L.; Cremer, J.; Haneya, A.; Panholzer, B. Acute Kidney Injury in Patients with Severe ARDS Requiring Extracorporeal Membrane Oxygenation: Incidence, Prognostic Impact and Risk Factors. J. Clin. Med. 2022, 11, 1079. https://doi.org/10.3390/jcm11041079
Pilarczyk K, Huenges K, Bewig B, Balke L, Cremer J, Haneya A, Panholzer B. Acute Kidney Injury in Patients with Severe ARDS Requiring Extracorporeal Membrane Oxygenation: Incidence, Prognostic Impact and Risk Factors. Journal of Clinical Medicine. 2022; 11(4):1079. https://doi.org/10.3390/jcm11041079
Chicago/Turabian StylePilarczyk, Kevin, Katharina Huenges, Burkhard Bewig, Lorenz Balke, Jochen Cremer, Assad Haneya, and Bernd Panholzer. 2022. "Acute Kidney Injury in Patients with Severe ARDS Requiring Extracorporeal Membrane Oxygenation: Incidence, Prognostic Impact and Risk Factors" Journal of Clinical Medicine 11, no. 4: 1079. https://doi.org/10.3390/jcm11041079
APA StylePilarczyk, K., Huenges, K., Bewig, B., Balke, L., Cremer, J., Haneya, A., & Panholzer, B. (2022). Acute Kidney Injury in Patients with Severe ARDS Requiring Extracorporeal Membrane Oxygenation: Incidence, Prognostic Impact and Risk Factors. Journal of Clinical Medicine, 11(4), 1079. https://doi.org/10.3390/jcm11041079