Abstract
Bone and muscle mass loss are known to occur simultaneously. The alpha-actinin three (ACTN3) genotype has been shown to potentially affect bone and muscle mass. In this study, we investigated the association between the ACTN3 genotype and bone and muscle mass loss in community-dwelling adults aged ≥ 60 years. This study was a cross-sectional analysis of data from 295 participants who participated in a community health checkup. The ACTN3 genotypes were classified as RR, RX, or XX types. Bone mass loss was defined as a calcaneal speed of sound T-score of <−1.32 and <−1.37, and muscle mass loss was defined as an appendicular skeletal muscle index of <7.0 kg/m2 and <5.7 kg/m2 in men and women, respectively. The percentages of XX, RX, and RR in the combined bone and muscle mass loss group were 33.8%, 30.8%, and 16.7%, respectively, with a significantly higher trend for XX. Multinomial logistic regression analysis showed that XX had an odds ratio of 3.00 (95% confidence interval 1.05–8.54) of being in the combined bone and muscle mass loss group compared to the RR group (covariates: age, sex, grip strength, and medications). The ACTN3 genotype of XX is associated with a higher rate of comorbid bone and muscle mass loss. Therefore, ACTN3 genotyping should be considered for preventing combined bone and muscle mass loss.
1. Introduction
The world’s population is aging rapidly, with the number of people aged 60 years and above projected to reach 1 billion by 2020 and 2.1 billion by 2050 [1]. Falls and fall-related fractures are common in the older population. One in three older adults falls each year [2], and approximately 10 percent of falls result in serious injuries [3]. In particular, hip fractures are often caused by falls, increasing the mortality rate and the need for care [4]. Although many factors contribute to falls and fractures, the loss of bone and muscle mass markedly increases the risk. In addition, bone and muscle mass loss are correlated [5,6,7]. Bone and muscle affect each other, with both mechanical stress and biochemical interactions thought to be involved [8]. A decrease in mechanical stress increases sclerostin and promotes bone resorption, whereas an increase in mechanical stress promotes bone formation [9]. Mechanical stress has been reported to increase protein synthesis and cause muscle hypertrophy [10]. Furthermore, bone and muscle have endocrine functions, with bone secreting osteokines and muscle secreting myokines, which enhance each other [11]. Recently, osteosarcopenia was defined as combined bone mass loss and sarcopenia [12,13,14]. The prevalence of osteosarcopenia has been reported to vary from 10.4% to 40.0% in different countries [15,16,17]. A meta-analysis showed that osteosarcopenia is associated with a significantly higher risk of falls, fractures, and mortality [18]. Preventing osteosarcopenia is important and requires investigation of the factors affecting both bone and muscle.
Some of the factors that affect bone and muscle mass include aging, physical inactivity, and vitamin D deficiency [19]. Bone mass and muscle mass are strongly influenced by genetic factors, with 60 to 70% being hereditary [20]. Alpha-actinin three (ACTN3) is a gene that affects bone and muscle mass [21]. ACTN3 is an actin-binding protein, and ACTN2 and 3 are major components of the z line in sarcomeres [22]. ACTN2 is expressed in all muscles, whereas ACTN3 is expressed only in Type II fibers [23,24]. North et al. identified a common stop-codon polymorphism (rs1815739; R577X) in ACTN3 [23]. Individuals homozygous for the X allele do not express ACTN3 in Type II muscle fibers, in contrast to individuals with the RX or RR genotypes. The XX genotype has fewer Type II fibers [24]. In addition, ACTN3 is expressed in osteoprogenitor cells [25]. Loss of ACTN3 may affect bone mass.
Studies examining ACTN3 genotypes and muscle mass have shown that individuals with XX and RX genotypes have less thigh muscle volume than those with the RR genotype [26]. In addition, a study involving older Korean adults showed that those with the XX genotype had lower skeletal muscle mass in the extremities than those with the RR genotype [27]. Studies examining the ACTN3 genotype and bone mass have shown that the XX and RX genotypes have lower bone mass than the RR genotype [28]. Thus, if the ACTN3 genotype is XX or RX, bone and muscle mass losses may occur simultaneously. This simultaneous loss is expected to increase the risk of falls and fractures. Previous studies have examined bone and muscle mass loss separately. However, these studies did not examine the association between comorbid bone and muscle mass loss and the ACTN3 genotype. We hypothesized that ACTN3 genotypes XX and RX would lead to concomitant loss of bone mass and muscle mass, as previously reported. The Asian Working Group for Sarcopenia defines older age as ≥60 or ≥65 years [29]. Menopause often occurs between the ages of 40 and 58 years, and bone mass density declines significantly after menopause [30]. This study aimed to determine the association between the ACTN3 genotype and comorbid bone and muscle mass loss in community-dwelling adults aged ≥60 years. If the ACTN3 genotype is associated with comorbid bone mass loss and muscle mass loss, ACTN3 genotyping could be used to provide early prevention and intervention for those at a high risk for falls and fractures.
2. Materials and Methods
2.1. Study Participants
This cross-sectional study used data from the Tarumizu Study 2018 and 2019 participants who consented to undergo ACTN3 genotyping. The Tarumizu Study is a community-based health check survey conducted jointly with Kagoshima University (Faculty of Medicine), Tarumizu City Office, and Tarumizu Chuo Hospital. The Tarumizu Study was open to citizens aged >40 years living in Tarumizu City by mailing a reply card. The surveys were conducted between July and December 2018 and June and December 2019. Among the Tarumizu Study 2018 and 2019 participants, 320 who were ≥60 and consented to ACTN3 genotyping were included in this study. Exclusion criteria were defined as individuals with missing data for bone mass (n = 12), muscle mass (n = 3), or walking speed (n = 1), and those with a history of stroke (n = 9). The final number of participants in the analysis was 295 (mean age 73.2 ± 6.5 years, females 63.4%). This study was approved by the Kagoshima University (Faculty of Medicine) Ethics Committee (Ref No. 170351, 190319), and informed consent was obtained from all participants prior to their inclusion in the study.
2.2. ACTN3 Genotyping
The ACTN3 genotype (rs1815739) was determined by collecting oral mucosal samples [26], and a DNA exercise-exercise gene test kit (Hersires International, Hiroshima, Japan) was used to analyze the samples. We ensured that participants fasted for 30 min prior to the test. Participants were asked to gargle with water and then use a sterile cotton swab (Tomy Works, Sakai, Japan) to rub the inside of their cheek for 1 min. EBS (Hiroshima, Japan) was used to extract DNA from the oral mucosa and determine the ACTN3 genotype. A Mag Max DNA Multi-Sample Ultra Kit (Thermo Fisher Scientific, Paisley, UK) and King Fisher Flex Purification System (Thermo Fisher Scientific were used to extract genomic DNA from the swabs. The ACTN3 genotype (rs1815739) was analyzed by polymerase chain reaction (PCR) with two pairs of primers (PCR-CTPP) using the KAPA2G Robust PCR Kit (Kapa Biosystems, Wilmington, MA, USA). ACTN3 genotypes were classified as XX, RX, and RR.
2.3. Measurement of Bone Mass
Bone mass was measured using quantitative ultrasound equipment, CM-200 (Furuno, Nishinomiya City, Japan) [31]. The measurement site was the right calcaneus, and we confirmed the absence of calcaneal fracture. Quality control and calibration were undertaken before measurements were performed. Participants placed their right foot on a foot-pad adjusted to the size of their foot, and two transducers were placed on either side of their heel. Sound waves were then transmitted through the calcaneus from one transducer and received by the other. The signal was transmitted to a computer for processing, display, and storage [32]. The device uses the speed of sound (SOS) as a parameter to assess the health of participants’ bones. In this study, the T-score generated from the SOS was used to classify bone health. The T-score values obtained were based on a Japanese population survey provided by the manufacturer. Bone mass loss was defined as T-score <−1.32 for men and <−1.37 for women, based on a previous study [31].
2.4. Measurement of Muscle Mass
We assessed appendicular skeletal muscle mass using multifrequency bioelectrical impedance analysis with the InBody 470 (InBody Japan, Tokyo, Japan) [33]. The InBody 470 analyzer adopts a tetrapolar, eight-point tactile electrode system that separately measures the impedance of the arms, trunk, and legs at three different frequencies (5, 50, and 250 kHz) for each segment. A surface of the hand electrode was placed in contact with each of the five fingers, while the participants’ heels and forefeet were placed on a circular foot electrode. Appendicular skeletal muscle index (ASMI; kg/m2) was calculated by dividing limb skeletal muscle mass by the square of height. We defined muscle mass loss as ASMI <7.0 kg/m2 in men and <5.7 kg/m2 in women based on the Asian Working Group for Sarcopenia 2019 criteria [29].
2.5. Physical Performance
Physical performance was assessed using grip strength and walking speed. A Smedley-type handheld dynamometer (Grip-D; Takei Ltd., Niigata, Japan) was used to determine the maximum grip strength of the participants’ dominant hand [34]. Participants were asked to walk 14 m (divided into two 2 m long end zones and a 10 m long middle zone) at their usual walking speed to calculate their walking speed (m/s) [35]. The measurement was performed using a photoelectric sensor-type measuring device (YW; Yagami Inc., Aichi, Japan) to automatically measure walking time.
2.6. Sociodemographic Characteristics
The following sociodemographic characteristics were investigated: age (years), sex, body mass index (BMI), medications (n/day), use of osteoporosis medications, medical history, Geriatric Depression Scale 15 (GDS15) score, fall history (last year), and exercise habits (at least twice a week). Medications, use of osteoporosis medications, and medical history were assessed by licensed doctors or nurses through an interview.
2.7. Statistical Analysis
The characteristics of the three groups of ACTN3 genotypes were compared using one-way analysis of variance for age, BMI, medications, GDS15, grip strength, and walking speed. Sex, osteoporosis medication, medical history, fall history, exercise habits, bone mass loss, and muscle mass loss were compared using the Mantel–Haenszel test for trend. Participants were classified into the following four groups based on bone and muscle mass cutoff values; normal, bone mass loss, muscle mass loss, and combined bone and muscle mass loss. The proportions of all groups in the three ACTN3 genotypes were analyzed using the Mantel–Haenszel test for trend. A multinomial logistic regression analysis was performed with the four groups divided by bone mass and muscle mass as the dependent variables and ACTN3 genotype as the independent variable. Age, sex, grip strength, and medications were included as covariates. IBM SPSS statistical software, version 27 (IBM Corp., Armonk, NY, USA) was used for data analysis. Statistical significance was set at p < 0.05.
3. Results
The characteristics of the ACTN3 genotype are listed in Table 1. The variables that showed significant differences among the three groups were muscle mass loss (p = 0.006) and grip strength (p = 0.015), with a higher percentage of muscle mass loss in the XX genotype group. Bone mass loss was not significantly different, and there were no other significant variables among the ACTN3 genotypes.

Table 1.
Characteristics of Participants by ACTN3 Genotype, Mean ± SD, or %.
Figure 1 shows the percentages of normal, bone mass loss, muscle mass loss, and combined bone and muscle mass loss in the three ACTN3 genotypes. Overall percentages of normal, bone mass loss, muscle mass loss, and combined bone and muscle mass loss groups were 26.1, 33.9, 12.2, and 27.8%, respectively. Significant trend differences were observed in the percentages of all ACTN3 genotypes (p = 0.004). The percentage of participants in the normal group was 18.9%, 25.9%, and 33.3% for XX, RX, and RR, respectively, with RR having the highest percentage. The percentage of combined bone and muscle mass loss was 33.8%, 30.8%, and 16.7% for XX, RX, and RR, respectively, and was the highest in XX.
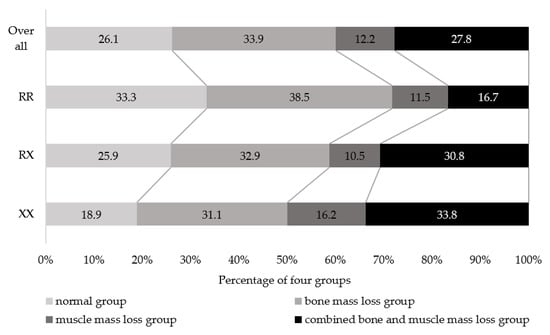
Figure 1.
Percentage of the four groups classified according to bone and muscle mass in ACTN3 genotypes. Mantel–Haenszel test for trend, p = 0.004.
Table 2 presents the results of the multinomial logistic regression analysis. The odds ratio of the combined bone and muscle mass loss group was significantly higher at 3.00 (95% confidence interval 1.05–8.54) than that for the normal group in XX, using RR as the reference. Bone mass loss and muscle mass loss groups were not associated with the ACTN3 genotype.

Table 2.
Odds ratio of the ACTN3 genotype in four groups classified by bone mass and muscle mass.
4. Discussion
This is the first study to examine the coexistence of bone and muscle mass loss with the ACTN3 genotype in community-dwelling adults aged ≥60 years. The results of this study suggest that bone and muscle mass loss are more likely to coexist in individuals with the XX genotype than in those with the RR genotype. However, there was no association between bone or muscle mass loss alone and the ACTN3 genotype. Thus, the ACTN3 genotype XX may be associated with combined bone and muscle mass loss.
In this study, the proportion of ACTN3 genotypes was 25.1% for XX, 48.5% for RX, and 26.4% for RR. In general, the proportions of ACTN3 genotypes in the Japanese population were 21.1–26.5% for type XX, 50.0–52.4% for type RX, and 20.3–26.9% for type RR [36,37,38,39], which are similar to the percentages of ACTN3 genotypes in this study. Thus, the participants of this study can be considered representative of the general Japanese population. In addition, the percentage of older adults with comorbid bone and muscle mass loss in previous studies has ranged from 21.4 to 27.9% [17,40]. The overall proportion of participants with combined bone and muscle mass loss in this study was 27.8%, similar to that reported in previous studies. Interestingly, this study indicated a higher percentage of combined bone and muscle mass loss in the XX genotype (33.8%) than that in the RR genotype (16.7%). Therefore, loss of bone and muscle mass may be more likely to coexist in the XX genotype than in the RR genotype. Conversely, those with the ACTN3 genotype RR had a higher percentage of stable combined bone and muscle mass at 33.3% compared with 18.9% in the XX genotype. A meta-analysis showed that RR of the ACTN3 genotype is more common in sprint and power athletes [41]. In addition, 12 weeks of resistance training in older adults showed that RR increased maximal strength by approximately 30% on bench press and 15% on leg extension compared to those in XX [42]. Bone mass is higher in RR than that in XX [25], and the percentage of people without osteoporosis who have reduced muscle mass is approximately 20% lower than that of those with osteoporosis [43]. The ACTN3 genotype RR may be protective against bone and muscle mass loss.
There are many common factors that cause bone and muscle mass loss, including sex hormone deficiencies, lifestyle factors such as inactivity and smoking, and comorbidities, such as genetic factors [44]. ACTN3 plays an important role in regulating protein synthesis and degradation signaling in the skeletal muscle and has been shown to affect muscle mass from early after birth [45]. Furthermore, ACTN3 knockout mice have been shown to have decreased bone mass due to decreased osteoblast activity and increased osteoclast activity [25]. In addition, the XX genotype may have increased osteocalcin, procollagen 1 N-terminal propeptide (P1NP), and β-isomerized C-terminal telopeptide (β-CTx) activity, indicating high bone metabolic turnover [46]. The ACTN3 genotype has a direct effect on the bone and muscle and is likely to coexist with bone and muscle mass loss. This study did not find an association between the ACTN3 genotype and bone mass or muscle mass loss alone. This may have been influenced by factors related to bone and muscle mass loss singly. Factors associated with bone mass loss include a history of fragility fractures and maternal hip fractures, whereas factors associated with muscle mass loss include low albumin levels, angiotensin-converting enzyme inhibitor use, and dyslipidemia [47]. However, these factors were not investigated, which is a limitation of this study. Additionally, several genes other than ACTN3 are active in both bone and muscles [48].
There are several limitations of our study that should be considered. As this was a cross-sectional study, it is unclear whether the ACTN3 genotype directly affects bone and muscle mass loss. We cannot discount the possibility that one occurs first and the other is affected by its decline. In addition, the severity and duration of bone and muscle mass loss were not investigated and should be included in future research. The participants in this study were respondents in a health check, who consented to ACTN3 genotyping, and were not randomly selected. Additionally, a sample size estimate was not calculated. In measuring bone mass, this study used SOS from quantitative ultrasound and not dual-energy X-ray absorptiometry, which is the gold standard for osteoporosis diagnosis. Furthermore, other potential covariates related to bone and muscle mass, such as nutrition, lifestyle, hormonal factors, physical activity, and other genes, remain to be considered. Bone and muscle mass are affected by sex, but the number of participants in this study was not sufficient to allow separate analysis by sex. Future studies should include a longitudinal design on a larger number of participants and examining the ACTN3 genotype and its effect on combined bone and muscle mass loss according to sex.
5. Conclusions
This study examined the association between the ACTN3 genotype and bone and muscle mass loss in community-dwelling older adults aged ≥60 years. Participants with ACTN3 genotype XX were more likely to have comorbid bone and muscle mass loss. Although various factors are involved in bone and muscle mass loss, the ACTN3 genotype is thought to be a major contributing factor. Combined bone and muscle mass loss may increase the risk of falls and fractures and may necessitate preventive measures for those with the XX genotype. Older adults with ACTN3 genotype XX may have combined bone and muscle mass loss, and exercise approaches and treatment strategies should be considered according to the ACTN3 genotype.
Author Contributions
Conceptualization, Y.T.; formal analysis, Y.T. and H.M.; funding acquisition, Y.T. and H.M.; investigation, Y.T., H.M., Y.N., Y.K., S.A., M.T., T.T., T.K. and M.O.; methodology, Y.T. and H.M.; project administration, H.M., T.T. and M.O.; resources, H.M., T.T. and M.O.; supervision, H.M., T.T. and M.O.; writing—original draft, Y.T.; writing—review and editing, H.M., Y.N., Y.K., S.A., M.T., T.T., T.K. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Research Funding for Longevity Sciences (29–42) from the National Center for Geriatrics and Gerontology (NCGG) and JSPS KAKENHI (Grant-in-Aid for Scientific Research [B]), Grant Number 19H03978, and research grants from the Japan Osteoporosis Foundation.
Institutional Review Board Statement
This study was approved by the Kagoshima University (Faculty of Medicine) Ethics Committee (Ref No. 170351, 190319), and informed consent was obtained from all participants prior to their inclusion in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
There are no linked research datasets for this study. The authors do not have permission to share the data.
Acknowledgments
The authors would like to thank Tarumizu Chuo Hospital and Tarumizu City office staff for their contributions to the study. We also thank all the participants in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Aging Overview. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 February 2022).
- Peeters, G.; van Schoor, N.M.; Lips, P. Fall risk: The clinical relevance of falls and how to integrate fall risk with fracture risk. Best Pract. Res. Clin. Rheumatol. 2009, 23, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E. Clinical practice. Preventing falls in elderly persons. N. Engl. J. Med. 2003, 348, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Leibson, C.L.; Tosteson, A.N.; Gabriel, S.E.; Ransom, J.E.; Melton, L.J. Mortality, disability, and nursing home use for persons with and without hip fracture: A population-based study. J. Am. Geriatr. Soc. 2002, 50, 1644–1650. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Hongo, M.; Mizutani, Y.; Shimada, Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. J. Bone Miner. Metab. 2013, 31, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, M.; Vallero, F.; Di Monaco, R.; Tappero, R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch. Gerontol. Geriatr. 2011, 52, 71–74. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; Tian, Q.; Papasian, C.J.; Hu, T.; Deng, H.W. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos. Int. 2016, 27, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Onorato, F.; Mastrogregori, A.; Rossi, D.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Role of physical activity in bone-muscle crosstalk: Biological aspects and clinical implications. J. Funct. Morphol. Kinesiol. 2021, 6, 55. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Osteocyte control of bone remodeling: Is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles? Osteoporos. Int. 2014, 25, 2685–2700. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A.; Robling, A.G. Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms. Bone 2015, 80, 24–36. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Loyola, W.; Phu, S.; Bani Hassan, E.; Brennan-Olsen, S.L.; Zanker, J.; Vogrin, S.; Conzade, R.; Kirk, B.; Al Saedi, A.; Probst, V.; et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): Definitions and characteristics. J. Am. Med. Dir. Assoc. 2020, 21, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Atlihan, R.; Kirk, B.; Duque, G. Non-pharmacological interventions in osteosarcopenia: A systematic review. J. Nutr. Health Aging 2021, 25, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Montero-Odasso, M.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of osteosarcopenia in older individuals with a history of falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Y.; Zhan, J.K.; Tang, Z.Y.; He, J.Y.; Tan, P.; Deng, H.Q.; Huang, W.; Liu, Y.S. Sarco-osteoporosis: Prevalence and association with frailty in chinese community-dwelling older adults. Int. J. Endocrinol. 2015, 2015, 482940. [Google Scholar] [CrossRef]
- Drey, M.; Sieber, C.C.; Bertsch, T.; Bauer, J.M.; Schmidmaier, R.; FiAT intervention group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin. Exp. Res. 2016, 28, 895–899. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, Y.; Teng, Y.; Long, Q.; Hao, Q.; Yu, X.; Yang, L.; Lv, Y.; Liu, J.; Zeng, Y.; et al. The analysis of osteosarcopenia as a risk factor for fractures, mortality, and falls. Osteoporos. Int. 2021, 32, 2173–2183. [Google Scholar] [CrossRef]
- Kirk, B.; Al Saedi, A.; Duque, G. Osteosarcopenia: A case of geroscience. Aging Med. 2019, 2, 147–156. [Google Scholar] [CrossRef]
- Karasik, D.; Kiel, D.P. Genetics of the musculoskeletal system: A pleiotropic approach. J. Bone Miner. Res. 2008, 23, 788–802. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. ACTN3, morbidity, and healthy aging. Front. Genet. 2018, 9, 15. [Google Scholar] [CrossRef]
- Houweling, P.J.; Papadimitriou, I.D.; Seto, J.T.; Pérez, L.M.; Coso, J.D.; North, K.N.; Lucia, A.; Eynon, N. Is evolutionary loss our gain? The role of ACTN3 p.Arg577Ter (R577X) genotype in athletic performance, ageing, and disease. Hum. Mutat. 2018, 39, 1774–1787. [Google Scholar] [CrossRef] [PubMed]
- North, K.N.; Yang, N.; Wattanasirichaigoon, D.; Mills, M.; Easteal, S.; Beggs, A.H. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat. Genet. 1999, 21, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.; De Bock, K.; Ramaekers, M.; Van den Eede, E.; Van Leemputte, M.; Hespel, P.; Thomis, M.A. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol. Genomics 2007, 32, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Schindeler, A.; McDonald, M.M.; Seto, J.T.; Houweling, P.J.; Lek, M.; Hogarth, M.; Morse, A.R.; Raftery, J.M.; Balasuriya, D.; et al. alpha-Actinin-3 deficiency is associated with reduced bone mass in human and mouse. Bone 2011, 49, 790–798. [Google Scholar] [CrossRef]
- Kiuchi, Y.; Makizako, H.; Nakai, Y.; Taniguchi, Y.; Tomioka, K.; Sato, N.; Wada, A.; Doi, T.; Kiyama, R.; Takenaka, T. Associations of alpha-actinin-3 genotype with thigh muscle volume and physical performance in older adults with sarcopenia or pre-sarcopenia. Exp. Gerontol. 2021, 154, 111525. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, I.; Kang, H. ACTN3 gene and susceptibility to sarcopenia and osteoporotic status in older Korean adults. BioMed Res. Int. 2017, 2017, 4239648. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Lim, S.T.; Kim, C.S. Association of ACTN3 polymorphisms with BMD, and physical fitness of elderly women. J. Phys. Ther. Sci. 2016, 28, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Subramaniam, S.; Chan, C.Y.; Soelaiman, I.N.; Mohamed, N.; Muhammad, N.; Ahmad, F.; Ng, P.Y.; Jamil, N.A.; Aziz, N.A.; Chin, K.Y. The performance of a calcaneal quantitative ultrasound device, CM-200, in stratifying osteoporosis risk among Malaysian population aged 40 years and above. Diagnostics 2020, 10, 178. [Google Scholar] [CrossRef]
- Chin, K.Y.; Soelaiman, I.N.; Mohamed, I.N.; Ibrahim, S.; Wan Ngah, W.Z. The effects of age, physical activity level, and body anthropometry on calcaneal speed of sound value in men. Arch. Osteoporos. 2012, 7, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kurose, S.; Nishikawa, S.; Nagaoka, T.; Kusaka, M.; Kawamura, J.; Nishioka, Y.; Sato, S.; Tsutsumi, H.; Kimura, Y. Prevalence and risk factors of sarcopenia in community-dwelling older adults visiting regional medical institutions from the Kadoma sarcopenia Study. Sci. Rep. 2020, 10, 19129. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Kubozono, T.; Kiyama, R.; Takenaka, T.; Kuwahata, S.; Tabira, T.; Kanoya, T.; Horinouchi, K.; Shimada, H.; Ohishi, M. Associations of social frailty with loss of muscle mass and muscle weakness among community-dwelling older adults. Geriatr. Gerontol. Int. 2019, 19, 76–80. [Google Scholar] [CrossRef]
- Kiuchi, Y.; Makizako, H.; Nakai, Y.; Tomioka, K.; Taniguchi, Y.; Kimura, M.; Kanouchi, H.; Takenaka, T.; Kubozono, T.; Ohishi, M. The association between dietary variety and physical frailty in community-dwelling older adults. Healthcare 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Miyamoto-Mikami, E.; Hirata, K.; Kimura, N.; Fuku, N. Association analysis of the ACTN3 R577X polymorphism with passive muscle stiffness and muscle strain injury. Scand. J. Med. Sci. Sport. 2018, 28, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Tobina, T.; Ichinoseki-Sekine, N.; Kakigi, R.; Tsuzuki, T.; Zempo, H.; Shiose, K.; Yoshimura, E.; Kumahara, H.; Ayabe, M.; et al. Role of selected polymorphisms in determining muscle fiber composition in Japanese men and women. J. Appl. Physiol. 2018, 124, 1377–1384. [Google Scholar] [CrossRef]
- Kikuchi, N.; Tsuchiya, Y.; Nakazato, K.; Ishii, N.; Ochi, E. Effects of the ACTN3 R577X genotype on the muscular strength and range of motion before and after eccentric contractions of the elbow flexors. Int. J. Sports Med. 2018, 39, 148–153. [Google Scholar] [CrossRef]
- Mikami, E.; Fuku, N.; Murakami, H.; Tsuchie, H.; Takahashi, H.; Ohiwa, N.; Tanaka, H.; Pitsiladis, Y.P.; Higuchi, M.; Miyachi, M.; et al. ACTN3 R577X genotype is associated with sprinting in elite Japanese athletes. Int. J. Sports Med. 2014, 35, 172–177. [Google Scholar] [CrossRef]
- Genaro, P.S.; Pereira, G.A.; Pinheiro, M.M.; Szejnfeld, V.L.; Martini, L.A. Influence of body composition on bone mass in postmenopausal osteoporotic women. Arch. Gerontol. Geriatr. 2010, 51, 295–298. [Google Scholar] [CrossRef]
- Alfred, T.; Ben-Shlomo, Y.; Cooper, R.; Hardy, R.; Cooper, C.; Deary, I.J.; Gunnell, D.; Harris, S.E.; Kumari, M.; Martin, R.M.; et al. ACTN3 genotype, athletic status, and life course physical capability: Meta-analysis of the published literature and findings from nine studies. Hum. Mutat. 2011, 32, 1008–1018. [Google Scholar] [CrossRef]
- Pereira, A.; Costa, A.M.; Izquierdo, M.; Silva, A.J.; Bastos, E.; Marques, M.C. ACE I/D and ACTN3 R/X polymorphisms as potential factors in modulating exercise-related phenotypes in older women in response to a muscle power training stimuli. Age 2013, 35, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Makizako, H.; Kiyama, R.; Tomioka, K.; Nakai, Y.; Kubozono, T.; Takenaka, T.; Ohishi, M. The association between osteoporosis and grip strength and skeletal muscle mass in community-dwelling older women. Int. J. Environ. Res. Public Health 2019, 16, 1228. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E. Determinants of Muscle and Bone Aging. J. Cell Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.T.; Roeszler, K.N.; Meehan, L.R.; Wood, H.D.; Tiong, C.; Bek, L.; Lee, S.F.; Shah, M.; Quinlan, K.G.R.; Gregorevic, P.; et al. ACTN3 genotype influences skeletal muscle mass regulation and response to dexamethasone. Sci. Adv. 2021, 7, eabg0088. [Google Scholar] [CrossRef] [PubMed]
- Levinger, I.; Yan, X.; Bishop, D.; Houweling, P.J.; Papadimitriou, I.; Munson, F.; Byrnes, E.; Vicari, D.; Brennan-Speranza, T.C.; Eynon, N. The influence of alpha-actinin-3 deficiency on bone remodelling markers in young men. Bone 2017, 98, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Brennan-Olsen, S.L.; Duque, G. Therapeutic approaches to osteosarcopenia: Insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19867009. [Google Scholar] [CrossRef]
- Trajanoska, K.; Rivadeneira, F.; Kiel, D.P.; Karasik, D. Genetics of bone and muscle interactions in umans. Curr. Osteoporos. Rep. 2019, 17, 86–95. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).