Use of the Visceral Adiposity Index as an Indicator of Chronic Kidney Disease in Older Adults: Comparison with Body Mass Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Body Adiposity Indices and Estimated Glomerular Filtration Rate (eGFR)
2.3. Statistical Analysis
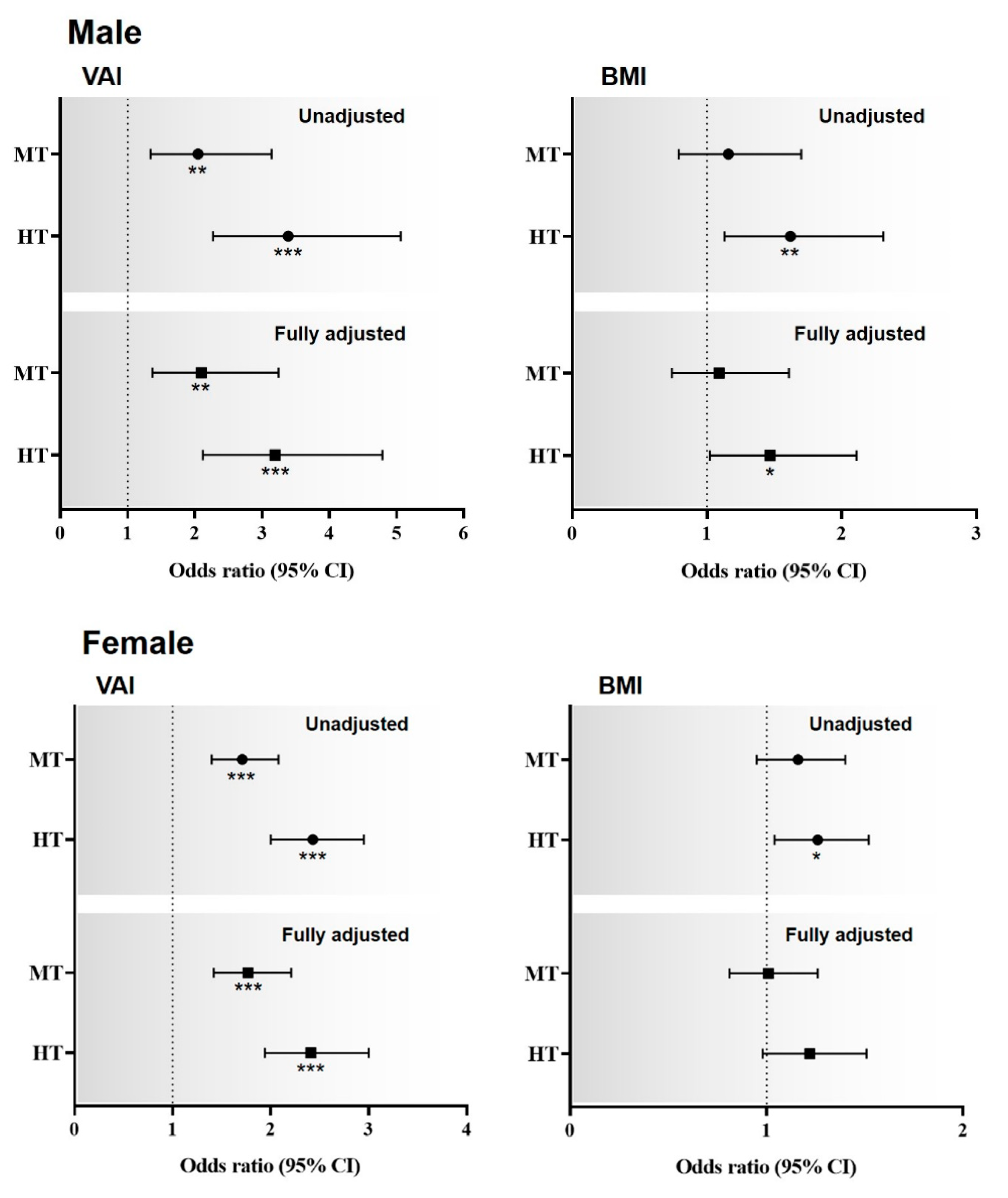
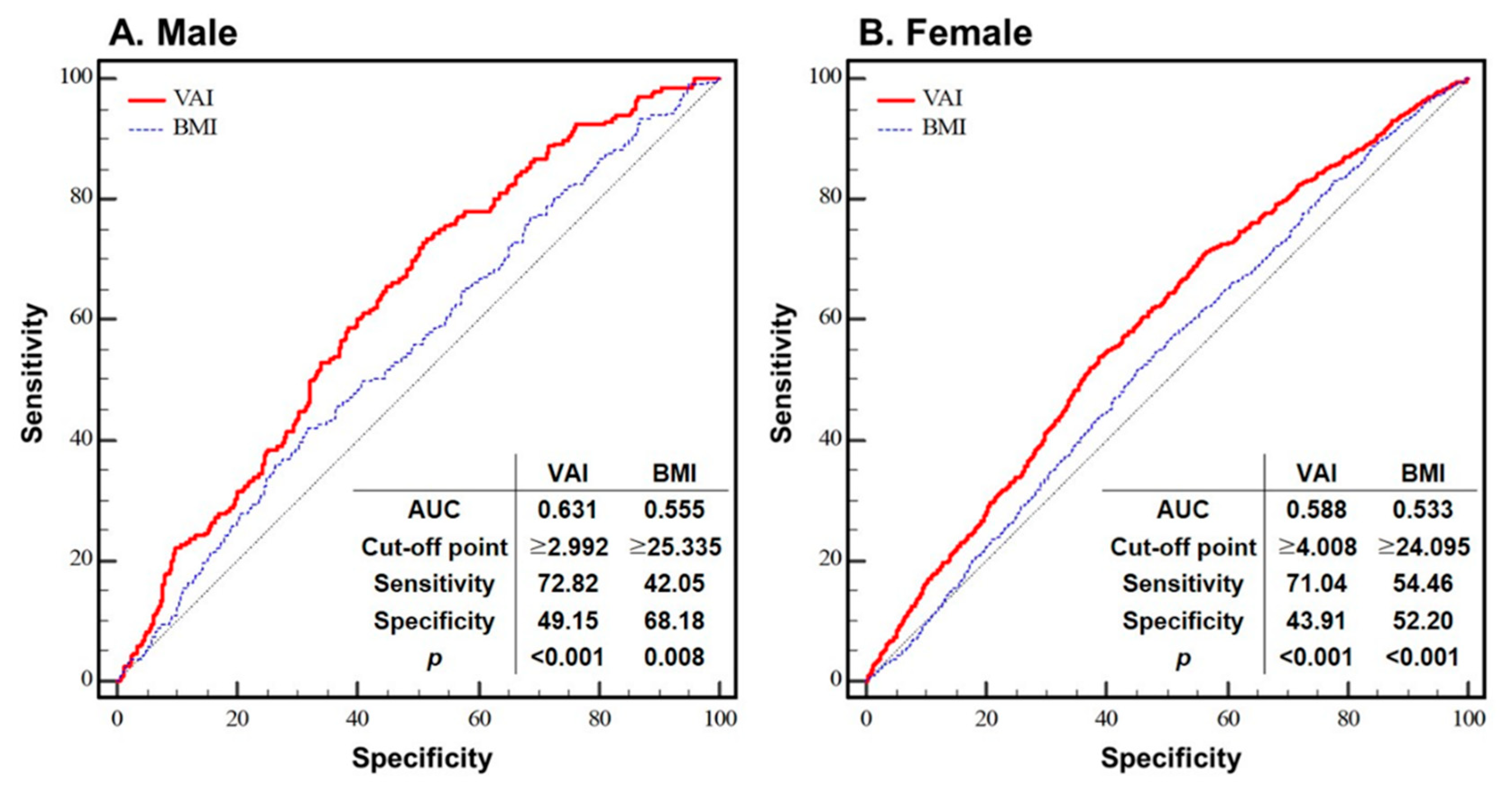
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD prevalence varies across the European general population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef]
- Abdulkader, R.C.R.M.; Burdmann, E.A.; Lebrão, M.L.; Duarte, Y.A.O.; Zanetta, D.M.T. Aging and decreased glomerular filtration rate: An elderly population-based study. PLoS ONE 2017, 12, e0189935. [Google Scholar] [CrossRef]
- Kang, H.-T.; Lee, J.; Linton, J.A.; Park, B.-J.; Lee, Y.-J. Trends in the prevalence of chronic kidney disease in Korean adults: The Korean national health and nutrition examination survey from 1998 to 2009. Nephrol. Dial. Transplant. 2013, 28, 927–936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.I.; Baek, H.; Jung, H.H. Prevalence of chronic kidney disease in Korea: The Korean national health and nutritional examination survey 2011–2013. J. Korean Med. Sci. 2016, 31, 915–923. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Mount, P.; Davies, M.; Choy, S.-W.; Cook, N.; Power, D. Obesity-Related chronic kidney disease—The role of lipid metabolism. Metabolites 2015, 5, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Câmara, N.O.S.; Iseki, K.; Kramer, H.; Liu, Z.-H.; Sharma, K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Oh, S.; Kim, E. Abdominal fat accumulation as a potential risk factor for low back pain in adult men. Int. J. Appl. Sports Sci. 2019, 31, 25–31. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Sairenchi, T.; Iso, H.; Irie, F.; Yamagishi, K.; Watanabe, H.; Tanaka, K.; Muto, T.; Ota, H. The dose-response relationship between body mass index and the risk of incident stage ≥ 3 chronic kidney disease in a general Japanese population: The Ibaraki prefectural health study (IPHS). J. Epidemiol. 2014, 24, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.L.; Molnar, M.Z.; Naseer, A.; Mikkelsen, M.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of Age and BMI with Kidney Function and Mortality: A Cohort Study. Lancet Diabetes Endocrinol. 2015, 3, 704–714. [Google Scholar] [CrossRef]
- Chang, T.-J.; Zheng, C.-M.; Wu, M.-Y.; Chen, T.-T.; Wu, Y.-C.; Wu, Y.-L.; Lin, H.-T.; Zheng, J.-Q.; Chu, N.-F.; Lin, Y.-M.; et al. Relationship between body mass index and renal function deterioration among the Taiwanese chronic kidney disease population. Sci. Rep. 2018, 8, 6908. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Tanaka, K.; Noh, J.; So, R.; Tsujimoto, T.; Sasai, H.; Kim, M.; Shoda, J. Abdominal obesity: Causal factor or simply a symptom of obesity-related health risk. Diabetes Metab. Syndr. Obes. 2014, 7, 289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Millán, D.; Vila, N.; Ibañez, P.; Gil, M.J.; Valentí, V.; et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- de Mutsert, R.; Gast, K.; Widya, R.; de Koning, E.; Jazet, I.; Lamb, H.; le Cessie, S.; de Roos, A.; Smit, J.; Rosendaal, F.; et al. Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: The Netherlands epidemiology of obesity study. Metab. Syndr. Relat. Disord. 2018, 16, 54–63. [Google Scholar] [CrossRef]
- Favre, G.; Legueult, K.; Pradier, C.; Raffaelli, C.; Ichai, C.; Iannelli, A.; Redheuil, A.; Lucidarme, O.; Esnault, V. Visceral fat is associated to the severity of COVID-19. Metabolism 2021, 115, 154440. [Google Scholar] [CrossRef]
- Kim, B.; Park, H.; Kim, G.; Isobe, T.; Sakae, T.; Oh, S. Relationships of fat and muscle mass with chronic kidney disease in older adults: A cross-sectional pilot study. Int. J. Environ. Res. Public Health 2020, 17, 9124. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Chen, Z.; Wang, X.; Zhang, L.; Nie, J.; Zheng, C.; Wang, J.; Shao, L.; Tian, Y.; et al. Comparison of visceral, body fat indices and anthropometric measures in relation to chronic kidney disease among Chinese adults from a large scale cross-sectional study. BMC Nephrol. 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 30827. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaqi, A.; Al-Naimi, F.; Qassmi, R.; Shi, Z.; Ganji, V.; Salih, R.; Bawadi, H. Visceral adiposity index is a better predictor of type 2 diabetes than body mass index in Qatari population. Medicine 2020, 99, e21327. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, G.; Wang, Z.; Sun, M.; Cao, M.; Zhu, Z.; Fu, Q.; Mao, J.; Shi, Y.; Yang, T. Visceral adiposity index may be a surrogate marker for the assessment of the effects of obesity on arterial stiffness. PLoS ONE 2014, 9, e104365. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Amato, M.C.; Di Marco, V.; Cammà, C.; Pizzolanti, G.; Barcellona, M.R.; Cabibi, D.; Galluzzo, A.; Sinagra, D.; Giordano, C.; et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2012, 35, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Bamba, R.; Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. The Visceral Adiposity Index Is a Predictor of Incident Chronic Kidney Disease: A Population-Based Longitudinal Study. Kidney Blood Press Res. 2020, 45, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Xiong, C.; Shao, X.; Gao, P.; Chen, H.; Ning, J.; Chen, Y.; Zou, Z.; Hong, G.; Li, X.; et al. Visceral adiposity index and chronic kidney disease in a non-diabetic population: A cross-sectional study. Diabetes Metab. Syndr. Obes. 2020, 13, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lai, S.-H.; Tsai, Y.-W.; Chang, S.-S. Visceral adiposity index as a predictor of chronic kidney disease in a relatively healthy population in Taiwan. J. Ren. Nutr. 2018, 28, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Zhao, Z.; Zhu, S.; Liu, X.; Zhou, C.; Shao, X.; Liang, Y.; Duan, C.; Holthöfer, H.; et al. Correlation of visceral adiposity index with chronic kidney disease in the people’s republic of china: To rediscover the new clinical potential of an old indicator for visceral obesity. Ther. Clin. Risk Manag. 2016, 12, 489–494. [Google Scholar] [CrossRef]
- Kim, B.; Kim, G.; Kim, E.; Park, J.; Isobe, T.; Sakae, T.; Oh, S. The a body shape index might be a stronger predictor of chronic kidney disease than BMI in a senior population. Int. J. Environ. Res. Public Health 2021, 18, 12874. [Google Scholar] [CrossRef] [PubMed]
- Bawadi, H.; Abouwatfa, M.; Alsaeed, S.; Kerkadi, A.; Shi, Z. Body shape index is a stronger predictor of diabetes. Nutrients 2019, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shimada, H.; Park, H.; Makizako, H.; Lee, S.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Suzuki, T. The association between kidney function and cognitive decline in community-dwelling, elderly Japanese people. J. Am. Med. Dir. Assoc. 2015, 16, 349.e1–349.e5. [Google Scholar] [CrossRef]
- Kim, B.; Ku, M.; Kiyoji, T.; Isobe, T.; Sakae, T.; Oh, S. Cardiorespiratory fitness is strongly linked to metabolic syndrome among physical fitness components: A retrospective cross-sectional study. J. Physiol. Anthropol. 2020, 39, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, G.; Kim, E.; Park, J.; Isobe, T.; Mori, Y.; Oh, S. The anthropometric measure ‘a body shape index’ may predict the risk of osteoporosis in middle-aged and older Korean people. Int. J. Environ. Res. Public Health 2022, 19, 4926. [Google Scholar] [CrossRef]
- Jackson, A.S.; Janssen, I.; Sui, X.; Church, T.S.; Blair, S.N. Longitudinal Changes in Body Composition Associated with Healthy Ageing: Men, Aged 20–96 Years. Br. J. Nutr. 2012, 107, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef]
- Batra, A.; Siegmund, B. The role of visceral fat. Dig Dis. 2012, 30, 70–74. [Google Scholar] [CrossRef]
- Poulos, S.P.; Hausman, D.B.; Hausman, G.J. The development and endocrine functions of adipose tissue. Mol. Cell Endocrinol. 2010, 323, 20–34. [Google Scholar] [CrossRef]
- Ragino, Y.I.; Stakhneva, E.M.; Polonskaya, Y.V.; Kashtanova, E.V. The role of secretory activity molecules of visceral adipocytes in abdominal obesity in the development of cardiovascular disease: A review. Biomolecules 2020, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Wahba, I.M.; Mak, R.H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Bagby, S.P. Obesity-initiated metabolic syndrome and the kidney: A recipe for chronic kidney disease? J. Am. Soc. Nephrol. 2004, 15, 2775–2791. [Google Scholar] [CrossRef] [PubMed]
- Schelling, J.R.; Sedor, J.R. The metabolic syndrome as a risk factor for chronic kidney disease: More than a fat chance? J. Am. Soc. Nephrol. 2004, 15, 2773–2774. [Google Scholar] [CrossRef] [PubMed]
- Mousapour, P.; Barzin, M.; Valizadeh, M.; Mahdavi, M.; Azizi, F.; Hosseinpanah, F. Predictive performance of lipid accumulation product and visceral adiposity index for renal function decline in non-diabetic adults, an 8.6-year follow-up. Clin. Exp. Nephrol. 2020, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 7736) | Male (n = 3479) | Female (n = 4257) | p Value | |
|---|---|---|---|---|
| eGFR, mg/dL † | 63.3 ± 19.3 | 73.5 ± 19.8 | 54.9 ± 14.1 | <0.001 |
| Visceral adiposity index † | 4.89 ± 3.47 | 3.87 ± 2.84 | 5.73 ± 3.71 | <0.001 |
| Body mass index, kg/m2 † | 24.23 ± 3.14 | 24.20 ± 3.22 | 24.25 ± 3.07 | =0.266 |
| Age, year † | 69.7 ± 6.4 | 69.7 ± 6.3 | 69.8 ± 6.5 | =0.741 |
| Height, cm | 158.9 ± 8.8 | 159.0 ± 8.8 | 158.9 ± 8.7 | =0.478 |
| Body weight, kg † | 61.3 ± 10.0 | 61.3 ± 10.3 | 61.3 ± 9.8 | =0.882 |
| Waist circumstance, cm † | 85.6 ± 8.9 | 85.6 ± 9.1 | 85.6 ± 8.8 | =0.625 |
| Creatinine, mg/dL † | 0.85 ± 0.31 | 0.86 ± 0.33 | 0.84 ± 0.29 | =0.053 |
| Triglyceride, mg/dL † | 131.2 ± 69.1 | 132.4 ± 74.3 | 130.2 ± 64.5 | =0.543 |
| HDLC, mg/dL | 49.1 ± 11.9 | 48.9 ± 11.9 | 49.2 ± 11.9 | =0.215 |
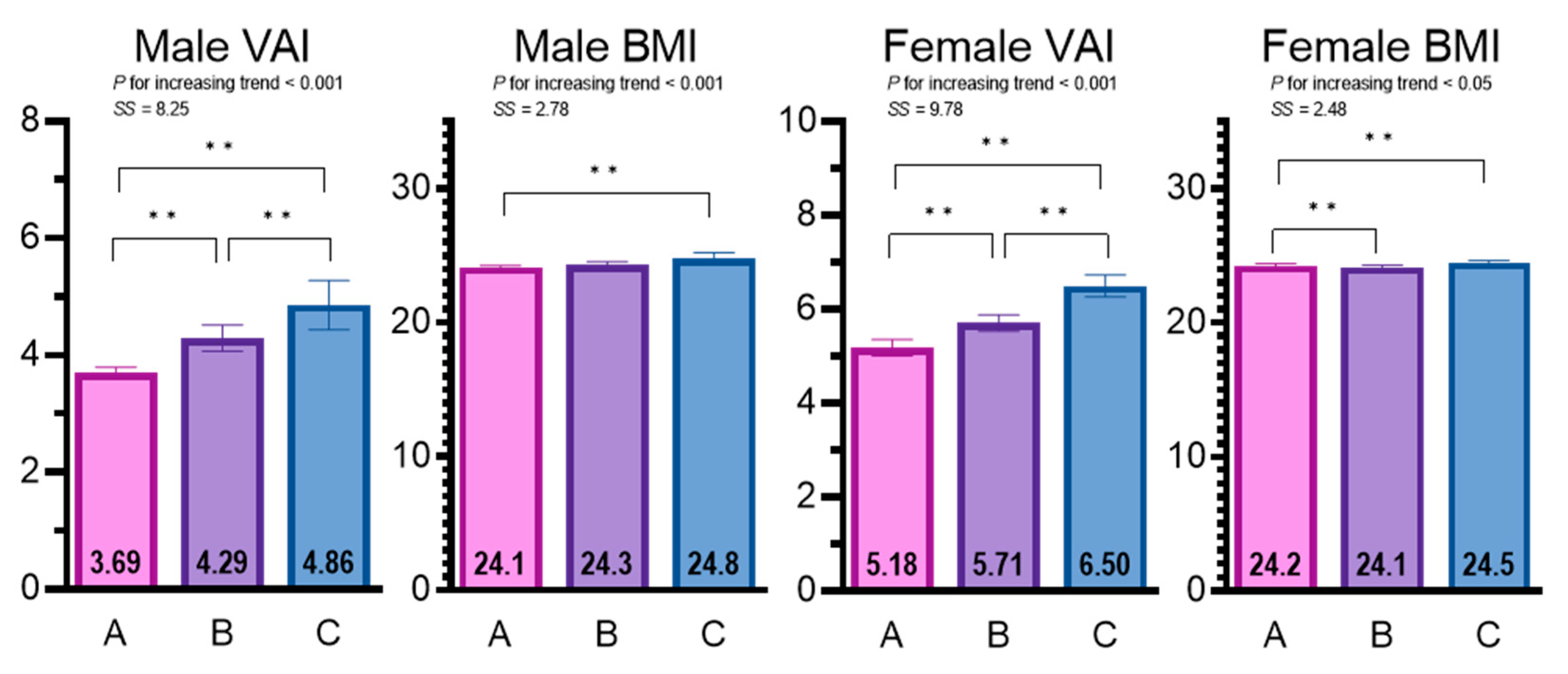
| eGFR Category (mL/min/1.73 m2) | p for Difference | SS ‡ | p for Trend ‡ | |||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| Male, n | 2594 | 690 | 195 | |||
| eGFR, mg/dL † | 81.5 ± 15.8 (80.8, 82.1) | 54.1 ± 4.2 (53.8, 54.4) | 36.6 ± 8.4 (35.4, 37.8) | A > B > C | −46.75 | <0.001 |
| Z score of VAI † | −0.06 ± 0.97 (−0.10, −0.03) | 0.15 ± 1.05 (0.07, 0.23) | 0.35 ± 1.05 (0.20, 0.50) | A < B < C | 8.25 | <0.001 |
| Z score of BMI | −0.02 ± 1.01 (−0.06, 0.02) | 0.03 ± 0.97 (−0.04, 0.10) | 0.18 ± 0.98 (0.04, 0.31) | A < C | 2.78 | <0.01 |
| Age, year | 69.4 ± 6.3 (69.2, 69.7) | 70.5 ± 6.2 (70.0, 71.0) | 70.6 ± 6.6 (69.7, 71.5) | A < B < C | 4.47 | <0.001 |
| Height, cm † | 157.2 ± 8.3 (156.9, 157.6) | 164.5 ± 8.1 (163.9, 165.1) | 162.9 ± 8.2 (161.7, 164.0) | A < B, A < C, B > C | 20.19 | <0.001 |
| Body mass, kg | 59.8 ± 9.9 (59.4, 60.1) | 65.9 ± 10.3 (65.1, 66.6) | 65.8 ± 10.3 (64.4, 67.3) | A < B, C | 15.37 | <0.001 |
| WC, cm | 84.7 ± 9.1 (84.3, 85.0) | 87.7 ± 8.6 (87.1, 88.3) | 89.8 ± 8.8 (88.6, 91.1) | A < B < C | 10.60 | <0.001 |
| Cre, mg/dL † | 0.75 ± 0.12 (0.74, 0.75) | 1.06 ± 0.08 (1.05, 1.07) | 1.67 ± 0.85 (1.55, 1.79) | A < B < C | 46.52 | <0.001 |
| TG, mg/dL | 130.5 ± 75.1 (127.6, 133.4) | 137.0 ± 73.9 (131.5, 142.5) | 142.0 ± 62.8 (133.1, 150.9) | NS | 4.15 | <0.001 |
| HDLC, mg/dL | 50.0 ± 12.0 (49.6, 50.5) | 46.3 ± 11.3 (45.5, 47.2) | 43.1 ± 10.5 (41.6, 44.6) | A < B < C | −10.08 | <0.001 |
| Female, n | 1482 | 1677 | 1098 | |||
| eGFR, mg/dL † | 70.2 ± 8.6 (69.8, 70.7) | 52.3 ± 4.3 (52.1, 52.5) | 38.2 ± 5.8 (37.8, 38.5) | A > B > C | −68.56 | <0.001 |
| Z score of VAI † | −0.15 ± 0.93 (−0.20, −0.10) | −0.01 ± 0.99 (−0.05, 0.04) | 0.21 ± 1.07 (0.14, 0.27) | A < B < C | 9.78 | <0.001 |
| Z score of BMI † | −0.01 ± 1.04 (−0.06, 0.04) | −0.04 ± 1.00 (−0.09, 0.01) | 0.07 ± 0.94 (0.02, 0.13) | A, B < C | 2.48 | <0.05 |
| Age, year | 69.3 ± 6.4 (69.0, 69.6) | 70.0 ± 6.5 (69.6, 70.3) | 70.1 ± 6.4 (69.8, 70.5) | A < B, C | 3.50 | <0.001 |
| Height, cm † | 154.1 ± 6.5 (153.8, 154.4) | 159.7 ± 8.7 (159.2, 160.1) | 164.1 ± 7.8 (163.6, 164.5) | A < B < C | 29.93 | <0.001 |
| Body mass, kg † | 57.6 ± 8.7 (57.1, 58.0) | 61.6 ± 9.4 (61.1, 62.0) | 66.0 ± 9.6 (65.4, 66.5) | A < B < C | 21.91 | <0.001 |
| WC, cm | 83.9 ± 8.7 (83.4, 84.3) | 85.4 ± 8.6 (85.0, 85.8) | 88.3 ± 8.5 (87.8, 88.8) | A < B < C | 12.81 | <0.001 |
| Cre, mg/dL † | 0.64 ± 0.06 (0.64, 0.64) | 0.83 ± 0.07 (0.83, 0.83) | 1.14 ± 0.41 (1.12, 1.17) | A < B < C | 68.08 | <0.001 |
| TG, mg/dL | 126.4 ± 64.3 (123.1, 129.6) | 130.2 ± 64.5 (127.1, 133.3) | 135.3 ± 64.5 (131.5, 139.1) | A < C | 4.21 | <0.001 |
| HDLC, mg/dL | 51.6 ± 11.9 (51.0, 52.2) | 49.2 ± 11.8 (48.6, 49.8) | 46.0 ± 11.3 (45.3, 46.6) | A > B > C | −12.55 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Kim, G.-M.; Oh, S. Use of the Visceral Adiposity Index as an Indicator of Chronic Kidney Disease in Older Adults: Comparison with Body Mass Index. J. Clin. Med. 2022, 11, 6297. https://doi.org/10.3390/jcm11216297
Kim B, Kim G-M, Oh S. Use of the Visceral Adiposity Index as an Indicator of Chronic Kidney Disease in Older Adults: Comparison with Body Mass Index. Journal of Clinical Medicine. 2022; 11(21):6297. https://doi.org/10.3390/jcm11216297
Chicago/Turabian StyleKim, Bokun, Gwon-Min Kim, and Sechang Oh. 2022. "Use of the Visceral Adiposity Index as an Indicator of Chronic Kidney Disease in Older Adults: Comparison with Body Mass Index" Journal of Clinical Medicine 11, no. 21: 6297. https://doi.org/10.3390/jcm11216297
APA StyleKim, B., Kim, G.-M., & Oh, S. (2022). Use of the Visceral Adiposity Index as an Indicator of Chronic Kidney Disease in Older Adults: Comparison with Body Mass Index. Journal of Clinical Medicine, 11(21), 6297. https://doi.org/10.3390/jcm11216297






