The Dawn of a New Era in Atopic Dermatitis Treatment
Abstract
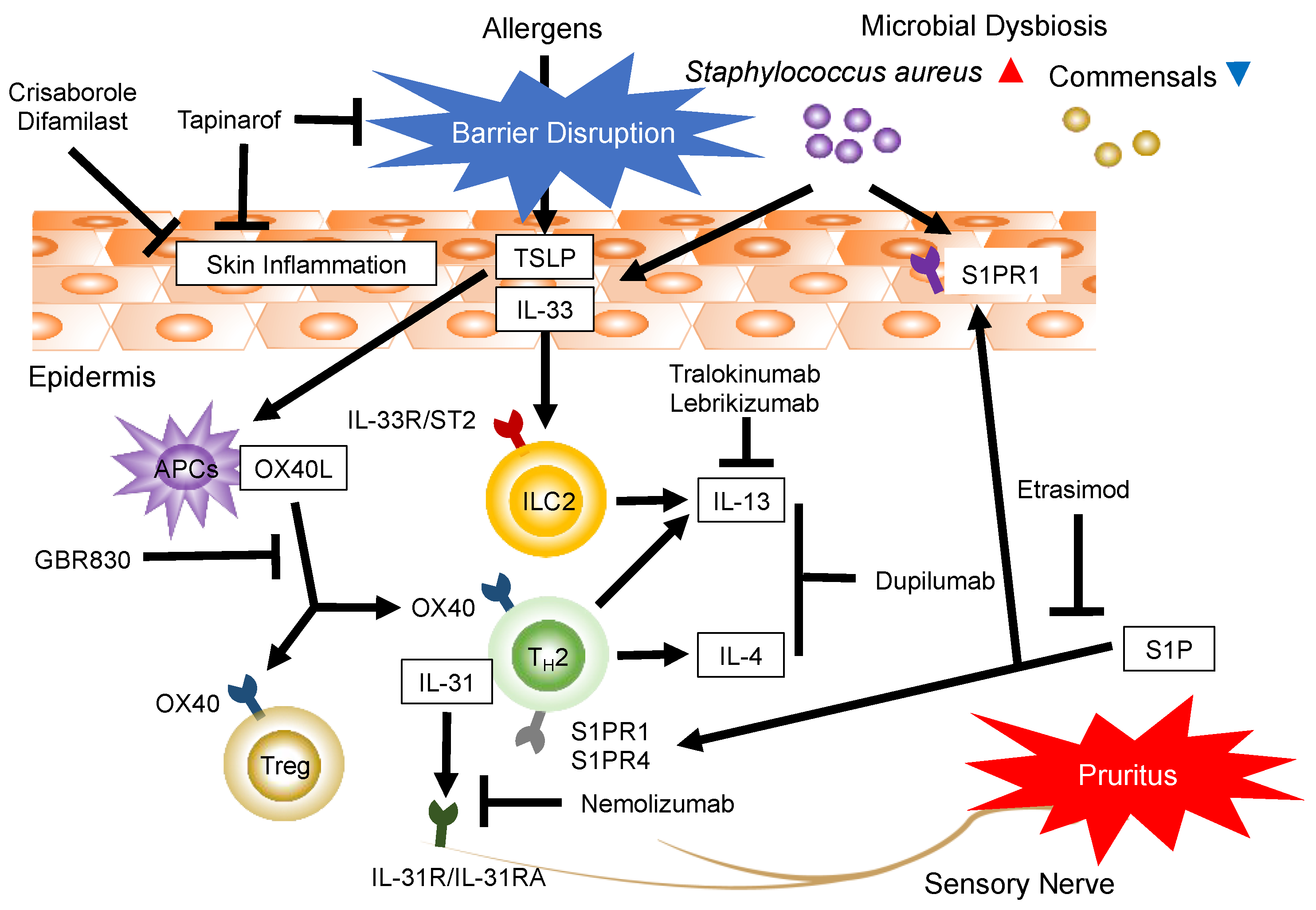
1. Introduction
2. Emerging Systemic/Topical Agents
3. Monoclonal Antibodies for Moderate-to-Severe AD
3.1. Targeting Th2 and Th2-Associated Cytokines: IL-4, IL-13, IL-33, Thymic Stromal Lymphopoietin, and OX40
3.2. Targeting Pruritus and the Th2-Associated Cytokine IL-31
3.3. Targeting Th17-Associated Cytokine IL-17
4. Targeting Immunomodulatory Effects and Sphingosine 1-Phosphate (S1P) Receptors (S1PRs)
5. Small-Molecule Inhibitors
5.1. Janus Kinase (JAK) Inhibitors
5.2. PDE4 Inhibitors
6. AhR-Modulating Agent
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Skin barrier and immune dysregulation in atopic dermatitis: An evolving story with important clinical implications. J. Allergy Clin. Immunol. Pract. 2014, 2, 371–379, quiz 380–371. [Google Scholar] [CrossRef] [PubMed]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Silverberg, J.I. Epidemiology of childhood atopic dermatitis. Clin. Dermatol. 2015, 33, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Chiesa Fuxench, Z.C.; Block, J.K.; Boguniewicz, M.; Boyle, J.; Fonacier, L.; Gelfand, J.M.; Grayson, M.H.; Margolis, D.J.; Mitchell, L.; Silverberg, J.I.; et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J. Investig. Dermatol. 2019, 139, 583–590. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Reed, M.L. A population-based survey of eczema prevalence in the United States. Dermatitis 2007, 18, 82–91. [Google Scholar] [CrossRef]
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Del Duca, E.; Diaz, A.; Gay-Mimbrera, J.; Zhang, N.; Wu, J.; Beaziz, J.; Estrada, Y.; Krueger, J.G.; Pavel, A.B.; et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J. Allergy Clin. Immunol. 2020, 147, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Gonzalez, J.; Shemer, A.; Malajian, D.; Xu, H.; Zheng, X.; Khattri, S.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suárez-Fariñas, M.; et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J. Allergy Clin. Immunol. 2015, 136, 104–115.e7. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Gonzalez, J.; Bonifacio, K.M.; Shemer, A.; Xiangyu, P.; Kunjravia, N.; Malajian, D.; Fuentes-Duculan, J.; Esaki, H.; Noda, S.; et al. Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J. Allergy Clin. Immunol. 2016, 137, 118–129.e5. [Google Scholar] [CrossRef]
- Brunner, P.M.; Suárez-Fariñas, M.; He, H.; Malik, K.; Wen, H.C.; Gonzalez, J.; Chan, T.C.; Estrada, Y.; Zheng, X.; Khattri, S.; et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci. Rep. 2017, 7, 8707. [Google Scholar] [CrossRef]
- Thijs, J.L.; Strickland, I.; Bruijnzeel-Koomen, C.A.F.M.; Nierkens, S.; Giovannone, B.; Knol, E.F.; Csomor, E.; Sellman, B.R.; Mustelin, T.; Sleeman, M.A.; et al. Serum biomarker profiles suggest that atopic dermatitis is a systemic disease. J. Allergy Clin. Immunol. 2018, 141, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Pavel, A.B.; Zhou, L.; Diaz, A.; Ungar, B.; Dan, J.; He, H.; Estrada, Y.D.; Xu, H.; Fernandes, M.; Renert-Yuval, Y.; et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J. Am. Acad. Dermatol. 2020, 82, 690–699. [Google Scholar] [CrossRef]
- He, H.; Li, R.; Choi, S.; Zhou, L.; Pavel, A.; Estrada, Y.D.; Krueger, J.G.; Guttman-Yassky, E. Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis. Ann. Allergy Asthma Immunol. 2020, 124, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Wichmann, K.; Bruder, M.; Ständer, S.; Wedi, B.; Kapp, A.; Werfel, T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008, 122, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Imamura, T.; Yokota, D.; Tsubouchi, H. Difamilast Ointment in Japanese Adult and Pediatric Patients with Atopic Dermatitis: A Phase III, Long-Term, Open-Label Study. Dermatol. Ther. 2022, 12, 1589–1601. [Google Scholar] [CrossRef]
- Saeki, H.; Baba, N.; Ito, K.; Yokota, D.; Tsubouchi, H. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: A phase III randomized double-blind, vehicle-controlled trial. Br. J. Dermatol. 2022, 186, 40–49. [Google Scholar] [CrossRef]
- Paller, A.S.; Stein Gold, L.; Soung, J.; Tallman, A.M.; Rubenstein, D.S.; Gooderham, M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021, 84, 632–638. [Google Scholar] [CrossRef]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Freitas, E.; Guttman-Yassky, E.; Torres, T. Tralokinumab for the Treatment of Atopic Dermatitis. Am. J. Clin. Dermatol. 2021, 22, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults with Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, eaax2945. [Google Scholar] [CrossRef]
- van de Veen, W.; Akdis, M. The use of biologics for immune modulation in allergic disease. J. Clin. Investig. 2019, 129, 1452–1462. [Google Scholar] [CrossRef]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Pavel, A.B.; Zhou, L.; Zhou, L.; Estrada, Y.D.; Zhang, N.; Xu, H.; Peng, X.; Wen, H.C.; Govas, P.; et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 482–493.e7. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Ungar, B.; Pavel, A.B.; Li, R.; Kimmel, G.; Nia, J.; Hashim, P.; Kim, H.J.; Chima, M.; Vekaria, A.S.; Estrada, Y.; et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 394–397. [Google Scholar] [CrossRef]
- Simpson, E.L.; Lacour, J.P.; Spelman, L.; Galimberti, R.; Eichenfield, L.F.; Bissonnette, R.; King, B.A.; Thyssen, J.P.; Silverberg, J.I.; Bieber, T.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaç, D.; Chu, C.-Y.; Hong, H.C.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021, 397, 2151–2168. [Google Scholar] [CrossRef]
- Samrao, A.; Berry, T.M.; Goreshi, R.; Simpson, E.L. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch. Dermatol. 2012, 148, 890–897. [Google Scholar] [CrossRef]
- Kim, B.S.; Howell, M.D.; Sun, K.; Papp, K.; Nasir, A.; Kuligowski, M.E. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J. Allergy Clin. Immunol. 2020, 145, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Sun, K.; Papp, K.; Venturanza, M.; Nasir, A.; Kuligowski, M.E. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: Results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J. Am. Acad. Dermatol. 2020, 82, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kabashima, K.; Oda, M.; Nagata, T. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J. Am. Acad. Dermatol. 2021, 85, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Murata, R.; Kaino, H.; Nagata, T. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J. Dermatol. 2020, 47, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e496. [Google Scholar] [CrossRef]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Fonacier, L.; Guttman-Yassky, E.; Ong, P.Y.; Silverberg, J.; Farrar, J.R. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann. Allergy Asthma Immunol. 2018, 120, 10–22.e2. [Google Scholar] [CrossRef]
- Choy, D.F.; Hsu, D.K.; Seshasayee, D.; Fung, M.A.; Modrusan, Z.; Martin, F.; Liu, F.T.; Arron, J.R. Comparative transcriptomic analyses of atopic dermatitis and psoriasis reveal shared neutrophilic inflammation. J. Allergy Clin. Immunol. 2012, 130, 1335–1343.e5. [Google Scholar] [CrossRef]
- Tazawa, T.; Sugiura, H.; Sugiura, Y.; Uehara, M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch. Dermatol. Res. 2004, 295, 459–464. [Google Scholar] [CrossRef]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef] [PubMed]
- Roediger, B.; Kyle, R.; Le Gros, G.; Weninger, W. Dermal group 2 innate lymphoid cells in atopic dermatitis and allergy. Curr. Opin. Immunol. 2014, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Hoyler, T.; Klose, C.S.; Souabni, A.; Turqueti-Neves, A.; Pfeifer, D.; Rawlins, E.L.; Voehringer, D.; Busslinger, M.; Diefenbach, A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 2012, 37, 634–648. [Google Scholar] [CrossRef]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef]
- Price, A.E.; Liang, H.E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 11489–11494. [Google Scholar] [CrossRef]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Liu, Y.J. Thymic stromal lymphopoietin: Master switch for allergic inflammation. J. Exp. Med. 2006, 203, 269–273. [Google Scholar] [CrossRef]
- Liu, Y.J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J. Allergy Clin. Immunol. 2007, 120, 238–244, quiz 245–236. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Stüber, E.; Neurath, M.; Calderhead, D.; Fell, H.P.; Strober, W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity 1995, 2, 507–521. [Google Scholar] [CrossRef]
- Sato, T.; Ishii, N.; Murata, K.; Kikuchi, K.; Nakagawa, S.; Ndhlovu, L.C.; Sugamura, K. Consequences of OX40-OX40 ligand interactions in langerhans cell function: Enhanced contact hypersensitivity responses in OX40L-transgenic mice. Eur. J. Immunol. 2002, 32, 3326–3335. [Google Scholar] [CrossRef]
- Ohshima, Y.; Tanaka, Y.; Tozawa, H.; Takahashi, Y.; Maliszewski, C.; Delespesse, G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997, 159, 3838–3848. [Google Scholar]
- Sugamura, K.; Ishii, N.; Weinberg, A.D. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat. Rev. Immunol. 2004, 4, 420–431. [Google Scholar] [CrossRef]
- Gramaglia, I.; Jember, A.; Pippig, S.D.; Weinberg, A.D.; Killeen, N.; Croft, M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 2000, 165, 3043–3050. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Weinberg, A.; Prell, R.A.; Vella, A.T. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J. Immunol. 2000, 164, 107–112. [Google Scholar] [CrossRef]
- Rogers, P.R.; Song, J.; Gramaglia, I.; Killeen, N.; Croft, M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 2001, 15, 445–455. [Google Scholar] [CrossRef]
- Chen, A.I.; McAdam, A.J.; Buhlmann, J.E.; Scott, S.; Lupher, M.L., Jr.; Greenfield, E.A.; Baum, P.R.; Fanslow, W.C.; Calderhead, D.M.; Freeman, G.J.; et al. Ox40-ligand has a critical costimulatory role in dendritic cell: T cell interactions. Immunity 1999, 11, 689–698. [Google Scholar] [CrossRef]
- Murata, K.; Ishii, N.; Takano, H.; Miura, S.; Ndhlovu, L.C.; Nose, M.; Noda, T.; Sugamura, K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000, 191, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Bilsborough, J.; Leung, D.Y.; Maurer, M.; Howell, M.; Boguniewicz, M.; Yao, L.; Storey, H.; LeCiel, C.; Harder, B.; Gross, J.A. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2006, 117, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, C.; Lüscher-Firzlaff, J.; Baron, J.M.; Lüscher, B. Signaling by IL-31 and functional consequences. Eur. J. Cell Biol. 2012, 91, 552–566. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Raap, U.; Gehring, M.; Kleiner, S.; Rüdrich, U.; Eiz-Vesper, B.; Haas, H.; Kapp, A.; Gibbs, B.F. Human basophils are a source of-and are differentially activated by-IL-31. Clin. Exp. Allergy 2017, 47, 499–508. [Google Scholar] [CrossRef]
- Kato, A.; Fujii, E.; Watanabe, T.; Takashima, Y.; Matsushita, H.; Furuhashi, T.; Morita, A. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J. Dermatol. Sci. 2014, 74, 229–235. [Google Scholar] [CrossRef]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Yamamura, K.; Uruno, T.; Shiraishi, A.; Tanaka, Y.; Ushijima, M.; Nakahara, T.; Watanabe, M.; Kido-Nakahara, M.; Tsuge, I.; Furue, M.; et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat. Commun. 2017, 8, 13946. [Google Scholar] [CrossRef]
- Kamikaseda, Y.; Uruno, T.; Kunimura, K.; Harada, A.; Saiki, K.; Oisaki, K.; Sakata, D.; Nakahara, T.; Kido-Nakahara, M.; Kanai, M.; et al. Targeted inhibition of EPAS1-driven IL-31 production by a small-molecule compound. J. Allergy Clin. Immunol. 2021, 148, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.R.; Gertz, M.E.; Keles, S.; Schäffer, A.A.; Sigmund, E.C.; Glocker, C.; Saghafi, S.; Pourpak, Z.; Ceja, R.; Sassi, A.; et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J. Allergy Clin. Immunol. 2015, 136, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.F.; Holland, S.M. Clinical manifestations of hyper IgE syndromes. Dis. Markers 2010, 29, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.F.; Freeman, A.F.; Engelhardt, K.R.; Holland, S.; Puck, J.M.; Grimbacher, B. An update on the hyper-IgE syndromes. Arthritis Res. Ther. 2012, 14, 228. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, J.C.; Lamborn, I.T.; Freeman, A.F.; Jing, H.; Favreau, A.J.; Matthews, H.F.; Davis, J.; Turner, M.L.; Uzel, G.; et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 2009, 361, 2046–2055. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, J.C.; Dove, C.G.; Su, H.C. Genetic, clinical, and laboratory markers for DOCK8 immunodeficiency syndrome. Dis. Markers 2010, 29, 131–139. [Google Scholar]
- Kunimura, K.; Yamamura, K.; Nakahara, T.; Kido-Nakahara, M.; Uruno, T.; Fukui, Y. Identification of a functional DOCK8 gene polymorphism associated with atopic dermatitis. Allergy 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73. [Google Scholar] [CrossRef]
- Toda, M.; Leung, D.Y.; Molet, S.; Boguniewicz, M.; Taha, R.; Christodoulopoulos, P.; Fukuda, T.; Elias, J.A.; Hamid, Q.A. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 2003, 111, 875–881. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Coant, N.; Sakamoto, W.; Mao, C.; Hannun, Y.A. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv. Biol. Regul. 2017, 63, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Nema, R.; Vishwakarma, S.; Agarwal, R.; Panday, R.K.; Kumar, A. Emerging role of sphingosine-1-phosphate signaling in head and neck squamous cell carcinoma. Onco Targets Ther. 2016, 9, 3269–3280. [Google Scholar]
- Henkel, F.D.R.; Friedl, A.; Haid, M.; Thomas, D.; Bouchery, T.; Haimerl, P.; de Los Reyes Jiménez, M.; Alessandrini, F.; Schmidt-Weber, C.B.; Harris, N.L.; et al. House dust mite drives proinflammatory eicosanoid reprogramming and macrophage effector functions. Allergy 2019, 74, 1090–1101. [Google Scholar]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef]
- Sakai, T.; Herrmann, N.; Maintz, L.; Nümm, T.J.; Welchowski, T.; Claus, R.A.; Gräler, M.H.; Bieber, T. Serum sphingosine-1-phosphate is elevated in atopic dermatitis and associated with severity. Allergy 2021, 76, 2592–2595. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105. [Google Scholar] [CrossRef]
- Igawa, S.; Choi, J.E.; Wang, Z.; Chang, Y.L.; Wu, C.C.; Werbel, T.; Ishida-Yamamoto, A.; Di Nardo, A. Human Keratinocytes Use Sphingosine 1-Phosphate and its Receptors to Communicate Staphylococcus aureus Invasion and Activate Host Defense. J. Investig. Dermatol. 2019, 139, 1743–1752.e5. [Google Scholar] [CrossRef]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef]
- Hill, R.Z.; Morita, T.; Brem, R.B.; Bautista, D.M. S1PR3 Mediates Itch and Pain via Distinct TRP Channel-Dependent Pathways. J. Neurosci. 2018, 38, 7833–7843. [Google Scholar] [CrossRef]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat 2013, 2, e24137. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef]
- Choy, E.H. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology 2019, 58, 953–962. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Torres, T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J. Dermatol. Treat. 2020, 31, 33–40. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Punzón, C.; Navarro, J.; Muñoz-Fernández, M.A.; Fresno, M. Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J. Pharmacol. Exp. Ther. 2001, 299, 753–759. [Google Scholar]
- Bäumer, W.; Hoppmann, J.; Rundfeldt, C.; Kietzmann, M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm. Allergy Drug Targets 2007, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zane, L.T.; Chanda, S.; Jarnagin, K.; Nelson, D.B.; Spelman, L.; Gold, L.S. Crisaborole and its potential role in treating atopic dermatitis: Overview of early clinical studies. Immunotherapy 2016, 8, 853–866. [Google Scholar] [CrossRef]
- Richardson, W.H.; Schmidt, T.M.; Nealson, K.H. Identification of an anthraquinone pigment and a hydroxystilbene antibiotic from Xenorhabdus luminescens. Appl. Environ. Microbiol. 1988, 54, 1602–1605. [Google Scholar] [CrossRef]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed]

| Agent | Target | Study Type | Study Duration | Dose | Reference | |
|---|---|---|---|---|---|---|
| Biologics | Dupilumab | IL-4Ra | Phase 3 | 16 weeks | 300 mg | [18] |
| Tralokinumab | IL-13 | Phase 2b | 16 weeks | 150, 300 mg | [19] | |
| Lebrikizumab | IL-13 | Phase 2b | 16 weeks | 125, 250 mg | [20] | |
| Etokimab | IL-33 | Phase 2a | 20 weeks | 300 mg | [21,22] | |
| Tezepelumab | TSLP | Phase 2a | 12 weeks | 280 mg | [23] | |
| GBR 830 | OX40 | Phase 2a | 16 weeks | 10 mg/kg | [24] | |
| Nemolizumab | IL-31Ra | Phase 3 | 16 weeks | 60 mg | [25] | |
| Secukinumab | IL-17A | Phase 2 | 16 weeks | 300 mg | [26] | |
| Etrasimod | S1PR 1/4/5 | Phase 2 | 16 weeks | 1, 2 mg | NCT04162769 | |
| Small-molecule inhibitors | Baricitinib | JAK 1/2 | Phase 3 | 16 weeks | 1, 2, 4 mg | [27] |
| Abrocitinib | JAK1 | Phase 3 | 12 weeks | 100, 200 mg | [28] | |
| Upadacitinib | JAK1 | Phase 3 | 16 weeks | 15, 30 mg | [29] | |
| Apremilast | PDE4 | Phase 2 | 12 weeks | 30, 40 mg | [30] |
| Agent | Target | Study Type | Study Duration | Dose | Reference |
|---|---|---|---|---|---|
| Ruxolitinib | JAK1/2 | Phase 2 | 12 weeks | 0.15, 0.5, 1.5% | [31,32] |
| Delgocitinib | Pan-JAK | Phase 3 | 28 weeks | 0.25, 0.5% | [33,34,35] |
| Crisaborole | PDE4 | Phase 3 | 4 weeks | 2.00% | [36] |
| Difamilast | PDE4 | Phase 3 | 4, 52 weeks | 0.3, 1.0% | [15,16] |
| Tapinarof | AhR | Phase 2b | 12 weeks | 0.5, 1.0% | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamura, K.; Nakahara, T. The Dawn of a New Era in Atopic Dermatitis Treatment. J. Clin. Med. 2022, 11, 6145. https://doi.org/10.3390/jcm11206145
Yamamura K, Nakahara T. The Dawn of a New Era in Atopic Dermatitis Treatment. Journal of Clinical Medicine. 2022; 11(20):6145. https://doi.org/10.3390/jcm11206145
Chicago/Turabian StyleYamamura, Kazuhiko, and Takeshi Nakahara. 2022. "The Dawn of a New Era in Atopic Dermatitis Treatment" Journal of Clinical Medicine 11, no. 20: 6145. https://doi.org/10.3390/jcm11206145
APA StyleYamamura, K., & Nakahara, T. (2022). The Dawn of a New Era in Atopic Dermatitis Treatment. Journal of Clinical Medicine, 11(20), 6145. https://doi.org/10.3390/jcm11206145





