A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
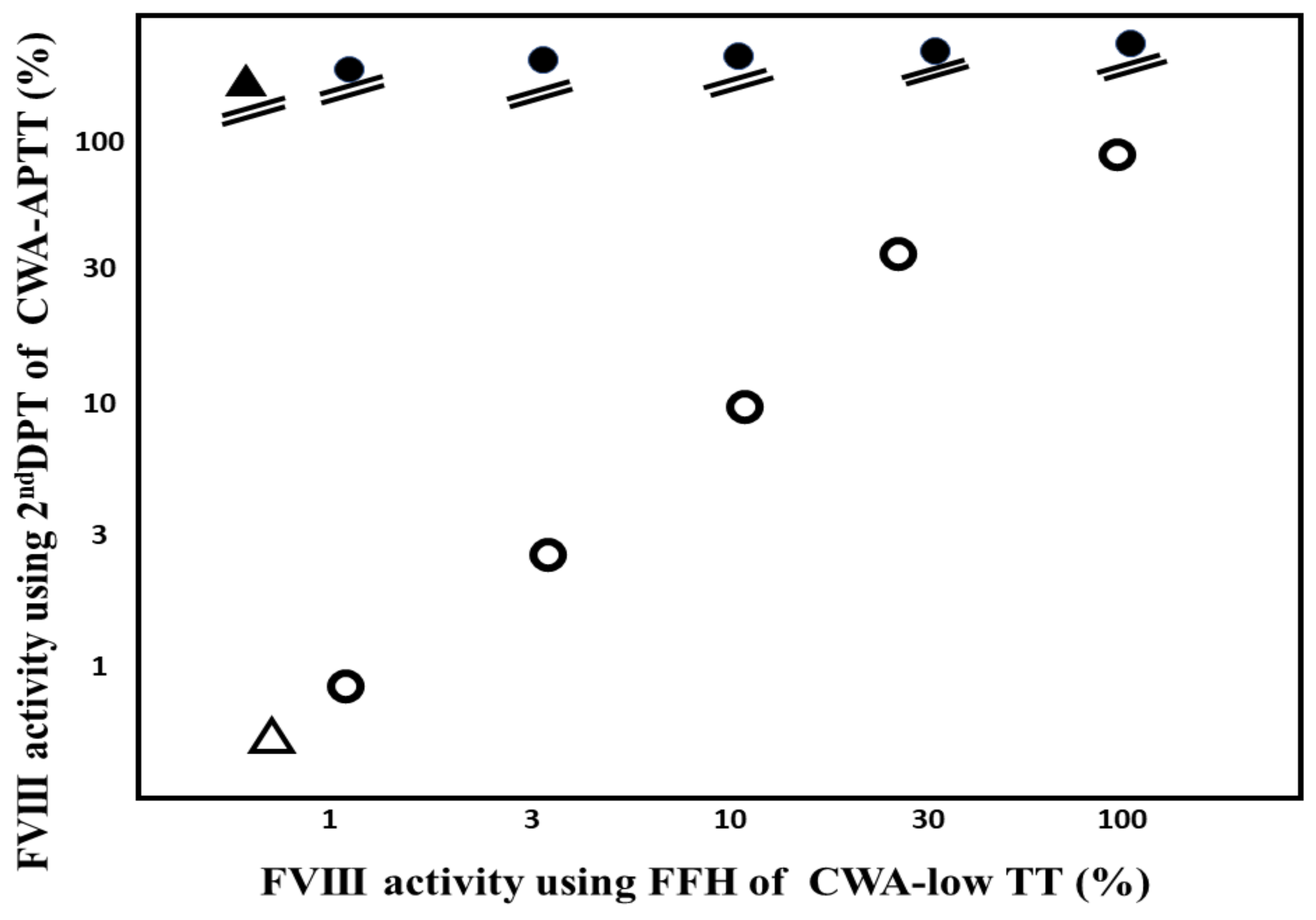
3. Results
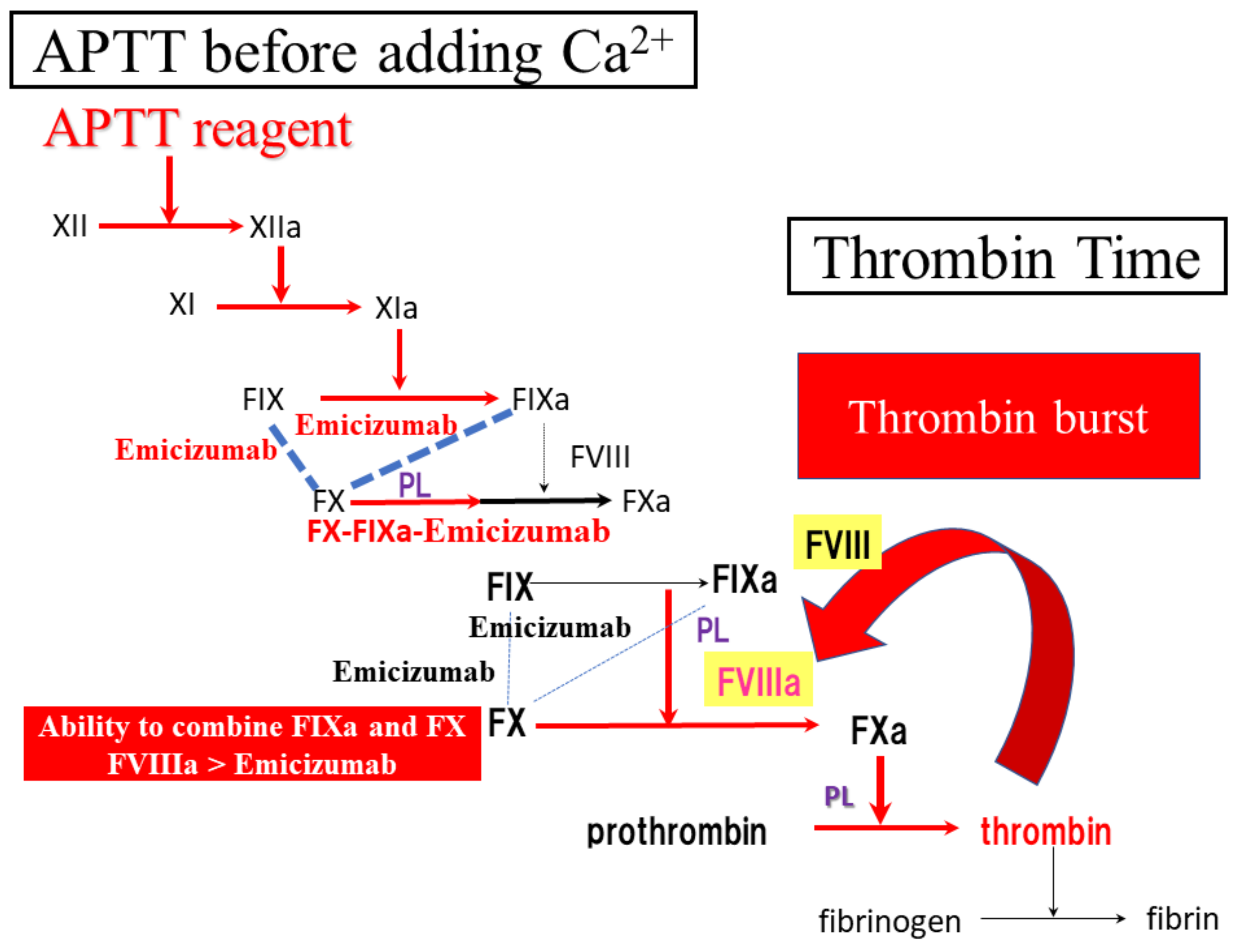
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hay, C.R.M.; Nissen, F.; Pipe, S.W. Mortality in congenital hemophilia A—A systematic literature review. J. Thromb. Haemost. 2021, 19, 6–20. [Google Scholar] [CrossRef] [PubMed]
- National Hemophilia Foundation. MASAC Recommendation Concerning Prophylaxis (Regular Administration of Clotting Factor Concentrate to Prevent Bleeding). Available online: http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=1007 (accessed on 1 June 2022).
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus Episodic Treatment to Prevent Joint Disease in Boys with Severe Hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Germini, F.; Noronha, N.; Philip, B.A.; Olasupo, O.; Pete, D.; Navarro, T.; Keepanasseril, A.; Matino, D.; Wit, K.; Parpia, S.; et al. Risk factors for bleeding in people living with hemophilia A and B treated with regular prophylaxis: A systematic review of the literature. J. Thromb. Haemost. 2022, 20, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Eckhardt, C.L.; van Velzen, A.S.; Vuong, C.; Coppens, M.; Castaman, G.; Hart, D.P.; Hermans, C.; Laros-van Gorkom, B.; Leebeek, F.W.G.; et al. Treatment-related risk factors for inhibitor development in non-severe hemophilia A after 50 cumulative exposure days: A case-control study. J. Thromb. Haemost. 2021, 19, 2171–2181. [Google Scholar] [CrossRef]
- Oldenburg, J.; Mahlangu, J.N.; Kim, B.; Schmitt, C.; Callaghan, M.U.; Young, G.; Santagostino, E.; Kruse-Jarres, R.; Negrier, C.; Kessler, C.; et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N. Engl. J. Med. 2017, 377, 809–818. [Google Scholar] [CrossRef]
- Mahlangu, J.; Oldenburg, J.; Paz-Priel, I.; Negrier, C.; Niggli, M.; Mancuso, M.E.; Schmitt, C.; Jiménez-Yuste, V.; Kempton, C.; Dhalluin, C.; et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N. Engl. J. Med. 2018, 379, 811–822. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nogami, K.; Shima, M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int. J. Hematol. 2017, 105, 174–183. [Google Scholar] [CrossRef]
- Tokutake, T.; Baba, H.; Shimada, Y.; Takeda, W.; Sato, K.; Hiroshima, Y.; Kirihara, T.; Shimizu, I.; Nakazawa, H.; Kobayashi, H.; et al. Exogenous Magnesium Chloride Reduces the Activated Partial Thromboplastin Times of Lupus Anticoagulant-Positive Patients. PLoS ONE 2016, 11, e0157835. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Nishioka, Y.; Nishio, M.; Abe, Y.; Nishioka, J.; Kamikura, Y.; Sase, T.; Kaneko, T.; Houdijk, W.P.M.; et al. Frequency of Abnormal Biphasic aPTT Clot Wave-forms in Patients with Underlying disorders Associated with Disseminated Intravascular Coagulation. Clin. Appl. Thromb. Hemost. 2006, 12, 185–192. [Google Scholar] [CrossRef]
- Byun, J.-H.; Jang, I.-S.; Kim, J.W.; Koh, E.-H. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016, 51, 171–174. [Google Scholar] [CrossRef]
- Bowyer, A.; Kitchen, S.; Maclean, R. Effects of emicizumab on APTT, one-stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia 2020, 26, 536–542. [Google Scholar] [CrossRef]
- Nakajima, Y.; Mizumachi, K.; Shimonishi, N.; Furukawa, S.; Yada, K.; Ogiwara, K.; Takeyama, M.; Shima, M.; Nogami, K. Comparisons of global coagulation potential and bleeding episodes in emicizumab-treated hemophilia A patients and mild hemophilia A patients. Int. J. Hematol. 2022, 115, 489–498. [Google Scholar] [CrossRef]
- Toh, C.H.; Samis, J.; Downey, C.; Walker, J.; Becker, L.; Brufatto, N.; Tejidor, L.; Jones, G.; Houdijk, W.; Giles, A.; et al. Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a Ca(++)-dependent complex of C-reactive protein with very-low-density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood 2002, 100, 2522–2529. [Google Scholar] [CrossRef]
- Shima, M. Understanding the hemostatic effects of recombinant factor VIIa by clot wave form analysis. Semin. Hematol. 2004, 41, 125–131. [Google Scholar] [CrossRef]
- Wada, H.; Ichikawa, Y.; Ezaki, M.; Shiraki, K.; Moritani, I.; Yamashita, Y.; Matsumoto, T.; Masuya, M.; Tawara, I.; Shimpo, H.; et al. Clot Waveform Analysis Demonstrates Low Blood Coagulation Ability in Patients with Idiopathic Thrombocytopenic Purpura. J. Clin. Med. 2021, 10, 5987. [Google Scholar] [CrossRef]
- Wada, H.; Ichikawa, Y.; Ezaki, E.; Matsumoto, T.; Yamashita, Y.; Shiraki, K.; Shimaoka, M.; Shimpo, H. The reevaluation of thrombin time using a clot waveform analysis. J. Clin. Med. 2021, 10, 4840. [Google Scholar] [CrossRef]
- Wada, H.; Matsumoto, T.; Ohishi, K.; Shiraki, K.; Shimaoka, M. Update on the Clot Waveform Analysis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620912027. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Toyoda, H.; Hirayama, M.; Yamashita, Y.; Katayama, N. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab: Comment. J. Thromb. Haemost. 2018, 16, 1665–1666. [Google Scholar] [CrossRef]
- Berntorp, E.; Salvagno, G.L. Standardization and Clinical Utility of Thrombin-Generation Assays. Semin. Thromb. Hemost. 2008, 34, 670–682. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Berntorp, E. Thrombin Generation Testing for Monitoring Hemophilia Treatment: A Clinical Perspective. Semin. Thromb. Hemost. 2010, 36, 780–790. [Google Scholar] [CrossRef]
- Nogami, K.; Soeda, T.; Matsumoto, T.; Kawabe, Y.; Kitazawa, T.; Shima, M. Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti-idiotype monoclonal antibodies. J. Thromb. Haemost. 2018, 16, 1383–1390. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Fujimoto, N.; Toyoda, J.; Abe, Y.; Ohishi, K.; Yamashita, Y.; Ikejiri, M.; Hasegawa, K.; Suzuki, K.; et al. An Evaluation of the Activated Partial Thromboplastin Time Waveform. Clin. Appl. Thromb. 2018, 24, 764–770. [Google Scholar] [CrossRef]
- Young, G.; Liesner, R.; Chang, T.; Sidonio, J.R.; Oldenburg, J.; Jiménez-Yuste, V.; Mahlangu, J.; Kruse-Jarres, R.; Wang, M.; Uguen, M.; et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood 2019, 134, 2127–2138. [Google Scholar] [CrossRef]
- Pipe, S.W.; Shima, M.; Lehle, M.; Shapiro, A.; Chebon, S.; Fukutake, K.; Key, N.S.; Portron, A.; Schmitt, C.; Podolak-Dawidziak, M.; et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): A multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019, 6, e295–e305. [Google Scholar] [CrossRef]
- Callaghan, M.U.; Negrier, C.; Paz-Priel, I.; Chang, T.; Chebon, S.; Lehle, M.; Mahlangu, J.; Young, G.; Kruse-Jarres, R.; Mancuso, M.E.; et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1–4 studies. Blood 2021, 137, 2231–2242. [Google Scholar] [CrossRef]
- Konstantinidi, A.; Sokou, R.; Parastatidou, S.; Lampropoulou, K.; Katsaras, G.; Boutsikou, T.; Gounaris, A.K.; Tsantes, A.E.; Iacovidou, N. Clinical Application of Thromboelastography/Thromboelastometry (TEG/TEM) in the Neonatal Population: A Narrative Review. Semin. Thromb. Hemost. 2019, 45, 449–457. [Google Scholar] [CrossRef]
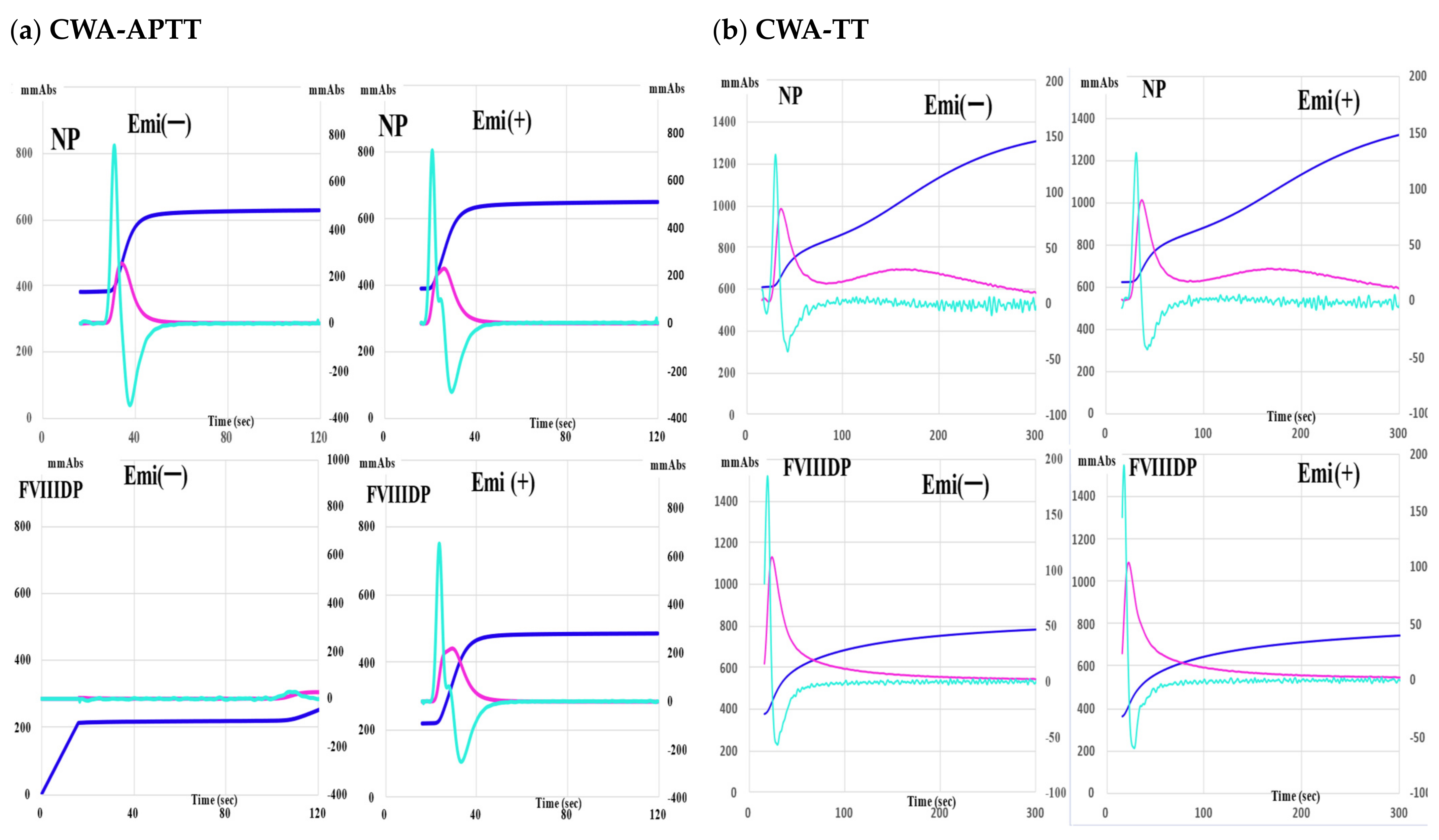

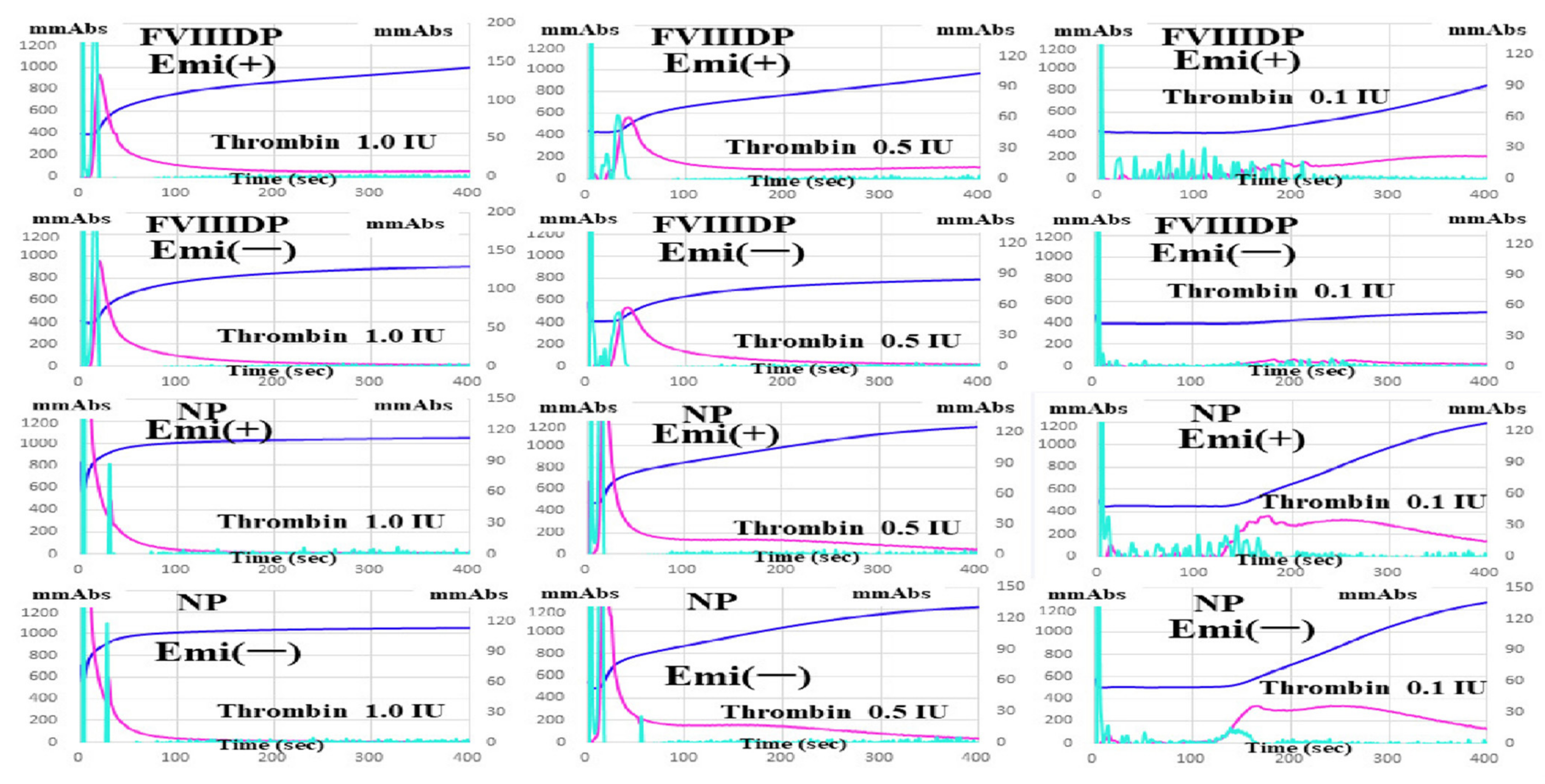
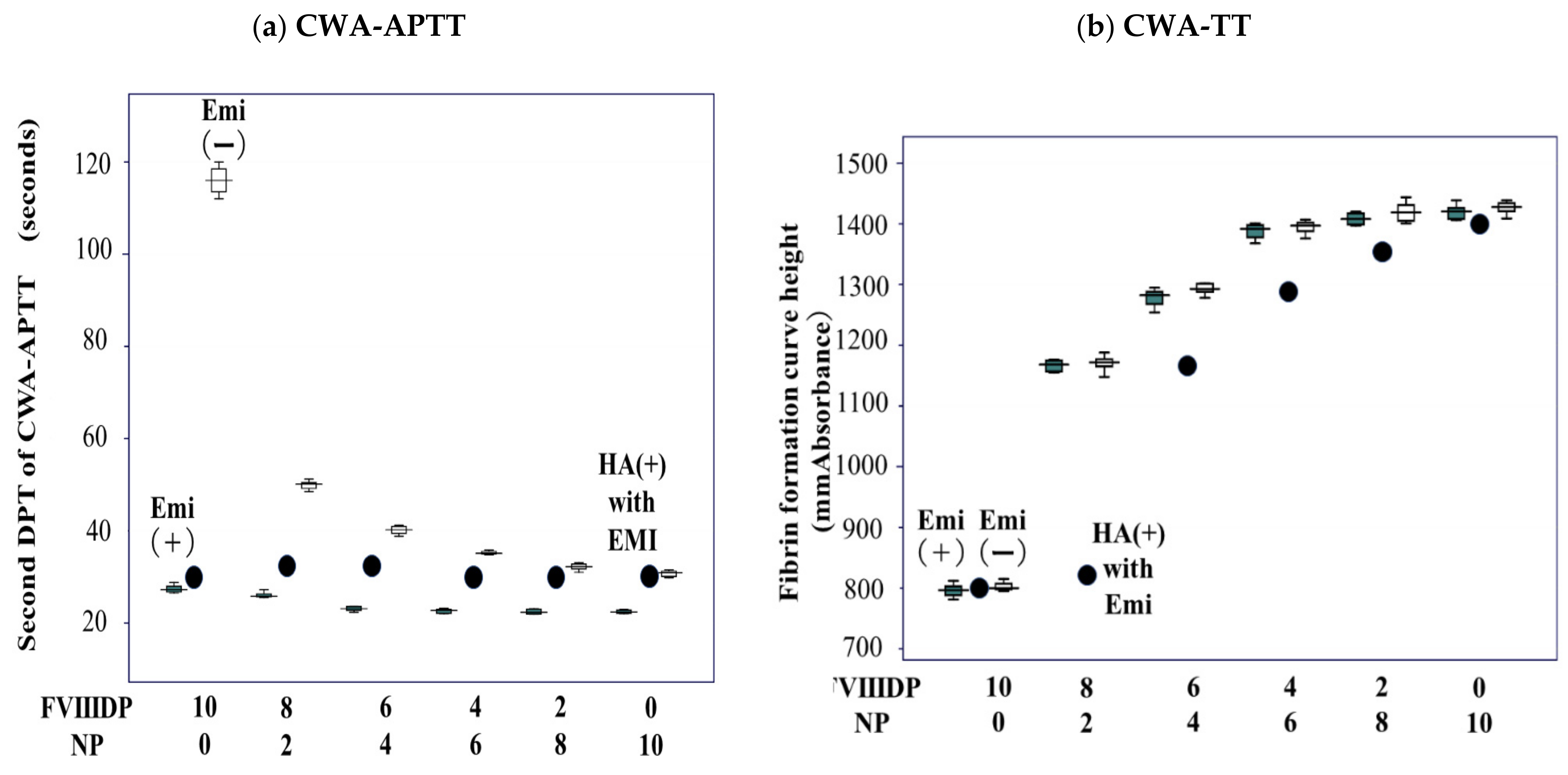
| FVIII-Deficient Plasma | Normal Plasma | |||
|---|---|---|---|---|
| Thrombin Concentration | With Emicizumab | Without Emicizumab | With Emicizumab | Without Emicizumab |
| (a) (mm Absorbance) | (b) (mm Absorbance) | (c) (mm Absorbance) | (d) (mm Absorbance) | |
| 1.0 IU/mL | 993 ± 31 ***### | 957 ± 49 ***### | 1043 ± 38 | 1047 ± 45 |
| 0.5 IU/mL | 893 ± 21 ***### | 776 ± 71 ***### | 1097 ± 25 | 1203 ± 35 |
| 0.1 IU/mL | 812 ± 39 ***### | 502 ± 35 ***### | 1156 ± 49 | 1203 ± 49 |
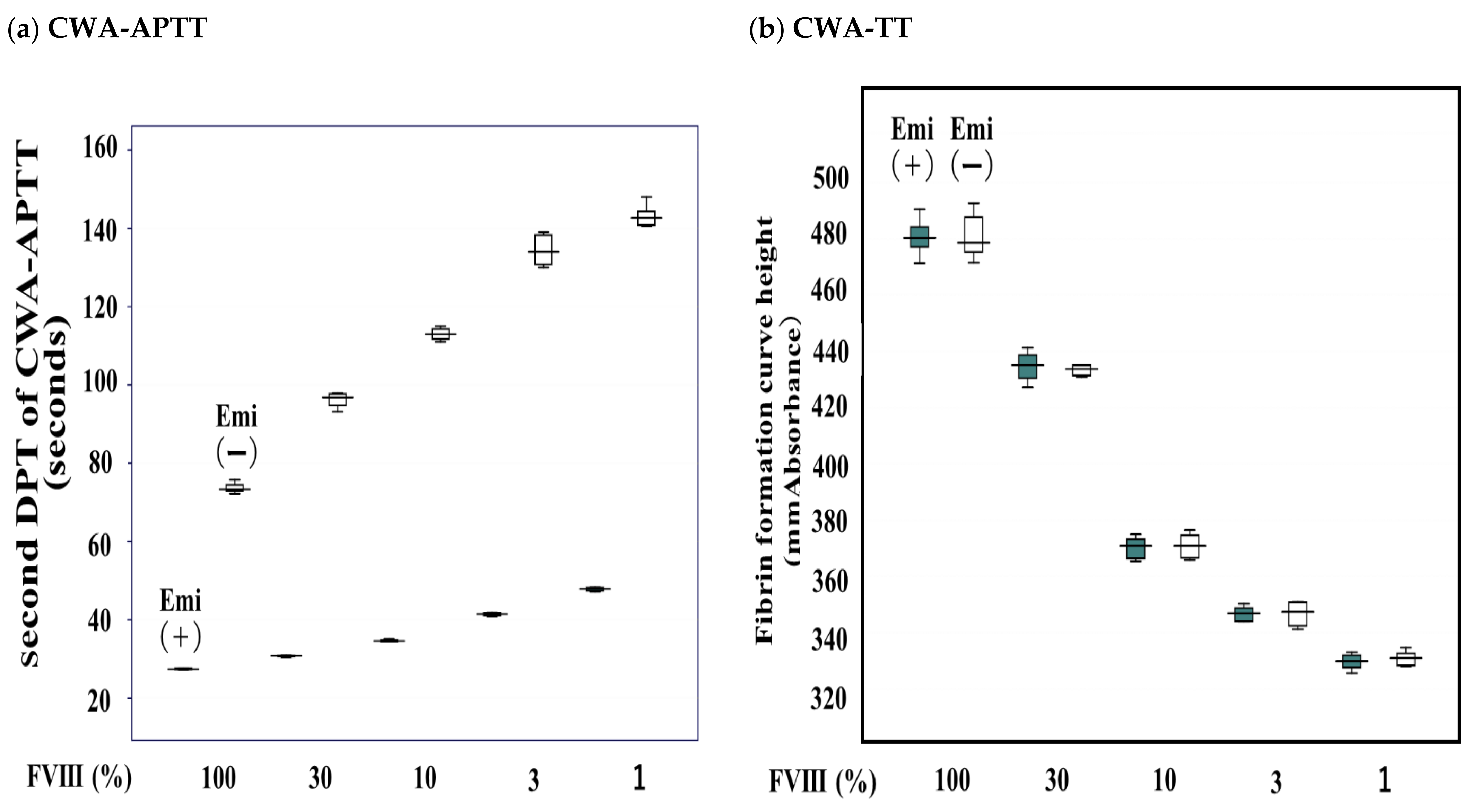
| FVIII Activity | FFH of CWA-TT (mm Absorbance) | FFT of CWA-TT (Second) | 1st DPH of CWA-TT (mm Absorbance) | 1st DPT of CWA-TT (Second) | ||||
|---|---|---|---|---|---|---|---|---|
| Emi (+) | Emi (−) | Emi (+) | Emi (−) | Emi (+) | Emi (−) | Emi (+) | Emi (−) | |
| 100% | 481 ± 6.88 | 434 ± 8.30 | 186 ± 3.37 | 184 ± 1.56 | 52.7 ± 1.47 | 53.7 ± 1.56 | 33.3 ± 1.26 | 32.5 ± 1.52 |
| 30% | 435 ± 5.46 | 433 ± 1.96 | 131 ± 6.57 | 127 ± 6.44 | 50.6 ± 2.85 | 49.4 ± 3.74 | 37.8 ± 2.05 | 35.4 ± 6.30 |
| 10% | 370 ± 4.00 | 371 ± 4.55 | 93.3 ± 4.47 | 90.6 ± 2.43 | 50.8 ± 1.34 | 49.3 ± 2.29 | 33.8 ± 0.91 | 33.6 ± 1.22 |
| 3% | 347 ± 2.71 | 347 ± 4.46 | 80.1 ± 3.02 | 81.2 ± 3.35 | 46.3 ± 3.82 | 47.2 ± 4.63 | 31.1 ± 2.42 | 30.7 ± 1.36 |
| 1% | 330 ± 2.91 | 331 ± 2.69 | 73.6 ± 2.04 | 74.8 ± 5.25 | 44.8 ± 2.86 | 46.4 ± 3.62 | 33.4 ± 1.30 | 33.5 ± 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, H.; Shiraki, K.; Matsumoto, T.; Suzuki, K.; Yamashita, Y.; Tawara, I.; Shimpo, H.; Shimaoka, M. A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab. J. Clin. Med. 2022, 11, 6142. https://doi.org/10.3390/jcm11206142
Wada H, Shiraki K, Matsumoto T, Suzuki K, Yamashita Y, Tawara I, Shimpo H, Shimaoka M. A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab. Journal of Clinical Medicine. 2022; 11(20):6142. https://doi.org/10.3390/jcm11206142
Chicago/Turabian StyleWada, Hideo, Katsuya Shiraki, Takeshi Matsumoto, Kei Suzuki, Yoshiki Yamashita, Isao Tawara, Hideto Shimpo, and Motomu Shimaoka. 2022. "A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab" Journal of Clinical Medicine 11, no. 20: 6142. https://doi.org/10.3390/jcm11206142
APA StyleWada, H., Shiraki, K., Matsumoto, T., Suzuki, K., Yamashita, Y., Tawara, I., Shimpo, H., & Shimaoka, M. (2022). A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab. Journal of Clinical Medicine, 11(20), 6142. https://doi.org/10.3390/jcm11206142







