Abstract
Tissue-specific stem cells exist in tissues and organs, such as skin and bone marrow. However, their pluripotency is limited compared to embryonic stem cells. Culturing primary cells on plastic tissue culture dishes can result in the loss of multipotency, because of the inability of tissue-specific stem cells to survive in feeder-less dishes. Recent findings suggest that culturing primary cells in medium containing feeder cells, particularly genetically modified feeder cells expressing growth factors, may be beneficial for their survival and proliferation. Therefore, the aim of this study was to elucidate the role of genetically modified human feeder cells expressing growth factors in maintaining the integrity of primary cultured human deciduous dental pulp cells. Feeder cells expressing leukemia inhibitory factor, bone morphogenetic protein 4, and basic fibroblast growth factor were successfully engineered, as evidenced by PCR. Co-culturing with mitomycin-C-treated feeder cells enhanced the proliferation of newly isolated human deciduous dental pulp cells, promoted their differentiation into adipocytes and neurons, and maintained their stemness properties. Our findings suggest that genetically modified human feeder cells may be used to maintain the integrity of primary cultured human deciduous dental pulp cells.
1. Introduction
Stem cells are largely classified into two groups: embryonic stem cells/induced pluripotent stem cells (ESCs/iPSCs) and tissue-specific stem cells (TSCs) (also known as somatic stem cells or progenitor cells) [1,2,3,4,5]. The former possess self-renewal and pluripotency properties, and are derived from early embryos through continuous cultivation of the inner cell mass of blastocysts (ESCs) or from differentiated cells (i.e., fibroblasts) after transfection with reprogramming factors (Yamanaka factors) (iPSCs) [2]. The latter exist in tissues and organs, such as skin, brain, and bone marrow. TSCs possess self-renewal ability, but with limited pluripotency compared to ESCs/iPSCs. Importantly, ESCs/iPSCs (but not TSCs) can form solid tumors (teratomas) when transplanted under the skin or renal capsule of immunocompromised mice [6,7,8,9]. The role of stem cells in regenerative medicine has received considerable attention, with promising results.
Human deciduous teeth are discarded by children aged 6–12 years old, after which they are replaced by adult teeth. Stem cells can be isolated from human deciduous teeth, and dental pulp cells derived from human deciduous teeth (hereafter referred to as human deciduous dental pulp cells [HDDPCs]) may be a useful resource for tooth regeneration [10,11]. Therefore, a comprehensive understanding of the properties of HDDPCs is required. However, most primary cultured HDDPCs are maintained in feeder-less plastic tissue culture dishes, often leading to loss of pluripotency, probably due to the inability of TSCs to survive on feeder-less plastic tissue culture dishes for prolonged periods [12,13].
In 1981, mouse ESCs were first derived from teratocarcinoma stem cells cultured in medium containing mitotically inactivated mouse embryonic fibroblast (MEF) feeder cells supplemented with fetal bovine serum (FBS) [14,15]. Feeder cells are known to produce growth factors, adhesion molecules, and extracellular matrix components for cell attachment [16]. MEFs are primary cells derived from mid-gestational fetuses that can be maintained for only a few passages before senescence, necessitating the use of mitomycin-C (MMC; a reagent used to inhibit the proliferation of dividing cells) [17]. STO cells (an immortalized cell line derived from mouse SIM embryonic fibroblasts [18]) are useful for establishing and maintaining mouse [19,20] and human ESCs [2]. STO cells are also useful for eliminating bacterial contamination during HDDPC isolation and propagation [21]. In contrast to MEFs, STO cells can continue to proliferate in vitro; however, their proliferation can be inhibited by MMC or gamma irradiation [17]. Moreover, feeder cells can be engineered to express and secrete growth factors, such as leukemia inhibitory factor (LIF), bone morphogenetic protein-4 (BMP4), and basic fibroblast growth factor (bFGF; also known as FGF-2), which are important for establishing and maintaining mouse and human ESCs [22,23,24,25,26,27]. These findings indicate that the addition of recombinant growth factors to the medium is unnecessary when ESCs are co-cultured with genetically engineered feeder cells capable of producing growth factors. Furthermore, feeder cells carrying drug resistance genes are useful for selecting genetically engineered cells [20,22].
However, little is known about how primary cultured cells, such as HDDPCs, can be maintained in vitro. Therefore, the aim of this study was to elucidate the role of genetically modified human feeder cells in maintaining the integrity of primary cultured HDDPCs. We hypothesized that co-culturing with feeder cells is important for maintaining the integrity of newly isolated HDDPCs. Considering the future use of HDDPC-derived stem cells in preclinical studies, human-derived feeder cells were used in this study to avoid xeno-contamination. Because human-derived feeder cells can be genetically engineered to express and secrete growth factors, we generated genetically modified (GM) HDDPCs through co-transfection with genes encoding LIF, BMP4, and bFGF, using a piggyBac (PB) transposon-based gene delivery system [28,29]. The role of GM HDDPCs in the growth, differentiation, and stemness properties of newly isolated HDDPCs was examined.
2. Materials and Methods
2.1. Cells
HDDPCs were isolated from human deciduous recovered from children aged 6–12 years old by digestion in a solution of 3 mg/mL of collagenase type I (#17100-017; Invitrogen, Carlsbad, CA, USA) and 4 mg/mL of dispase (#410810077; Roche Applied Science, Basel, Switzerland) for 30–60 min at 37 °C. The isolated HDDPCs were maintained in 60-mm gelatin-coated dishes (#4010-020; Iwaki Glass Co., Ltd., Tokyo, Japan) containing minimum essential medium α (MEMα) supplemented with L-glutamine and phenol red (#135-15175; Wako Pure Chemical Industries, Ltd., Osaka, Japan), 20% heat-inactivated FBS (#SFMB30-2239; Equitech Bio Inc., Kerrville, TX, USA), 50 U/mL of penicillin, and 50 mg/mL of streptomycin (#15140-122; Invitrogen) (hereafter referred to as MEMα/20% FBS) at 37 °C under 5% CO2 for >7 d. The medium was changed every 3 days. Primary cultured cells were obtained after approximately 10 passages and frozen using CellBanker cell freezing medium (#CB021; Takara Bio Inc., Shiga, Japan). The isolated HDDPCs were used in co-cultivation assays with MMC-treated GM HDDPCs. HDDPCs [29] that had been passaged for 20 generations, were used as GM feeder cells. These cells were maintained under the same conditions as the newly isolated HDDPCs.
All HDDPC experiments were performed according to the guidelines and protocols approved by the Ethical Committee for the Use and Experimentation of Graduate School of Dental Science, Asahi University (No. 34007; dated on 16 March 2022).
2.2. Construction of PB Transposon Vectors
PB-based expression vectors (Figure 1A) were constructed using a standard cloning procedure. pTrans [30] is a vector for the expression of PB transposase under the control of the chicken β-actin (CAG) promoter [31]. pT-bFN-3 is a PB-based vector that carries a bFGF expression unit under the control of the CAG promotor and a neomycin resistance gene (neo) expression unit under the control of the mouse phosphoglycerate kinase (Pgk) promoter. pT-PBILB-4 is a PB-based vector that carries a BMP4/LIF expression unit under the control of the mouse Pgk promoter and a blasticidin S-resistance gene (bsr) expression unit under the control of the CAG promoter. BMP4/LIF expression is mediated by the internal ribosome entry site, a multicistronic element enabling CAP-independent protein translation [32]. pT-FLAG-E7 is a PB-based vector that carries a bovine papilloma virus-derived E7 expression unit under the control of the CAG promoter. E7 is an immortalization gene responsible for continuous cell proliferation. pCE-29 [33] is a plasmid vector for the expression of enhanced green fluorescent protein (EGFP) cDNA under the control of the CAG promoter. All plasmids were grown in E. coli DH5α and purified using a Macherey-Nagel plasmid purification kit as previously described [34].
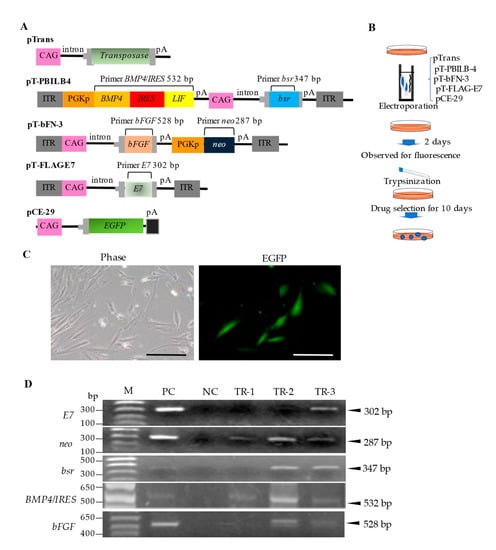
Figure 1.
Isolation of GM HDDPCs via transfection using a PB transposon-based gene delivery system. (A) Plasmid construction. pTrans is a vector for the expression of PB transposase under the control of the CAG promoter. pT-bFN-3 is a PB-based vector that carries a bFGF expression unit under the control of the CAG promoter and a neo expression unit under the control of the mouse Pgk promoter. pT-PBILB-4 is a PB-based vector that carries a BMP4/LIF expression unit under the control of the mouse Pgk promoter and a bsr expression unit under the control of the CAG promoter. pT-FLAG-E7 is a PB-based vector that carries a bovine papilloma virus-derived E7 expression unit under the control of the CAG promoter. pCE-29 is a plasmid vector for the expression of EGFP cDNA under the control of the CAG promoter. (B) Flowchart of the isolation procedure. (C) Fluorescence 2 d after transfection. Phase, photograph taken under light; EGFP, photograph taken under UV light. Scale bars, 50 μm. (D) PCR analysis of transfected clones (TR-1–3). Abbreviations: bFGF: basic fibroblast growth factor; BMP4: bone morphogenetic protein-4; bsr: blasticidin S-resistance gene; CAG: chicken β-actin promoter; EGFP: enhanced green fluorescent protein; GM: genetically modified; HDDPC: human deciduous dental pulp cells; IRES: internal ribosomal entry site; ITR: inverted terminal repeat; LIF: leukemia inhibitory factor; M: molecular size markers; NC: negative control (genomic DNA from non-transfected HDDPCs); pA: poly (A) addition site; PB: piggyBac; PC: positive control (5 ng of each plasmid); Pgk: phosphoglycerate kinase; Pgkp: Pgk promoter.
2.3. Generation of GM HDDPCs via Transfection with PB-Based Vectors
HDDPCs [26] (5 × 105 cells) were electroporated in 100 µL of R solution (Invitrogen) containing pTrans, pT-bFN-3, pT-PBILB-4, pT-FLAG-E7, and pCE-29 (1 μg each) using the Neon Transfection System (#MPK10096; Invitrogen), as shown in Figure 1B. The cells were examined for EGFP-derived fluorescence 2 d after transfection. The cells were then trypsinized and reseeded in 60-mm gelatin-coated dishes containing MEMα/20% FBS supplemented with 800 μg/mL of G418 (#631307; TaKaRa Bio Inc.) and 400 μg/mL of bsr (#3513-03-9; Merck, Darmstadt, Germany). After 7−10 d of selection, the emerging colonies were picked using a 3-mm paper disc (#3030-917; Whatmann, Buckinghamshire, UK) dipped in 0.25% trypsin in calcium- and magnesium-free Dulbecco’s modified phosphate-buffered saline (DPBS), as previously described [29]. The cell-containing paper discs were transferred to a gelatin-coated 48-well plate (#3830-048; Iwaki Glass Co., Ltd.) containing 200 μL of drug-free MEMα/20% FBS and cultured for 7–10 d at 37 °C under 5% CO2. The cells were further propagated in a stepwise manner and frozen for molecular immunocytochemical analysis.
2.4. Preparation of MMC-Treated Feeder Cells
GM HDDPCs (105 cells) (Section 2.3) were incubated in a 60-mm gelatin-coated dish containing 4 mL of MEMα/20% FBS supplemented with 4 μg/mL of MMC (#M4287; Sigma-Aldrich, St. Louis, MO, USA), for 4 h at 37 °C under 5% CO2, to inhibit cell proliferation. After treatment, cells were washed three times with DPBS, trypsinized, aliquoted (104 dissociated cells) into cryotubes (#430488; Corning, Glendale, AZ, USA) containing 400 μL of CellBanker (#CB021; TaKaRa Bio Inc.), and deep-frozen.
2.5. PCR Analysis
Genomic DNA was extracted by adding 300 μL of lysis buffer [0.125 μg/mL of proteinase K, 0.125 μg/mL of Pronase E, 0.32 M sucrose, 10 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and 1% (v/v) Triton X-100] to transfected HDDPCs (~105 cells) in a 1.5-mL tube, followed by incubation for 2–3 d at 37 °C and phenol/chloroform extraction. The supernatant was isopropanol precipitated. The precipitated DNA was then dissolved in 20 μL of sterile water and stored at 4 °C. PCR was performed in a total reaction volume of 10 μL containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.25 mM of each dNTP, 1 mM of each primer (forward and reverse) (Table 1), 2 μL of genomic DNA (~5 ng), and 0.5 U of rTaq polymerase (#R001; Takara Shuzo Co., Ltd., Tokyo, Japan). The PCR conditions were as follows: 40 cycles at 96 °C for 10 s, 56 °C for 1 min, and 72 °C for 2 min. E7-S and E7-RV primers were used to detect pT-FLAG-E7, which yielded a 302-bp product from the upper region of E7. Similarly, neo-3S and neo-3RV primers were used to detect the neo expression unit in pT-bFN-3, which yielded a 287-bp product from neo. Additionally, bsr-S and bsr-RV primers were used to detect the bsr expression unit in pT-PBILB-4, which yielded a 347-bp product from bsr. BMP4/LIF-S and BMP4/LIF-RV primers were used to detect the fragment containing the BMP4/LIF expression unit in pT-PBILB-4, which yielded a 532-bp product from BMP4/LIF. bFGF-S and bFGF-RV primers were used to detect the bFGF expression unit in pT-bFN-3, which yielded a 528-bp product from bFGF. Genomic DNA (0.5 μg) from untransfected HDDPCs was used as a negative control; 5 ng of each plasmid listed in Figure 1A were used as positive controls. The PCR products (5 µL) were separated on a 2% agarose gel and visualized with ethidium bromide.

Table 1.
PCR primers.
2.6. Immunocytochemical Staining
Immunocytochemical staining was performed to detect the transgenic products. Briefly, GM HDDPCs were seeded in a gelatin-coated 24-well plate (#3820-024; Iwaki Glass Co., Ltd.) containing MEMα/20% FBS. Cells at 60–70% confluence were fixed in 4% paraformaldehyde (PFA) in DPBS for 5 min at 24 °C, washed three times with DPBS, and permeabilized with 0.1% Triton X-100 (#T8787; Sigma-Aldrich) in DPBS for 3 min at 24 °C. Cells were washed three times with DPBS containing 1% normal goat serum (NGS) (Invitrogen) (hereafter referred to as PBS/NGS) and blocked by incubation with 20% AquaBlock (#PP82; East Coast Biologics, Inc., North Berwick, USA) for 30 min at room temperature. Cells were washed three times with PBS/NGS and stained with primary antibodies against BMP4 (clone H-134, 1:200) (#sc-9081; Santa Cruz Biotechnology, Dallas, TX, USA), LIF (1:200) (#SAB2701974; Sigma-Aldrich), or bFGF (1:200) (#N5413; Sigma-Aldrich) overnight at 4 °C. After washing 3 times with PBS/NGS, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G γ-chain secondary antibody (1:200) (#AP124JA4; Millipore-Chemicon, Darmstadt, Germany) for approximately 2 h at 4 °C. After washing with PBS/NGS 3 times, nuclear staining was performed with 4′,6-diamidino-2-phenylindole (#H-1200; Vector Laboratories, Burlingame, CA, USA) for 10 min at room temperature. Fluorescence was examined using an Olympus BX60 fluorescence microscope (#BX60; Olympus, Tokyo, Japan).
2.7. Cell Growth Assay
To determine the effect of MMC-treated GM HDDPCs on the proliferation of freshly isolated HDDPCs, frozen stocks of MMC-treated GM HDDPCs were thawed 4 d prior to the cell growth assay. For the experimental group, MMC-treated GM HDDPCs (104 cells) and freshly isolated HDDPCs (4 × 104 cells) were mixed at a ratio of 1:5 in a volume of 180 μL. A 10 μL aliquot of cell suspension was plated on a gelatin-coated 24-well plate containing 1 mL of drug-free MEMα/20% FBS as the first passage. After culturing for 6–10 d, cells were harvested via trypsinization and counted using a disposable hemocytometer (#521-10; Funakoshi, Tokyo, Japan). The final cell number was calculated by subtracting the number of MMC-treated GM HDDPCs (104 cells) initially plated from the total cell number. Two wells per line were examined and the average cell number was plotted. Cells were mixed with MMC-treated GM HDDPCs (104 cells), pelleted, and resuspended in 180 μL of drug-free MEMα/20% FBS. A 10 μL aliquot of cell suspension was plated on a 24-well plate as the second passage. This was repeated for up to 10 passages. For the control group, freshly isolated HDDPCs (5 × 104 cells) alone were prepared in a volume of 180 μL. A 10 μL aliquot of cell suspension was plated on a gelatin-coated 24-well plate containing 1 mL of drug-free MEMα/20% FBS as the first passage. After culturing for 6–10 d, cells were harvested via trypsinization and counted using a hemocytometer (#521-10; Funakoshi Co., Ltd., Tokyo, Japan). Two wells per line were examined and the average cell number was plotted. Cells were pelleted and resuspended in 180 μL of drug-free MEMα/20% FBS. A 10 μL aliquot of cell suspension was plated on a 24-well plate as the second passage. This was repeated for up to 10 passages.
2.8. In vitro Differentiation Assay
To determine the effect of MMC-treated GM HDDPCs on the differentiation of freshly prepared HDDPCs, frozen stocks of MMC-treated GM HDDPCs were thawed 4 d prior to the assay. For the experimental group, MMC-treated GM HDDPCs (1 × 104 cells) and freshly isolated HDDPCs (5 × 104 cells) were mixed at a ratio of 1:5 in a volume of 180 μL. A 100 μL aliquot of cell suspension was plated on a gelatin-coated 24-well plate containing 1 mL of drug-free MEMα/20% FBS. For the control group, freshly isolated HDDPCs (6 × 104 cells) alone were prepared in a volume of 180 μL. A 100 μL aliquot of cell suspension was plated on a gelatin-coated 24-well plate containing 1 mL of drug-free MEMα/20% FBS. When the cells reached 80–90% confluence, the medium was changed to a differentiation-inducing medium, as described below.
To induce osteogenic differentiation, cells were cultured in osteogenic differentiation medium (#KBDSTC103; DS Pharma, Osaka, Japan) for ~5 d at 37 °C under 5% CO2. Cells were fixed in 4% PFA for 5 min at 24 °C and washed three times with DPBS, followed by Alizarin Red S staining (#ARD-A1; PG Research, Tokyo, Japan) for 30 min at room temperature.
To induce neurogenic differentiation, cells were cultured in mesenchymal stem cell neurogenic differentiation medium (#C-28015; PromoCell, Heidelberg, Germany) for 7 day at 37 °C under 5% CO2. After fixation in 4% PFA for 5 min at room temperature, the cells were incubated with 0.1% Cresyl violet solution (#038-0482; Wako Pure Chemical Industries Ltd.) for 30 min at room temperature to stain the cytoplasm of neurons with Nissl.
2.9. Alkaline Phosphatase (ALP) Assay
To determine the effect of MMC-treated GM HDDPCs on ALP expression by freshly prepared HDDPCs, frozen stocks of MMC-treated GM HDDPCs were thawed 4 d prior to the assay. For the experimental group, MMC-treated GM HDDPCs (1 × 103 cells) were seeded in a gelatin-coated 24-well plate containing 1 mL of drug-free MEMα/20% FBS, followed by the addition of freshly isolated HDDPCs (5 × 103 cells) and cultured for 7 d. After trypsinization, the cell suspension (~5 × 103 cells) was reseeded in a gelatin-coated 24-well plate containing MMC-treated GM HDDPCs (1 × 103 cells) and cultured for 7 d. This procedure was repeated for 20 passages. For the control group, freshly isolated HDDPCs (~5 × 103) alone were seeded in a gelatin-coated 24-well plate and cultured for 7 d. After trypsinization, the cell suspension (~5 × 103 cells) was reseeded in a gelatin-coated 24-well plate. This was repeated for at least 20 passages. Cells at 80–90% confluency were fixed in 4% PFA for 5 min at 24 °C, followed by cytochemical staining for ALP activity using the Leukocyte Alkaline Phosphatase Kit (#ALP-TK1; Sigma-Aldrich, St. Louis, MO, USA). ALP activity was visualized by the appearance of red-brown products upon ALP-mediated conversion of α-naphthol coupled with a diazonium salt.
3. Results
3.1. Preparation of GM HDDPCs
HDDPCs were transfected with PB-based transposon vectors (Figure 1A), which are effective for acquiring stable mammalian transfectants [28], to generate stable transfectants expressing several growth factors and an immortalization gene. HDDPCs were electroporated with a PB transposase expression vector, pTrans, three PB transposons (pT-bFN-3, pT-PBILB-4, and pT-FLAG-E7), and an EGFP expression vector, pCE-29 (used to monitor transfection efficiency), using the Neon Transfection System (Figure 1B). Approximately 40% of the cells (1/2 tested) exhibited EGFP-derived fluorescence after 2 d of transfection (Figure 1C). To acquire stable transfectants, transfected cells were treated with G418 and bsr for 7–10 d. The emerging colonies were picked using a trypsin-dipped paper disc, as described by Nakayama et al. [35], transferred to a 48-well plate containing drug-free MEMα/20% FBS, and propagated for 7–10 d. Three clones were successfully obtained (TR-1–3).
PCR was performed to confirm the presence of the introduced genes in the established clones. TR-1 contained neo and BMP4/LIF, whereas both TR-2 and TR-3 contained E7, neo, bsr, BMP4/LIF, and bFGF (Figure 1D). TR-2 had a higher proliferation rate in vitro than TR-3; therefore, TR-2 was selected for subsequent experiments. Immunocytochemical staining using antibodies against BMP4, LIF, and bFGF confirmed the expression of these proteins in TR-2 (Figure 2A).
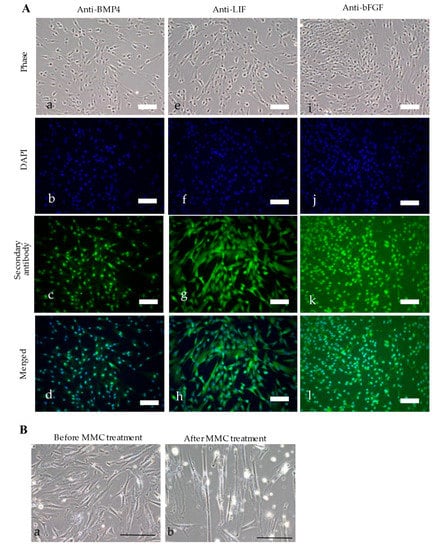
Figure 2.
Characterization of TR-2 feeder cells. (A) Immunocytochemical staining of TR-2 feeder cells. Cells were immunostained with (a–c), and (d) anti-BMP, (e–h) anti-LIF, or (i), (j), (k), and (l) anti-bFGF antibodies. Scale bars = 50 μm. (B) Morphological changes in TR-2 feeder cells (a) before and (b) 35 d after MMC treatment. Scale bars = 100 μm. bFGF: basic fibroblast growth factor; BMP4: bone morphogenetic protein-4; DAPI: 4′,6-diamidino-2-phenylindole; LIF: leukemia inhibitory factor; MMC: mitomycin-C.
TR-2 was incubated in MEMα/20% FBS containing MMC for 4 h at 37 °C to determine the effect of MMC on TR-2 morphology. There was no change in cell behavior (e.g., detachment from the dish surface); however, MMC-treated TR-2 (hereafter referred to as “TR-2 feeder cells”) exhibited a slightly enlarged morphology (Figure 2B).
3.2. HDDPC-Derived Feeder Cells Enhanced the Proliferation of HDDPCs
Primary HDDPCs were cultured with or without TR-2 feeder cells, and morphological changes were evaluated after 10 passages. In the presence or absence of TR-2 feeder cells, the morphology of HDDPCs remained fibroblastic after 4 d of culture (nine passages) (Figure 3A). However, HDDPCs cultured with TR-2 feeder cells had a higher proliferation rate than those cultured without TR-2 feeder cells (Figure 3B), indicating that TR-2 feeder cells enhanced the proliferation of HDDPCs.
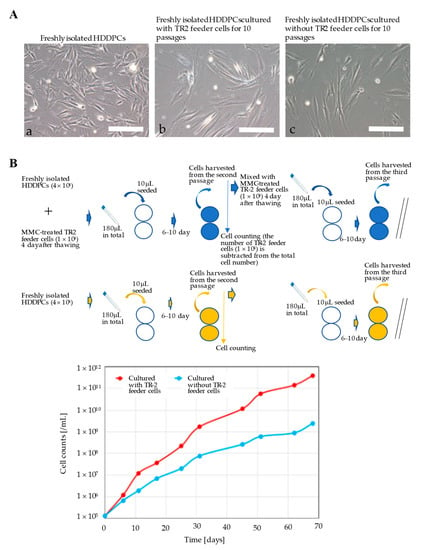
Figure 3.
Characterization of freshly isolated primary HDDPCs cultured with or without TR-2 feeder cells (I). (A) Morphology of primary HDDPCs (a) before transfection, (b) cultured with TR-2 feeder cells for 10 passages, and (c) cultured without TR-2 feeder cells for 10 passages. Scale bars = 100 μm. (B) Proliferation of primary HDDPCs with or without MMC-treated TR-2 feeder cells. The assay is shown schematically in the upper column. In the lower column, the proliferation rate after 10 passages is shown. Primary HDDPCs cultured with TR-2 feeder cells had a higher proliferation rate than those cultured without TR-2 feeder cells. HDDPC: human deciduous dental pulp cells; MMC: mitomycin-C.
3.3. HDDPC-Derived Feeder Cells Maintained the Pluripotency of HDDPCs
Primary HDDPCs were cultured with or without TR-2 feeder cells for 10 passages, and then subjected to osteogenic or neurogenic differentiation induction to determine whether TR-2 feeder cells supported the pluripotency of HDDPCs. HDDPCs cultured with TR-2 feeder cells exhibited higher osteogenic differentiation (as determined by the appearance of heavily calcified deposits) after 5 d of culture than those cultured without TR-2 feeder cells (Figure 4A). Similarly, HDDPCs cultured with TR-2 feeder cells exhibited higher neurogenic differentiation after 7 d of culture than those cultured without TR-2 feeder cells (as evidenced by the greater number of elongated axons and dendrites with Nissl bodies around the nucleus compared to those cultured without TR-2 feeder cells) (Figure 4A).
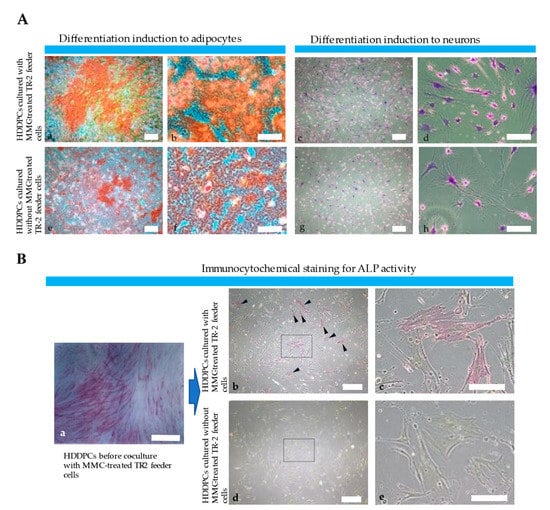
Figure 4.
Characterization of freshly isolated primary HDDPCs cultured with or without TR-2 feeder cells (II). (A) Osteogenic and neurogenic differentiation of primary HDDPCs cultured in the (a–c), and (d) presence or (e–g), and (h) absence of TR-2 feeder cells. (a,b,e,f) Alizarin red staining 5 d after incubation in osteogenic medium. (c,d,g,h) Nissl staining 7 d after incubation in neurogenic medium. HDDPCs cultured with TR-2 feeder cells exhibited better differentiation than those cultured without TR-2 feeder cells. Scale bars = 100 μm [(a,c,e,g)] and 50 μm [(b,d,f,h)]. (B) Expression of ALP (a stem cell marker). Cytochemical ALP staining of HDDPCs (a) before culturing with TR-2 feeder cells, (b,c) cultured with TR-2 feeder cells for 20 passages, and (d,e) cultured without TR-2 feeder cells for 20 passages. The former maintained ALP activity [arrowheads (b)]. The boxes in (b,d) are enlarged and shown as (c,e), respectively. Scale bars = 100 μm [(a,b,d)] and 50 μm [(c,e)]. ALP: alkaline phosphatase; HDDPC: human deciduous dental pulp cells; MMC: mitomycin-C.
3.4. HDDPC-Derived Feeder Cells Maintained the Stemness of HDDPCs
Previously, we demonstrated that HDDPCs with high ALP, OCT3/4, and SOX2 expression are easily reprogrammed into iPSCs, with pluripotent capabilities [29], indicating that HDDPCs with high ALP activity have stemness properties. Based on these findings, we performed immunocytochemical staining of ALP in HDDPCs cultured with or without TR-2 feeder cells for 20 passages. HDDPCs cultured with TR-2 feeder cells exhibited mosaic ALP staining (Figure 4B), like parental HDDPCs, indicating a mixture of stem and non-stem cells. Conversely, HDDPCs cultured without TR-2 feeder cells exhibited negative ALP staining (Figure 4B). Our findings suggest that HDDPCs cultured with TR-2 feeder cells possess stemness properties and can be easily reprogrammed into iPSCs.
4. Discussion
Since the first establishment of mouse ESCs using mitotically inactivated feeder cells in 1981 [36,37], mitosis-incompetent murine or human embryonic fibroblast cells have been used to generate and maintain human and rat ESCs/iPSCs and mouse iPSCs [2,3,38,39,40,41,42]. Additionally, feeder cells are effective in maintaining various types of juvenile cells, such as hematopoietic progenitor cells [11,12,13,14], limbal epithelial progenitor cells [43,44,45,46], spermatogonial progenitor cells from adult mammalian testis [7,47], and stem cells from human corneal or oral epithelial cells [5,6]. Although MEFs are commonly used as feeder cells, there have been no reports of the creation of feeder cells that are more suitable for stem cell maintenance than MEFs. In this study, we produced feeder cells derived from HDDPCs that have enough ability to maintain undifferentiated cells.
Continuous culture in feeder-less plastic tissue culture dishes can result in the gradual loss of pluripotency of TSCs [15,16] and ESCs/iPSCs [48]. Since the first report that the cytokine LIF can maintain the self-renewal and pluripotency of mouse ESCs in the absence of feeder cells [19,49], the beneficial effects of adding specific growth factors, such as BMP4 and bFGF, into the culture medium of ESCs/iPSCs have been reported [50,51]. Researchers have generated genetically engineered cells overexpressing growth factors to achieve cost-effective ESCs/iPSCs culture systems [52,53]. Horie et al. [54] demonstrated that GM fibroblasts (STO cells) expressing E-cadherin (an adhesion molecule involved in cell-to-cell interactions) [55] maintained the pluripotency of mouse ESCs, indicating the importance of scaffold formation between feeder cells and ESCs. In this study, GM HDDPCs (TR-2 feeder cells) simultaneously expressing three growth factors (bFGF, LIF, and BMP4) and an immortalization gene were generated to support the proliferation and differentiation of primary HDDPCs. Furthermore, 10% FBS has been frequently employed for maintaining differentiated cells, but when cultivating cells enriched with stem cells, 20% FBS has been preferred in order to achieve stable growth rates [56]. Notably, contamination with oral bacteria was common during the primary culture of HDDPCs. To prevent contamination, antibiotics were used consistently in our experiments, and they did not appear to affect the pluripotency of HDDPCs.
In this study, a PB-based gene delivery system was used to construct TR-2 feeder cells, enabling the isolation of stable transfectants from various cell types [57]. PB-based gene delivery is simple because researchers can use only two types of nucleic acids: a PB transposase (Trans) expression vector and a transposon vector carrying a gene of interest flanked by two inverted terminal repeats. When the nucleic acids are placed inside a cell, PB Trans binds to the inverted terminal repeats to allow the gene of interest to be individually integrated into host chromosomal sites containing the TTAA sequence, which is duplicated on the two flanks of the integrated fragment [58,59]. Dental pulp cells have been used as a material for gene transfection, and bone differentiation can be promoted by introducing OCT3/4 and other genes [60]. In this study, four constructs (three transposons and one non-transposon plasmid) were introduced into HDDPCs in vitro and three clones (TR-1–3) were obtained. Among the clones, TR-2 was found to contain all four genes of interest (BMP4, LIF, bFGF, and E7) (Figure 1B) in its genome, and successfully expressed BMP4, LIF, and bFGF via immunocytochemical assay (Figure 2A). These results show that the PB-based gene delivery system was effective in generating GM HDDPCs with multiple genes.
Notably, HDDPCs obtained after 1–4 passages often showed unstable growth rates, possibly due to the various types of cells included with the HDDPCs. After culturing in the conditions used in this study, HDDPCs retained stem-like properties even after 10 passages [61], and exhibited stable growth rates [62]. The growth assay shows that, after 10 passages, HDDPCs co-cultured with TR-2 feeder cells had a higher proliferation rate than those cultured without TR-2 feeder cells (Figure 3B). HDDPCs cultured with TR-2 feeder cells had better differentiation potential than those cultured without TR-2 feeder cells (Figure 4A), indicating that GM human feeder cells are important for maintaining the integrity of primary cultured HDDPCs. Continuous culture of primary HDDPCs in feeder-less medium resulted in a loss of ALP activity (Figure 4B), indicating a loss of stem cells (initially included in the isolation of HDDPCs). A previous study showed that primary HDDPCs comprise various cells, including stem and non-stem cells [63]. As ALP and OCT-3/4 are markers of ESCs/iPSCs [34,59,63], ALP(−)/OCT-3/4(−) cells may be considered non-stem cells, whereas ALP(−)/OCT-3/4(+), ALP(+)/OCT-3/4(−), and ALP(+)/OCT-3/4(+) cells may be considered stem cells [63]. This study indicates that the secretion of growth factors from TR-2 feeder cells may be beneficial for stem cell survival. Notably, expression of CD73, CD90, and STRO-1, all of which are recognized as markers for mesenchymal stem cells (MSCs), has been reported in HDDPCs [64]. Unfortunately, we did not test for expression of these proteins in the HDDPCs used in the present study. HDDPCs should be comprehensively checked for the expression of stem-cell-specific markers, including MSC-related markers, in future studies.
The use of feeder cells is labor intensive, as the feeder layer needs to be seeded a day before stem cell culture, and MMC treatment or gamma irradiation is required to inhibit cell proliferation. Therefore, an alternative feeder-free culture system for human ESCs/iPSCs has been developed using a medium containing 2i (GSK3 and MAPK inhibitor) and LIF [65], heparin [66], DNA aptamer capable of binding to a bFGF receptor [67], or human plasma protein-based hydrogel [68]. Commercially available media (StemFlex [#A3349401; Thermo Fisher Scientific], mTeSR™1 [#ST-85850; Veritas Japan Co., Ltd., Yokohama, Japan], and StemFit Basic04 Complete Type (#ASB04CT; Ajinomoto, Tokyo, Japan) can also be used to culture human ESCs/iPSCs. Human ESCs/iPSCs were established or maintained on tissue culture surface coated with extracellular matrix proteins, such as fibronectin, vitronectin, and laminin [65,69], or on commercially available Matrigel (#354234; Corning, NY, USA), which is liquid at low temperatures and a gel at 37 °C [65,70]. Porous membranes [71], synthetic substrates [72], and DAS nanocrystalline graphene [73] have also been used in feeder-free culture systems for human ESCs/iPSCs. To our knowledge, there have been few reports on the successful cultivation of human dental pulp-derived stem cells in the absence of feeder cells. Although some reagents have been developed to support these juvenile cells, their mechanisms of action are unclear, and they may be expensive. Therefore, GM feeder cells may serve as an effective and cheap alternative for maintaining stem cells.
5. Conclusions
Our findings show that co-cultivation of primary HDDPCs with MMC-treated TR-2 feeder cells can maintain the integrity of primary HDDPCs, including proliferation rate, differentiation ability, and expression of stemness markers. As TR-2 feeder cells express growth factors that are potentially beneficial for maintaining juvenile cells, such as stem cells, progenitor cells, and ESCs/iPSCs, they may be useful for maintaining other types of juvenile cells that are difficult to maintain in the absence of feeder cells or require specific growth medium.
Author Contributions
Conceptualization, M.S., E.I., and I.S.; investigation, N.I., S.O., N.K., H.N., T.M. (Takeyasu Maeda), T.S. and Y.K. (Yoshito Kakihara); funding acquisition, E.I., M.S., T.M. (Tomoya Murakami) and I.S.; resources, T.M., M.T. and Y.T.; writing—original draft preparation, M.S. and I.S.; writing—review and editing, E.I., Y.K. (Yuki Kiyokawa), K.S. and Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by a grant (no. 15K07695 for M.S.; no. 21K10165 for E.I.; no. 22K17081 for T.M.; and no. 26670883 and 22H03277 for I.S.) from the Ministry of Education, Science, Sports, and Culture, Japan.
Institutional Review Board Statement
Human deciduous were extracted from healthy individuals in accordance with the guidelines set by the ethical committee for the use and experimentation of the Niigata University Graduate School of Medical and Dental Science (permission no. 2017-0185 dated 16 October 2017, and SD00798 dated 12 September 2017).
Informed Consent Statement
Informed consent was obtained from the subjects or their parents before they entered the study.
Data Availability Statement
Not applicable.
Acknowledgments
We thank R. Tsien and M. Ohtsuka for providing the PB-based vectors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, J.B.; Lee, J.E.; Park, J.H.; Kim, S.J.; Kim, M.K.; Roh, S.I.; Yoon, H.S. Establishment and Maintenance of Human Embryonic Stem Cell Lines on Human Feeder Cells Derived from Uterine Endometrium Under Serum-Free Condition. Biol. Reprod. 2005, 72, 42–49. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.J.; Oh, E.J.; Moon, S.Y.; Roh, S.I.; Kim, C.G.; Yoon, H.S. Establishment and Maintenance of Human Embryonic Stem Cells on STO, a Permanently Growing Cell Line. Biol. Reprod. 2003, 69, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, I.; Inada, E.; Iwase, Y.; Noguchi, H.; Murakami, T.; Soda, M.; Kubota, N.; Hasegawa, H.; Akasaka, E.; Matsumoto, Y.; et al. Choice of Feeders Is Important When First Establishing iPSCs Derived from Primarily Cultured Human Deciduous Tooth Dental Pulp Cells. Cell Med. 2015, 8, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Hayashi, R.; Takagi, R.; Yamato, M.; Takayanagi, H.; Tano, Y.; Nishida, K. A Novel Method of Culturing Human Oral Mucosal Epithelial Cell Sheet Using Post-Mitotic Human Dermal Fibroblast Feeder Cells and Modified Keratinocyte Culture Medium for Ocular Surface Reconstruction. Br. J. Ophthalmol. 2010, 94, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, F.; Inoue, T.; Li, Y.; Hori, Y.; Maeda, N.; Tano, Y.; Nishida, K. New Culture Technique of Human Eliminable Feeder-Assisted Target Cell Sheet Production. Biochem. Biophys. Res. Commun. 2010, 399, 373–378. [Google Scholar] [CrossRef]
- Seandel, M.; James, D.; Shmelkov, S.V.; Falciatori, I.; Kim, J.; Chavala, S.; Scherr, D.S.; Zhang, F.; Torres, R.; Gale, N.W.; et al. Generation of Functional Multipotent Adult Stem Cells from GPR125+ Germline Progenitors. Nature 2007, 449, 346–350. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial Cultivation of Strains of Human Epidermal Keratinocytes: The Formation of Keratinizing Colonies from Single Cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of Silver on Burn Wound Infection Control and Healing: Review of the Literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Flores-Guzmán, P.; Fernández-Sánchez, V.; Mayani, H. Concise Review: Ex Vivo Expansion of Cord Blood-Derived Hematopoietic Stem and Progenitor Cells: Basic Principles, Experimental Approaches, and Impact in Regenerative Medicine. Stem Cells Transl. Med. 2013, 2, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.N.; Ng, J.; Niu, T.; Yang, H.; Mcmannis, J.D.; Karandish, S.; Kaur, I.; Fu, P.; Del Angel, M.; Messinger, R.; et al. Superior Ex Vivo Cord Blood Expansion Following Co-culture with Bone Marrow-Derived Mesenchymal Stem Cells. Bone Marrow Transplant. 2006, 37, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Salati, S.; Lisignoli, G.; Manferdini, C.; Pennucci, V.; Zini, R.; Bianchi, E.; Norfo, R.; Facchini, A.; Ferrari, S.; Manfredini, R. Co-culture of Hematopoietic Stem/Progenitor Cells with Human Osteblasts Favours Mono/Macrophage Differentiation at the Expense of the Erythroid Lineage. PLoS ONE 2013, 8, e53496. [Google Scholar] [CrossRef][Green Version]
- Rosler, E.; Brandt, J.; Chute, J.V.; Hoffman, R. Co Cultivation of Umbilical Cord Blood Cells with Endothelial Cells Leads to Extensive Amplification of Competent CD34+CD38−Cells. Exp. Hematol. 2000, 28, 841–852. [Google Scholar] [CrossRef]
- Kawada, H.; Ando, K.; Tsuji, T.; Shimakura, Y.; Nakamura, Y.; Chargui, J.; Hagihara, M.; Itagaki, H.; Shimizu, T.; Inokuchi, S.; et al. Rapid Ex Vivo Expansion of Human Umbilical Cord Hematopoietic Progenitors Using a Novel Culture System. Exp. Hematol. 1999, 27, 904–915. [Google Scholar] [CrossRef]
- Tateno, H.; Saito, S.; Hiemori, K.; Kiyoi, K.; Hasehira, K.; Toyoda, M.; Onuma, Y.; Ito, Y.; Akutsu, H.; Hirabayashi, J. Alpha2-6 Sialylation Is a Marker of the Differentiation Potential of Human Mesenchymal Stem Cells. Glycobiology 2016, 26, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Wunder, E.; Sovalat, H.; Bourderont, D.; Baerenzung, M.; Bachorz, J.; Hénon, P. Mature Accessory Cells Influence Long-Term Growth of Human Hematopoietic Progenitors on a Murine Stromal Cell Feeder Layer. Stem Cells 1998, 16, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R.; Evans, M.J. Differentiation of Clonal Lines of Teratocarcinoma Cells: Formation of Embryoid Bodies In Vitro. Proc. Natl. Acad. Sci. USA 1975, 72, 1441–1445. [Google Scholar] [CrossRef]
- Ware, L.M.; Axelrad, A.A. Inherited Resistance to N- and B-Tropic Murine Leukemia Viruses In Vitro: Evidence That Congenic Mouse Strains SIM and SIM.R Differ at the Fv-1 Locus. Virology 1972, 50, 339–348. [Google Scholar] [CrossRef]
- Smith, A.G.; Heath, J.K.; Donaldson, D.D.; Wong, G.G.; Moreau, J.; Stahl, M.; Rogers, D. Inhibition of Pluripotential Embryonic Stem Cell Differentiation by Purified Polypeptides. Nature 1988, 336, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, I.; Sato, M.; Iwase, Y.; Inada, E.; Nomura, T.; Akasaka, E.; Yamasaki, Y.; Noguchi, H. Generation of Mouse STO Feeder Cell Lines That Confer Resistance to Several Types of Selective Drugs. Cell Med. 2012, 3, 97–102. [Google Scholar] [CrossRef]
- Murakami, T.; Saitoh, I.; Inada, E.; Kurosawa, M.; Iwase, Y.; Noguchi, H.; Terao, Y.; Yamasaki, Y.; Hayasaki, H.; Sato, M. STO Feeder Cells Are Useful for Propagation of Primarily Cultured Human Deciduous Dental Pulp Cells by Eliminating Contaminating Bacteria and Promoting Cellular Outgrowth. Cell Med. 2013, 6, 75–81. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.P.; Bradley, A. The Wnt-1 (int-1) Proto-Oncogene Is Required for Development of a Large Region of the Mouse Brain. Cell 1990, 62, 1073–1085. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Chen, D.; Lin, X.; Li, J.; Wang, J.; Peng, Y.; Wang, S.; Wang, Y. Leukemia Inhibitory Factor-Expressing Human Embryonic Lung Fibroblasts as Feeder Cells for Human Embryonic Germ Cells. Cells Tissues Organs. 2007, 186, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Hanwate, M.; Deb, K.; Sharma, V.; Totey, S. FGF2 Secreting Human Fibroblast Feeder Cells: A Novel Culture System for Human Embryonic Stem Cells. Mol. Reprod. Dev. 2008, 75, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.; Gao, S.; Cohen, M.; Jaconi, M.; Bergstrom, R.; Holm, F.; Galan, A.; Sanchez, E.; Irion, O.; Dubuisson, J.B.; et al. Immortalized Human Skin Fibroblast Feeder Cells Support Growth and Maintenance of Both Human Embryonic and Induced Pluripotent Stem Cells. Hum. Reprod. 2009, 24, 2567–2581. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Y.; Zhang, P.; He, L.; Nan, X.; Yue, W.; Pei, X. Human Fetal Liver Stromal Cells That Overexpress bFGF Support Growth and Maintenance of Human Embryonic Stem Cells. PLoS ONE 2010, 5, e14457. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, G.R.; Choi, H.I.; Park, N.H.; Chung, H.T.; Han, I.S. Overexpression of Bone Morphogenetic Protein 4 in STO Fibroblast Feeder Cells Represses the Proliferation of Mouse Embryonic Stem Cells In Vitro. Exp. Mol. Med. 2012, 44, 457–463. [Google Scholar] [CrossRef][Green Version]
- Sato, M.; Inada, E.; Saitoh, I.; Watanabe, S.; Nakamura, S. PiggyBac-Based Non-viral In Vivo Gene Delivery Useful for Production of Genetically Modified Animals and Organs. Pharmaceutics 2020, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Inada, E.; Saitoh, I.; Watanabe, S.; Aoki, R.; Miura, H.; Ohtsuka, M.; Murakami, T.; Sawami, T.; Yamasaki, Y.; Sato, M. PiggyBac Transposon-Mediated Gene Delivery Efficiently Generates Stable Transfectants Derived from Cultured Primary Human Deciduous Tooth Dental Pulp Cells (HDDPCs) and HDDPC-Derived iPS Cells. Int. J. Oral Sci. 2015, 7, 144–154. [Google Scholar] [CrossRef]
- Sato, M.; Inada, E.; Saitoh, I.; Matsumoto, Y.; Ohtsuka, M.; Miura, H.; Nakamura, S.; Sakurai, T.; Watanabe, S. A Combination of Targeted Toxin Technology and the PiggyBac-Mediated Gene Transfer System Enables Efficient Isolation of Stable Transfectants in Nonhuman Mammalian Cells. Biotechnol. J. 2015, 10, 143–153. [Google Scholar] [CrossRef]
- Hitoshi, N.; Ken-ichi, Y.; Jun-ichi, M. Efficient Selection for High-Expression Transfectants with a Novel Eukaryotic Vector. Gene 1991, 108, 193–200. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal Initiation of Translation of Eukaryotic mRNA Directed by a Sequence Derived from Poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ishikawa, A.; Kimura, M. Direct Injection of Foreign DNA into Mouse Testis as a Possible In Vivo Gene Transfer System via Epididymal Spermatozoa. Mol. Reprod. Dev. 2002, 61, 49–56. [Google Scholar] [CrossRef]
- Sato, M.; Akasaka, E.; Saitoh, I.; Ohtsuka, M.; Nakamura, S.; Sakurai, T.; Watanabe, S. A Simplified Protocol for the Semi-Large-Scale Recovery of Plasmids from Escherichia coli Grown on Agar Plates. J. Biomed. Sci. Eng. 2012, 5, 406–408. [Google Scholar] [CrossRef]
- Nakayama, A.; Sato, M.; Shinohara, M.; Matsubara, S.; Yokomine, T.; Akasaka, E.; Yoshida, M.; Takao, S. Efficient Transfection of Primarily Cultured Porcine Embryonic Fibroblasts Using the Amaxa Nucleofection System™. Cloning Stem Cells 2007, 9, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a Pluripotent Cell Line from Early Mouse Embryos Cultured in Medium Conditioned by Teratocarcinoma Stem Cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of Germline-Competent Induced Pluripotent Stem Cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Ueda, S.; Kawamata, M.; Teratani, T.; Shimizu, T.; Tamai, Y.; Ogawa, H.; Hayashi, K.; Tsuda, H.; Ochiya, T. Establishment of Rat Embryonic Stem Cells and Making of Chimera Rats. PLoS ONE 2008, 3, e2800. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tong, C.; Mehrian-Shai, R.; Jia, L.; Wu, N.; Yan, Y.; Maxson, R.E.; Schulze, E.N.; Song, H.; Hsieh, C.L.; et al. Germline Competent Embryonic Stem Cells Derived from Rat Blastocysts. Cell 2008, 135, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, W.; Zhu, S.; Zhu, J.; Shi, Y.; Lin, T.; Hao, E.; Hayek, A.; Deng, H.; Ding, S. Generation of Rat and Human Induced Pluripotent Stem Cells by Combining Genetic Reprogramming and Chemical Inhibitors. Cell Stem Cell 2009, 4, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Li, W.; Hayashida, Y.; He, H.; Chen, S.Y.; Tseng, D.Y.; Kheirkhah, A.; Tseng, S.C. Human Amniotic Epithelial Cells as Novel Feeder Layers for Promoting Ex Vivo Expansion of Limbal Epithelial Progenitor Cells. Stem Cells 2007, 25, 1995–2005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omoto, M.; Miyashita, H.; Shimmura, S.; Higa, K.; Kawakita, T.; Yoshida, S.; McGrogan, M.; Shimazaki, J.; Tsubota, K. The Use of Human Mesenchymal Stem Cell-Derived Feeder Cells for the Cultivation of Transplantable Epithelial Sheets. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Inoue, T.; Takamatsu, F.; Maeda, N.; Ohashi, Y.; Nishida, K. Development of Genetically Modified Eliminable Human Dermal Fibroblast Feeder Cells for Ocular Surface Regeneration Medicine. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7522–7531. [Google Scholar] [CrossRef]
- Ang, L.P.; Jain, P.; Phan, T.T.; Reza, H.M. Human Umbilical Cord Lining Cells as Novel Feeder Layer for Ex Vivo Cultivation of Limbal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4697–4704. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Q.; Huang, Y.; Huang, Q.; Jiang, L.; Guo, L. Low microRNA-199a Expression in Human Amniotic Epithelial Cell Feeder Layers Maintains Human-Induced Pluripotent Stem Cell Pluripotency via Increased Leukemia Inhibitory Factor Expression. Acta Biochim. Biophys. Sin. 2012, 44, 197–206. [Google Scholar] [CrossRef]
- Shuwei, L.; Ge, L.; Guangxiu, L. The Comparison of Human Embryonic Stem Cells Cultured in Feeder-Containing Culture System and Feeder-Free Culture System. Cell Res. 2008, 18, S121. [Google Scholar]
- Williams, R.L.; Hilton, D.J.; Pease, S.; Willson, T.A.; Stewart, C.L.; Gearing, D.P.; Wagner, E.F.; Metcalf, D.; Nicola, N.A.; Gough, N.M. Myeloid Leukaemia Inhibitory Factor Maintains the Developmental Potential of Embryonic Stem Cells. Nature 1988, 336, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Carpenter, M.K.; Inokuma, M.S.; Chiu, C.P.; Harris, C.P.; Waknitz, M.A.; Itskovitz-Eldor, J.; Thomson, J.A. Clonally Derived Human Embryonic Stem Cell Lines Maintain Pluripotency and Proliferative Potential for Prolonged Periods of Culture. Dev. Biol. 2000, 227, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Nichols, J.; Chambers, I.; Smith, A. BMP Induction of Id Proteins Suppresses Differentiation and Sustains Embryonic Stem Cell Self-Renewal in Collaboration with STAT3. Cell 2003, 115, 281–292. [Google Scholar] [CrossRef]
- Meng, G.L.; Zur Nieden, N.I.; Liu, S.Y.; Cormier, J.T.; Kallos, M.S.; Rancourt, D.E. Properties of Murine Embryonic Stem Cells Maintained on Human Foreskin Fibroblasts Without LIF. Mol. Reprod. Dev. 2008, 75, 614–622. [Google Scholar] [CrossRef]
- Eiselleova, L.; Peterkova, I.; Neradil, J.; Slaninova, I.; Hampl, A.; Dvorak, P. Comparative Study of Mouse and Human Feeder Cells for Human Embryonic Stem Cells. Int. J. Dev. Biol. 2008, 52, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Ito, A.; Kiyohara, T.; Kawabe, Y.; Kamihira, M. E-Cadherin Gene-Engineered Feeder Systems for Supporting Undifferentiated Growth of Mouse Embryonic Stem Cells. J. Biosci. Bioeng. 2010, 110, 582–587. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F.; Berx, G. The Cell-Cell Adhesion Molecule E-Cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Naziruddin, B.; Jackson, A.; Shimoda, M.; Ikemoto, T.; Fujita, Y.; Chujo, D.; Takita, M.; Kobayashi, N.; Onaca, N.; et al. Characterization of human pancreatic progenitor cells. Cell Transplant. 2010, 19, 879–886. [Google Scholar] [CrossRef]
- Miura, H.; Inoko, H.; Inoue, I.; Okada, Y.; Tanaka, M.; Sato, M.; Ohtsuka, M. PiggyBac-Mediated Generation of Stable Transfectants with Surface Human Leukocyte Antigen Expression from a Small Number of Cells. Anal. Biochem. 2013, 437, 29–31. [Google Scholar] [CrossRef]
- Fraser, M.J.; Cary, L.; Boonvisudhi, K.; Wang, H.G.H. Assay for Movement of Lepidopteran Transposon IFP2 in Insect Cells Using a Baculovirus Genome as a Target DNA. Virology 1995, 211, 397–407. [Google Scholar] [CrossRef]
- Fraser, M.J.; Ciszczon, T.; Elick, T.; Bauser, C. Precise Excision of TTAA-Specific Lepidopteran Transposons PiggyBac (IFP2) and Tagalong (TFP3) from the Baculovirus Genome in Cell Lines from Two Species of Lepidoptera. Insect Mol. Biol. 1996, 5, 141–151. [Google Scholar] [CrossRef]
- Atari, M.; Gil-Recio, C.; Fabregat, M.; García-Fernández, D.; Barajas, M.; Carrasco, M.A.; Jung, H.S.; Alfaro, F.H.; Casals, N.; Prosper, F.; et al. Dental pulp of the third molar: A new source of pluripotent-like stem cells. J. Cell Sci. 2012, 125, 3343–3356. [Google Scholar] [CrossRef] [PubMed]
- Inada, E.; Saitoh, I.; Kubota, N.; Soda, M.; Matsueda, K.; Murakami, T.; Sawami, T.; Kagoshima, A.; Yamasaki, Y.; Sato, M. Alkaline phosphatase and OCT-3/4 as useful markers for predicting susceptibility of human deciduous teeth-derived dental pulp cells to reprogramming factor-induced iPS cells. J. Investig. Clin. Dent. 2017, 8, e12236. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Maeda, K.; Koriyama, M.; Inada, E.; Saitoh, I.; Miura, H.; Ohtsuka, M.; Nakamura, S.; Sakurai, T.; Watanabe, S.; et al. The piggyBac-Based Gene Delivery System Can Confer Successful Production of Cloned Porcine Blastocysts with Multigene Constructs. Int. J. Mol. Sci. 2016, 17, 1424. [Google Scholar] [CrossRef] [PubMed]
- Inada, E.; Saitoh, I.; Kubota, N.; Iwase, Y.; Kiyokawa, Y.; Noguchi, H.; Yamasaki, Y.; Sato, M. RNA Analysis Based on a Small Number of Manually Isolated Fixed Cells (RNA-snMIFxC) to Profile Stem Cells from Human Deciduous Tooth-Derived Dental Pulp Cells. Biol. Proced. Online 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Toldrà, R.; Dosta, P.; Montori, S.; Ramos, V.; Atari, M.; Borrós, S. Improvement of osteogenesis in dental pulp pluripotent-like stem cells by oligopeptide-modified poly(β-amino ester)s. Acta Biomater. 2017, 53, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-Free Growth of Undifferentiated Human Embryonic Stem Cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef]
- Furue, M.K.; Na, J.; Jackson, J.P.; Okamoto, T.; Jones, M.; Baker, D.; Hata, R.; Moore, H.D.; Sato, J.D.; Andrews, P.W. Heparin Promotes the Growth of Human Embryonic Stem Cells in a Defined Serum-Free Medium. Proc. Natl. Acad. Sci. USA 2008, 105, 13409–13414. [Google Scholar] [CrossRef]
- Hayata, Y.; Ueki, R.; Sando, S. Feeder-Free Human Induced Pluripotent Stem Cell Culture Using a DNA Aptamer-Based Mimic of Basic Fibroblast Growth Factor. Methods Mol. Biol. 2021, 2312, 301–305. [Google Scholar] [CrossRef]
- Lewis, F.C.; Bryan, N.; Hunt, J.A. A Feeder-Free, Human Plasma-Derived Hydrogel for Maintenance of a Human Embryonic Stem Cell Phenotype In Vitro. Cell Regen. 2012, 1, 6. [Google Scholar] [CrossRef][Green Version]
- Amit, M.; Shariki, C.; Margulets, V.; Itskovitz-Eldor, J. Feeder Layer- and Serum-Free Culture of Human Embryonic Stem Cells. Biol. Reprod. 2004, 70, 837–845. [Google Scholar] [CrossRef]
- Li, Y.; Lin, C.; Wang, L.; Liu, Y.; Mu, X.; Ma, Y.; Li, L. Maintenance of Human Embryonic Stem Cells on Gelatin. Chin. Sci. Bull. 2009, 54, 4214–4220. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, S.E.; Lee, J.H.; Lim, D.S.; Kim, K.S.; Chung, H.M.; Lee, S.H. A Novel Culture Technique for Human Embryonic Stem Cells Using Porous Membranes. Stem Cells 2007, 25, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Kim, J.K.; Lahann, J.; Krebsbach, P.H. Derivation and Long-Term Culture of Transgene-Free Human Induced Pluripotent Stem Cells on Synthetic Substrates. Stem Cells Transl. Med. 2014, 3, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Nam, D.; Choi, J.K.; Araúzo-Bravo, M.J.; Kwon, S.Y.; Zaehres, H.; Lee, T.; Park, C.Y.; Kang, H.W.; Schöler, H.R.; et al. Establishment of Feeder-Free Culture System for Human Induced Pluripotent Stem Cell on DAS Nanocrystalline Graphene. Sci. Rep. 2016, 6, 20708. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).