Functional Outcomes after Selective Clamping in Robot-Assisted Partial Nephrectomy
Abstract
1. Introduction
2. Materials and Methods
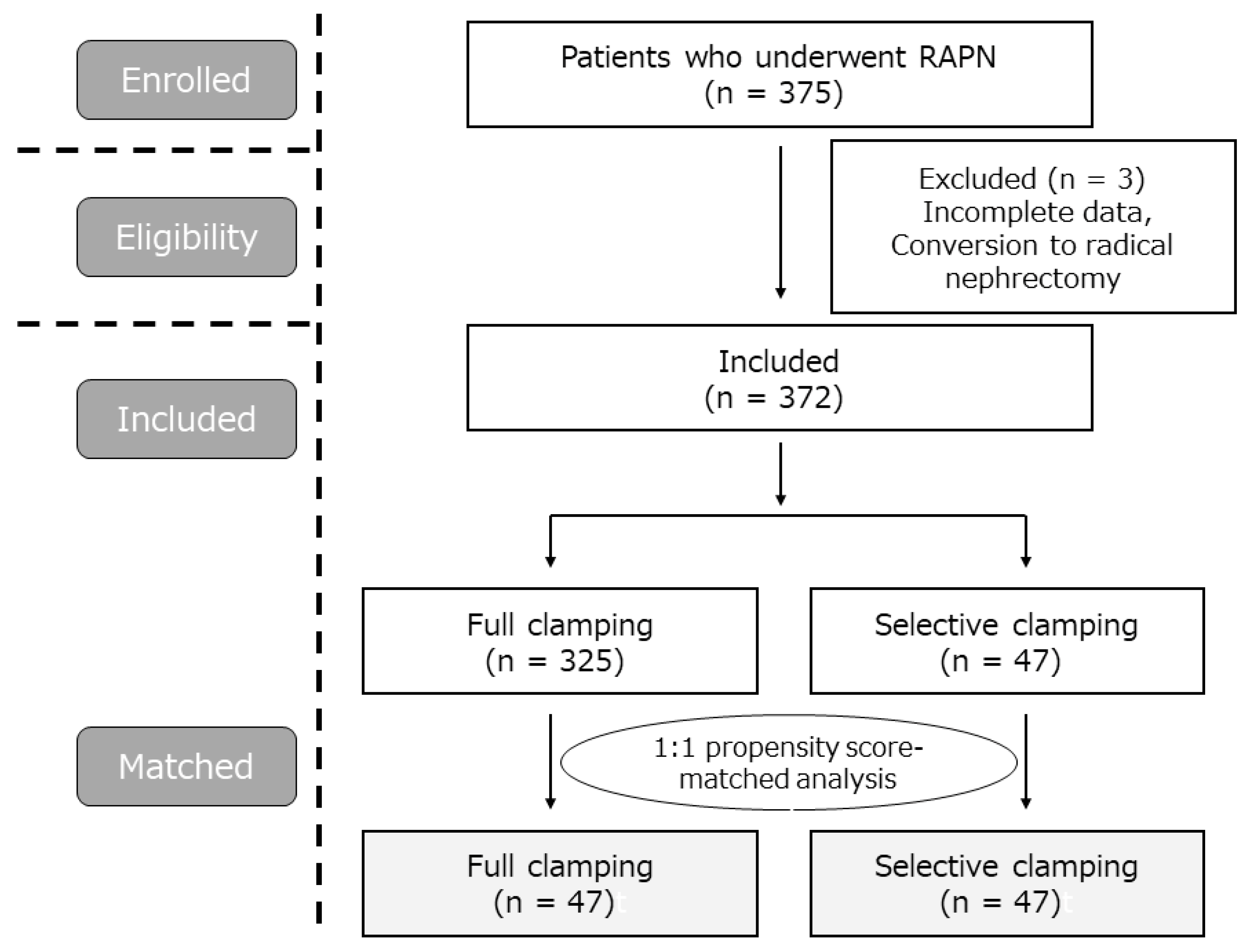
2.1. Patient Population
2.2. Surgery
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Perioperative Outcomes
3.3. Renal Functional Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scosyrev, E.; Messing, E.M.; Sylvester, R.; Campbell, S.; Van Poppel, H. Renal function after nephron-sparing surgery versus radical nephrectomy: Results from EORTC randomized trial 30904. Eur. Urol. 2014, 65, 372–377. [Google Scholar] [CrossRef]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A.; Colombel, M.; Klotz, L.; Skinner, E.; Keane, T.; et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2011, 59, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Medina, L.G.; Gill, T.; Abreu, A.; Sotelo, R.; Artibani, W.; Gill, I.S. Impact of Surgical Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 258–274. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 2012, 79, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Badani, K.K.; Kothari, P.D.; Okhawere, K.E.; Eun, D.; Hemal, A.; Abaza, R.; Porter, J.; Lovallo, G.; Ahmed, M.; Munver, R.; et al. Selective clamping during robot-assisted partial nephrectomy in patients with a solitary kidney: Is it safe and does it help? BJU Int. 2020, 125, 893–897. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; McClintock, T.R.; Stifelman, M.D. Near-infrared fluorescence imaging with intraoperative administration of indocyanine green for robotic partial nephrectomy. Curr. Urol. Rep. 2015, 16, 20. [Google Scholar] [CrossRef]
- Furukawa, J.; Miyake, H.; Hinata, N.; Muramaki, M.; Tanaka, K.; Fujisawa, M. Renal Functional and Perioperative Outcomes of Selective Versus Complete Renal Arterial Clamping During Robot-Assisted Partial Nephrectomy: Early Single-Center Experience With 39 Cases. Surg. Innov. 2016, 23, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Harke, N.; Schoen, G.; Schiefelbein, F.; Heinrich, E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: A single-surgeon matched-pair study. World J. Urol. 2014, 32, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Komninos, C.; Shin, T.Y.; Tuliao, P.; Han, W.K.; Chung, B.H.; Choi, Y.D.; Rha, K.H. Renal function is the same 6 months after robot-assisted partial nephrectomy regardless of clamp technique: Analysis of outcomes for off-clamp, selective arterial clamp and main artery clamp techniques, with a minimum follow-up of 1 year. BJU Int. 2015, 115, 921–928. [Google Scholar] [CrossRef]
- Martin, G.L.; Warner, J.N.; Nateras, R.N.; Andrews, P.E.; Humphreys, M.R.; Castle, E.P. Comparison of total, selective, and nonarterial clamping techniques during laparoscopic and robot-assisted partial nephrectomy. J. Endourol. 2012, 26, 152–156. [Google Scholar] [CrossRef]
- Mattevi, D.; Luciani, L.G.; Mantovani, W.; Cai, T.; Chiodini, S.; Vattovani, V.; Puglisi, M.; Malossini, G. Fluorescence-guided selective arterial clamping during RAPN provides better early functional outcomes based on renal scan compared to standard clamping. J. Robot. Surg. 2019, 13, 391–396. [Google Scholar] [CrossRef]
- McClintock, T.R.; Bjurlin, M.A.; Wysock, J.S.; Borofsky, M.S.; Marien, T.P.; Okoro, C.; Stifelman, M.D. Can selective arterial clamping with fluorescence imaging preserve kidney function during robotic partial nephrectomy? Urology 2014, 84, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Paulucci, D.J.; Rosen, D.C.; Sfakianos, J.P.; Whalen, M.J.; Abaza, R.; Eun, D.D.; Krane, L.S.; Hemal, A.K.; Badani, K.K. Selective arterial clamping does not improve outcomes in robot-assisted partial nephrectomy: A propensity-score analysis of patients without impaired renal function. BJU Int. 2017, 119, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Taweemonkongsap, T.; Suk-Ouichai, C.; Chotikawanich, E.; Jitpraphai, S.; Woranisarakul, V.; Ramart, P.; Phinthusophon, K.; Amornvesukit, T.; Leewansangtong, S.; Srinualnad, S.; et al. The Impact of Arterial Clamping Technique in Robot-Assisted Partial Nephrectomy on Renal Function and Surgical Outcomes: Six-Year Experience at Siriraj Hospital, Thailand. Urol. Int. 2018, 100, 301–308. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Yuan, J.; Jiang, Y. Comparison of selective and main renal artery clamping in partial nephrectomy of renal cell cancer: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e11856. [Google Scholar] [CrossRef]
- Desai, M.M.; de Castro Abreu, A.L.; Leslie, S.; Cai, J.; Huang, E.Y.; Lewandowski, P.M.; Lee, D.; Dharmaraja, A.; Berger, A.K.; Goh, A.; et al. Robotic partial nephrectomy with superselective versus main artery clamping: A retrospective comparison. Eur. Urol. 2014, 66, 713–719. [Google Scholar] [CrossRef]
- Long, J.A.; Fiard, G.; Giai, J.; Teyssier, Y.; Fontanell, A.; Overs, C.; Poncet, D.; Descotes, J.L.; Rambeaud, J.J.; Moreau-Gaudry, A.; et al. Superselective Ischemia in Robotic Partial Nephrectomy Does Not Provide Better Long-term Renal Function than Renal Artery Clamping in a Randomized Controlled Trial (EMERALD): Should We Take the Risk? Eur. Urol. Focus 2021, 8, 769–776. [Google Scholar] [CrossRef]
- Takahara, K.; Sumitomo, M.; Fukaya, K.; Jyoudai, T.; Nishino, M.; Hikichi, M.; Nukaya, T.; Zennami, K.; Ichino, M.; Fukami, N.; et al. Predictors for trifecta achievement of robot-assisted partial nephrectomy in high-complexity tumors (Preoperative Aspects and Dimensions Used for an Anatomical score >/=10). Asian J. Endosc. Surg. 2020, 13, 390–396. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The RENAL nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Khalifeh, A.; Autorino, R.; Hillyer, S.P.; Laydner, H.; Eyraud, R.; Panumatrassamee, K.; Long, J.A.; Kaouk, J.H. Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomy cases: A single surgeon experience. J. Urol. 2013, 189, 1236–1242. [Google Scholar] [CrossRef] [PubMed]


| Pre-Matching | Post-Matching | |||||
|---|---|---|---|---|---|---|
| Median (IQR) or n (%) | Full Clamping (n = 325) | Selective Clamping (n = 47) | p Value | Full Clamping (n = 47) | Selective Clamping (n = 47) | p Value |
| Age | 62 (53–70) | 60 (51–67) | 0.390 | 58 (46–65) | 60 (51–67) | 0.222 |
| Sex (%) | ||||||
| Male | 236 (72.6) | 33 (70.2) | 0.729 | 33 (70.2) | 33 (70.2) | 1.000 |
| Female | 89 (27.4) | 14 (29.8) | 14 (29.8) | 14 (29.8) | ||
| BMI, kg/m2 | 24 (22–26) | 24 (22–26) | 0.749 | 24 (22–27) | 24 (22–26) | 0.349 |
| ASA score | ||||||
| 1 | 101 (31.1) | 22 (46.8) | 0.074 | 27 (57.4) | 22 (46.8) | 0.698 |
| 2 | 217 (66.8) | 24 (51.1) | 19 (40.4) | 24 (51.1) | ||
| 3 | 7 (2.2) | 1 (2.1) | 1 (2.1) | 1 (2.1) | ||
| eGFR, mL/min/1.73 m2 | 69.6 (58.9–80.2) | 66.2 (51.9–77.1) | 0.115 | 66.5 (56.5–78.2) | 66.2 (51.9–77.1) | 0.675 |
| Tumor side | ||||||
| Right | 158 (48.6) | 31 (66.0) | 0.029 | 30 (63.8) | 31 (66.0) | 1.000 |
| Left | 167 (51.4) | 16 (34.0) | 17 (36.2) | 16 (34.0) | ||
| Approach | ||||||
| Transperitoneal | 156 (48.0) | 27 (57.4) | 0.275 | 22 (46.8) | 27 (57.4) | 0.409 |
| Retroperitoneal | 169 (52.0) | 20 (42.6) | 25 (53.2) | 20 (42.6) | ||
| Tumor size, mm | 29 (22–37) | 30 (22–35) | 0.834 | 25 (20–33) | 30 (22–35) | 0.193 |
| RENAL score | ||||||
| 4–6 | 149 (45.8) | 15 (31.9) | 0.186 | 21 (44.7) | 15 (31.9) | 0.136 |
| 7–9 | 155 (47.7) | 29 (61.7) | 26 (55.3) | 29 (61.7) | ||
| 10–12 | 21 (6.5) | 3 (6.4) | 0 (0) | 3 (6.4) | ||
| Hilar tumor | 59 (18.2) | 10 (21.3) | 0.688 | 8 (17.0) | 10 (21.3) | 0.794 |
| Cystic tumor | 48 (14.8) | 8 (17.0) | 0.665 | 5 (10.6) | 8 (17.0) | 0.552 |
| Post-Matching | |||
|---|---|---|---|
| Median (IQR) or n (%) | Full Clamping (n = 47) | Selective Clamping (n = 47) | p Value |
| Surgical time, min | 165 (143–198) | 152 (136–180) | 0.208 |
| Console time, min | 114 (88–132) | 102 (90–132) | 0.623 |
| WIT, min | 16 (12–19) | 16 (13–19) | 0.738 |
| EBL, mL | 30 (15–100) | 60 (30–112) | 0.046 |
| Negative surgical margins | 47 (100) | 47 (100) | 1.000 |
| Pathology, clear cell carcinoma | 13 (27.7) | 14 (29.8) | 1.000 |
| All grades of complications | 2 (4.3) | 7 (14.9) | 0.158 |
| Clavien–Dindo ≥ grade 3 | 1 (2.1) | 3 (6.4) | 0.617 |
| Trifecta achievement | 44 (93.6) | 38 (80.9) | 0.120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahara, K.; Kusaka, M.; Nukaya, T.; Takenaka, M.; Zennami, K.; Ichino, M.; Sasaki, H.; Sumitomo, M.; Shiroki, R. Functional Outcomes after Selective Clamping in Robot-Assisted Partial Nephrectomy. J. Clin. Med. 2022, 11, 5648. https://doi.org/10.3390/jcm11195648
Takahara K, Kusaka M, Nukaya T, Takenaka M, Zennami K, Ichino M, Sasaki H, Sumitomo M, Shiroki R. Functional Outcomes after Selective Clamping in Robot-Assisted Partial Nephrectomy. Journal of Clinical Medicine. 2022; 11(19):5648. https://doi.org/10.3390/jcm11195648
Chicago/Turabian StyleTakahara, Kiyoshi, Mamoru Kusaka, Takuhisa Nukaya, Masashi Takenaka, Kenji Zennami, Manabu Ichino, Hitomi Sasaki, Makoto Sumitomo, and Ryoichi Shiroki. 2022. "Functional Outcomes after Selective Clamping in Robot-Assisted Partial Nephrectomy" Journal of Clinical Medicine 11, no. 19: 5648. https://doi.org/10.3390/jcm11195648
APA StyleTakahara, K., Kusaka, M., Nukaya, T., Takenaka, M., Zennami, K., Ichino, M., Sasaki, H., Sumitomo, M., & Shiroki, R. (2022). Functional Outcomes after Selective Clamping in Robot-Assisted Partial Nephrectomy. Journal of Clinical Medicine, 11(19), 5648. https://doi.org/10.3390/jcm11195648





