Subclinical Myocardial Fibrosis in Systemic Lupus Erythematosus as Assessed by Pulse-Cancellation Echocardiography: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient Involvement
2.3. Study Outcomes
2.4. Study Procedures
2.4.1. Pulse-Cancellation Echocardiography
2.4.2. Speckle-Tracking Echocardiography
2.4.3. CVD Risk Assessment
2.4.4. SLE Assessment
2.4.5. Laboratory Parameters
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of SLE Patients
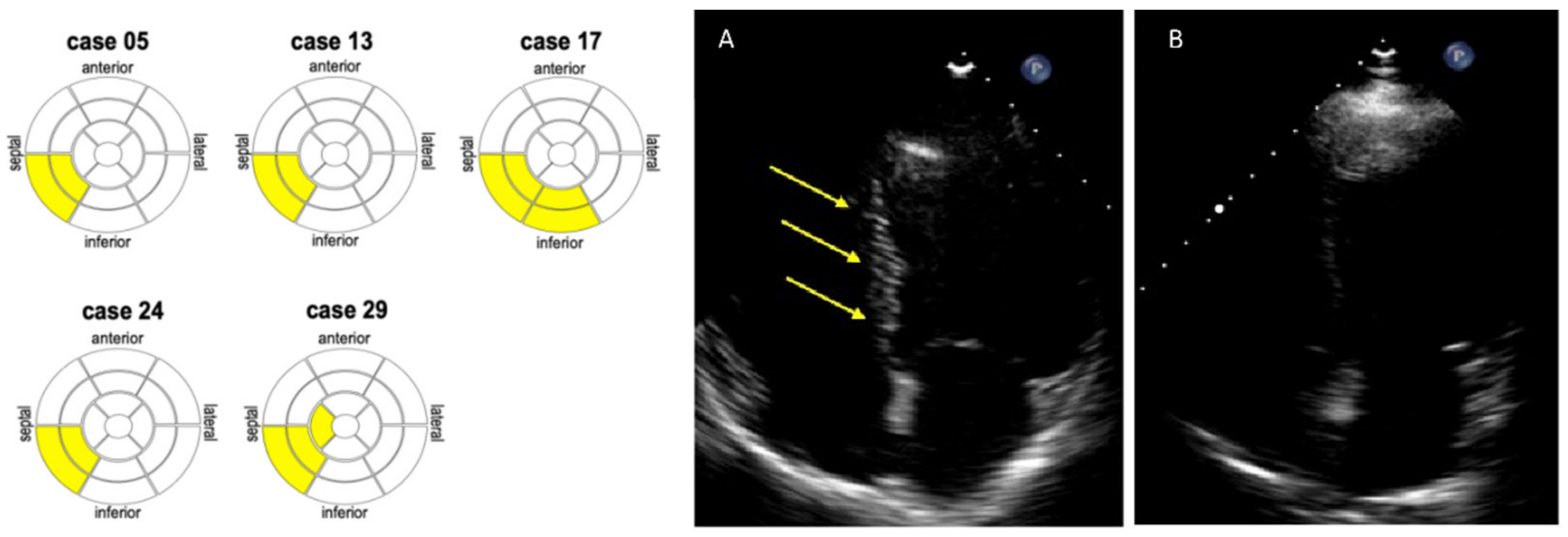
3.2. Detection of Myocardial Scars by eSCAR in SLE Patients
3.3. Association of Myocardial Scar by eSCAR with Impaired Myocardial Strain in SLE Patients
3.4. Association of Myocardial Scars by eSCAR with Glucocorticoids and Anti-dsDNA
3.5. Association of Myocardial Scars by eSCAR with SLE Flares
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bengtsson, A.A.; Rylander, L.; Hagmar, L.; Nived, O.; Sturfelt, G. Risk factors for developing systemic lupus erythematosus: A case-control study in southern Sweden. Rheumatology 2002, 41, 563–571. [Google Scholar] [CrossRef]
- Palmieri, V.; Migliaresi, P.; Orefice, M.; Lupo, T.; Di Minno, M.; Valentini, G.; Celentano, A. High prevalence of subclinical cardiovascular abnormalities in patients with systemic lupus erythematosus in spite of a very low clinical damage index. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Manzi, S.; Meilahn, E.N.; Rairie, J.E.; Conte, C.G.; Medsger, T.A.; Jansen-McWilliams, L.; D’Agostino, R.B.; Kuller, L.H. Age-specific Incidence Rates of Myocardial Infarction and Angina in Women with Systemic Lupus Erythematosus: Comparison with the Framingham Study. Am. J. Epidemiol. 1997, 145, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.; McGwin, J.G.; Vila, L.M.; Kaslow, R.A.; Alarcon, G.S.; Reveille, J.D.; LUMINA Study Group. Associated factors and impact of myocarditis in patients with SLE from LUMINA, a multiethnic US cohort. Rheumatology 2007, 47, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.; Petersen, J.; Ullman, S.; Junker, P.; Voss, A.; Rasmussen, J.M.; Tarp, U.; Poulsen, L.H.; Hansen, G.V.O.; Skaarup, B.; et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus. II. Disease mortality and clinical factors of prognostic value. Clin. Rheumatol. 1998, 17, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Tanwani, J.; Tselios, K.; Gladman, D.D.; Su, J.; Urowitz, M.B. Lupus myocarditis: A single center experience and a comparative analysis of observational cohort studies. Lupus 2018, 27, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, W.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A.; et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018, 137, 961–972. [Google Scholar] [CrossRef]
- Comarmond, C.; Cacoub, P. Myocarditis in auto-immune or auto-inflammatory diseases. Autoimmun. Rev. 2017, 16, 811–816. [Google Scholar] [CrossRef]
- Winau, L.; Baydes, R.H.; Braner, A.; Drott, U.; Burkhardt, H.; Sangle, S.; D’Cruz, D.P.; Carr-White, G.; Marber, M.; Schnoes, K.; et al. High-sensitive troponin is associated with subclinical imaging biosignature of inflammatory cardiovascular involvement in systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 1590–1598. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Chan, A.K.; Brown, K.A.; Chan, C.W.; Reynolds, H.G.; Tsang, S.; Davis, R.B. Impact of Unrecognized Myocardial Scar Detected by Cardiac Magnetic Resonance Imaging on Event-Free Survival in Patients Presenting with Signs or Symptoms of Coronary Artery Disease. Circulation 2006, 113, 2733–2743. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.H.; Ismail, N.A.; Dweck, M.R.; et al. Association of Fibrosis with Mortality and Sudden Cardiac Death in Patients with Nonischemic Dilated Cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef]
- Wu, E.; Judd, R.M.; Vargas, J.D.; Klocke, F.J.; Bonow, R.O.; Kim, R.J. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet 2001, 357, 21–28. [Google Scholar] [CrossRef]
- Wagner, A.; Mahrholdt, H.; Holly, T.A.; Elliott, M.D.; Regenfus, M.; Parker, M.; Klocke, F.J.; Bonow, R.O.; Kim, R.J.; Judd, R.M. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet 2003, 361, 374–379. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Bianconcini, M.; Marziliano, N.; Parrini, I.; Conte, M.R.; Siniscalchi, C.; Faden, G.; Faggiano, P.; Pigazzani, F.; Grassi, F.; et al. Scar Detection by Pulse-Cancellation Echocardiography. JACC: Cardiovasc. Imaging 2016, 9, 1239–1251. [Google Scholar] [CrossRef]
- Petri, M.; Buyon, J.; Kim, M. Classification and definition of major flares in SLE clinical trials. Lupus 1999, 8, 685–691. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Tuttolomondo, D.; Guaricci, A.I.; Di Giannuario, G. Pulse-Cancellation Echocardiography for Clinical Evaluation of Myocardial Scar Burden. Curr. Cardiol. Rep. 2021, 23, 100. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Du Toit, R.; Herbst, P.G.; van Rensburg, A.; Snyman, H.W.; Reuter, H.; Doubell, A.F. Speckle tracking echocardiography in acute lupus myocarditis: Comparison to conventional echocardiography. Echo Res. Pract. 2017, 4, 9–19. [Google Scholar] [CrossRef]
- Farag, S.I.; Bastawisy, R.B.; Hamouda, M.A.; Hassib, W.A.; Wahdan, H.A. Value of speckle tracking echocardiography for early detection of left ventricular dysfunction in patients with systemic lupus erythematosus. J. Cardiovasc. Echogr. 2020, 30, 140–145. [Google Scholar] [CrossRef]
- Zen, M.; Bassi, N.; Nalotto, L.; Canova, M.; Bettio, S.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Disease activity patterns in a monocentric cohort of SLE patients: A seven-year follow-up study. Clin. Exp. Rheumatol. 2012, 30, 856–863. [Google Scholar]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Puntmann, V.O.; D’Cruz, D.; Smith, Z.; Pastor, A.; Choong, P.; Voigt, T.; Carr-White, G.; Sangle, S.; Schaeffter, T.; Nagel, E. Native Myocardial T1 Mapping by Cardiovascular Magnetic Resonance Imaging in Subclinical Cardiomyopathy in Patients with Systemic Lupus Erythematosus. Circ. Cardiovasc. Imaging 2013, 6, 295–301. [Google Scholar] [CrossRef]
- Burkard, T.; Trendelenburg, M.; Daikeler, T.; Hess, C.; Bremerich, J.; Haaf, P.; Buser, P.; Zellweger, M.J. The heart in systemic lupus erythematosus—A comprehensive approach by cardiovascular magnetic resonance tomography. PLoS ONE 2018, 13, e0202105. [Google Scholar] [CrossRef]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular Magnetic Resonance, Fibrosis, and Prognosis in Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef]

| SLE Patients (n = 27) | Controls (n = 32) | p-Value | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Age, years | 45 ± 11 | 46 ± 7 | 0.797 |
| Male sex, n (%) | 3 (11) | 0 (0) | 0.090 |
| BMI, kg/m2 | 23 ± 3 | 23 ± 4 | 0.999 |
| Current smokers, n (%) | 10 (37) | 8 (25) | 0.399 |
| Hypertension, n (%) | 8 (30) | 3 (9) | 0.091 |
| Hypercholesterolemia, n (%) | 4 (15) | 6 (19) | 0.728 |
| Standard echocardiography | |||
| LV EDV index, mL/m2 | 53.8 ± 11 | 49.1 ± 6.9 | 0.04 |
| LV ESV index, mL/m2 | 20.9 ± 5.2 | 17.9 ± 3.7 | 0.01 |
| LV EF, % | 61.2 ± 4.2 | 63.7 ± 2.9 | 0.009 |
| LV mass index, g/m2 | 64 ± 14.7 | 65 ± 17.6 | 0.87 |
| LAVI, mL/m2 | 22.8 ± 6.9 | 24 ± 6.3 | 0.49 |
| E velocity (cm/s) | 74.3 ± 21.7 | 77.9 ± 17.8 | 0.47 |
| A velocity (cm/s) | 60 ± 18.6 | 66.6 ± 17.6 | 0.16 |
| Deceleration time, ms | 183.8 ± 74.5 | 182.5 ± 60.7 | 0.94 |
| E/A ratio | 1.3 ± 0.6 | 1.2 ± 0.4 | 0.72 |
| E/e’ ratio | 6.9 ± 2.5 | 6.7 ± 2.1 | 0.85 |
| TRPG, mmHg | 17.5 ± 4.1 | 19.4 ± 4.3 | 0.29 |
| TAPSE, mmHg | 24 ± 7.2 | 24.3 ± 2.7 | 0.82 |
| s’ tricuspidal velocity, cm/s | 10.3 ± 5.1 | 13.2 ± 1.7 | 0.01 |
| Speckle tracking echocardiography | |||
| GLS global (%) | −21 ± 2 | −23.9 ± 1.8 | <0.0001 |
| GLS 4-chamber (%) | −21.5 ± 2.7 | −22.8 ± 1.9 | 0.03 |
| GLS 2-chamber (%) | −21.6 ± 2.4 | −22.8 ± 2.1 | 0.04 |
| GLS 3-chamber (%) | −20.9 ± 2.6 | −22.5 ± 2.4 | 0.01 |
| GLS base (%) | −19 ± 2.6 | −22.8 ± 2.9 | <0.0001 |
| GLS mid (%) | −19.5 ± 2 | −23.5 ± 3.4 | <0.0001 |
| GLS apex (%) | −25.1 ± 3 | −25.5 ± 3.3 | 0.6 |
| GLS anterior (%) | −21.9 ± 2.4 | −23.8 ± 4.3 | 0.03 |
| GLS antero-septal (%) | −22.6 ± 3.2 | −25.8 ± 3.6 | 0.001 |
| GLS infero-septal (%) | −20.9 ± 2.5 | −23.5 ± 2.8 | <0.0001 |
| GLS inferior (%) | −21.2 ± 2.4 | −25 ± 3.5 | <0.0001 |
| GLS infero-lateral (%) | −20.3 ± 2.6 | −22.6 ± 2.7 | 0.001 |
| GLS antero-lateral (%) | −21.4 ± 2.7 | −23.5 ± 2.7 | 0.004 |
| Myocardial fibrosis (eSCAR) | |||
| eSCAR, n (%) | 5 (19) | 0 (0) | 0.01 |
| eSCAR anterior, n (%) | 0 (0) | 0 (0) | ND |
| eSCAR antero-septal, n (%) | 0 (0) | 0 (0) | ND |
| eSCAR infero-septal, n (%) | 5 (19) | 0 (0) | 0.01 |
| eSCAR inferior, n (%) | 1 (4) | 0 (0) | 0.29 |
| eSCAR infero-lateral, n (%) | 0 (0) | 0 (0) | ND |
| eSCAR antero-lateral, n (%) | 0 (0) | 0 (0) | ND |
| eSCAR, n (%) | 5 (19) | 0 (0) | 0.01 |
| eSCAR anterior, n (%) | 0 (0) | 0 (0) | ND |
| eSCAR antero-septal, n (%) | 0 (0) | 0 (0) | ND |
| eSCAR infero-septal, n (%) | 5 (19) | 0 (0) | 0.01 |
| eSCAR-Positive (n = 5) | eSCAR-Negative (n = 22) | p-Value | |
|---|---|---|---|
| Standard echocardiography | |||
| LV EDV index, mL/m2 | 56.7 ± 18.5 | 53.3 ± 9.3 | 0.53 |
| LV ESV index, mL/m2 | 22.4 ± 8 | 20.7 ± 4.7 | 0.53 |
| LV EF, % | 60.7 ± 3.2 | 61.3 ± 4.4 | 0.76 |
| LV mass index, g/m2 | 67.7 ± 20.7 | 63.3 ± 13.6 | 0.55 |
| LAVI, mL/m2 | 19.8 ± 7.7 | 23.5 ± 6.7 | 0.28 |
| E velocity (cm/s) | 76.2 ± 15.1 | 73.8 ± 23.1 | 0.82 |
| A velocity (cm/s) | 56.5 ± 20.6 | 60.8 ± 18.6 | 0.64 |
| Deceleration time, ms | 225.2 ± 30.8 | 175.1 ± 78.4 | 0.17 |
| E/A ratio | 1.5 ± 0.6 | 1.2 ± 0.5 | 0.37 |
| E/e’ ratio | 7.8 ± 3.7 | 6.7 ± 2.2 | 0.40 |
| TRPG, mmHg | 22 ± 2.4 | 25.2 ± 7.6 | 0.46 |
| TAPSE, mmHg | 12 ± 1.8 | 9.9 ± 5.5 | 0.47 |
| s’ tricuspidal velocity, cm/s | 56.7 ± 18.5 | 53.3 ± 9.3 | 0.53 |
| Speckle tracking echocardiography | |||
| GLS global (%) | −18.4 ± 1.5 | −21.6 ± 1.7 | 0.001 |
| GLS 4-chamber (%) | −18.2 ± 2.2 | −22.2 ± 2.3 | 0.002 |
| GLS 2-chamber (%) | −18.9 ± 1.9 | −22.2 ± 2.1 | 0.003 |
| GLS 3-chamber (%) | −19.8 ± 3.6 | −21.1 ± 2.4 | 0.31 |
| GLS base (%) | −15.7 ± 2.4 | −19.7 ± 2.2 | 0.001 |
| GLS mid (%) | −17.3 ± 1.4 | −20 ± 1.9 | 0.005 |
| GLS apex (%) | −23.1 ± 1.1 | −25.5 ± 3.1 | 0.1 |
| GLS anterior (%) | −18.8 ± 1.9 | −22.5 ± 2 | 0.001 |
| GLS antero-septal (%) | −20.7 ± 2.1 | −23.1 ± 3.3 | 0.13 |
| GLS infero-septal (%) | −17.3 ± 2.1 | −21.7 ± 1.9 | <0.0001 |
| GLS inferior (%) | −18.5 ± 2.1 | −21.7 ± 2.2 | 0.006 |
| GLS infero-lateral (%) | −18.3 ± 3.3 | −20.7 ± 2.4 | 0.05 |
| GLS antero-lateral (%) | −18.8 ± 2.7 | −21.9 ± 2.4 | 0.01 |
| eSCAR-Positive (n = 5) | eSCAR-Negative (n = 22) | p-Value | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| Age, years | 41 (33, 50) | 45 (39, 54) | 0.151 |
| Female sex, n (%) | 4 (80) | 20 (91) | 0.999 |
| Obesity, n (%) | 0 (0) | 1 (5) | 0.999 |
| Smoking (ever), n (%) | 3 (60) | 7 (32) | 0.326 |
| Hypertension, n (%) | 2 (40) | 6 (27) | 0.616 |
| Dyslipidemia, n (%) | 0 (0) | 4 (18) | 0.561 |
| SLE characteristics | |||
| Disease duration, years | 11 (3, 24) | 13 (8, 21) | 0.901 |
| Age at diagnosis, years | 23 (17, 45) | 29 (23, 34) | 0.289 |
| SLEDAI score | 5 (2.5, 10.5) | 2.0 (2.0, 6.0) | 0.161 |
| SDI score | 0.5 (0.0, 1.8) | 1.0 (0.0, 2.0) | 0.973 |
| Arthritis, n (%) | 3 (60) | 15 (68) | 0.999 |
| Neuropsychiatric manifestations, n (%) | 0 (0) | 3 (14) | 0.999 |
| Lupus nephritis, n (%) | 1 (20) | 11 (50) | 0.342 |
| Mucocutaneous manifestations, n (%) | 3 (60) | 12 (55) | 0.999 |
| Cytopenia, n (%) | 3 (60) | 8 (36) | 0.370 |
| Antiphospholipid syndrome, n (%) | 1 (20) | 5 (23) | 0.999 |
| Serositis, n (%) | 0 (0) | 3 (14) | 0.999 |
| Laboratory data | |||
| Hemoglobin, g/dL | 12.9 (12.6, 13.5) | 13.0 (12.0, 14.1) | 0.731 |
| Creatinine, mg/dL | 0.81 (0.55, 1.51) | 0.67 (0.60, 0.74) | 0.377 |
| eGFR, mL/min per 1.73 m2 | 103 (87, 121) | 100 (69, 161) | 0.454 |
| ESR, mm/h | 15 (6, 28) | 15 (6, 21) | 0.433 |
| CRP, mg/L | 1.5 (0.2, 2.8) | 2.0 (1.0, 4.0) | 0.271 |
| Complement C3, g/L | 71 (60, 91) | 87 (70, 99) | 0.142 |
| Complement C4, g/L | 12 (6, 30) | 15 (8, 18) | 0.901 |
| Autoantibodies | |||
| Antinuclear Ab (≥1:80) IIF, n (%) | 5 (100) | 22 (100) | 0.999 |
| Antinuclear Ab titer, dilution | 1:1280 (1:640, 1:1280) | 1:320 (1:160, 1:640) | 0.019 |
| Anti-dsDNA Ab IIF, n (%) | 4 (80) | 15 (68) | 0.540 |
| Anti-dsDNA Ab titer, IU/mL | 127 (55, 286) | 21 (0, 54) | 0.037 |
| Anti-Smith Ab, n (%) | 2 (40) | 4 (18) | 0.303 |
| Anti-Ro/SSA Ab, n (%) | 2 (40) | 9 (41) | 0.999 |
| Anti-La/SSB Ab, n (%) | 0 (0) | 6 (27) | 0.555 |
| Anti-U1RNP Ab, n (%) | 2 (40) | 4 (18) | 0.303 |
| Antiphospholipid Ab, n (%) | 2 (40) | 9 (41) | 0.999 |
| SLE medications | |||
| Glucocorticoids, n (%) | 4 (80) | 12 (55) | 0.618 |
| Current prednisone dose, mg day | 11 (1, 27) | 3 (0, 5) | 0.067 |
| Cumulative prednisone dose, g | 36 (6, 61) | 19 (5, 26) | 0.212 |
| Hydroxychloroquine, n (%) | 3 (60) | 19 (86) | 0.221 |
| Methotrexate, n (%) | 1 (20) | 3 (14) | 0.999 |
| Azathioprine, n (%) | 0 (0) | 2 (9) | 0.999 |
| Mycophenolate, n (%) | 1 (20) | 9 (41) | 0.621 |
| Belimumab, n (%) | 2 (40) | 5 (23) | 0.580 |
| Prior cyclophosphamide, n (%) | 0 (0) | 3 (14) | 0.999 |
| Prior rituximab, n (%) | 2 (40) | 2 (9) | 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giollo, A.; Vinco, G.; Cioffi, G.; Frizzera, F.; Quinternetto, A.; Bergamini, C.; Dal Porto, M.; Orsolini, G.; Zen, M.; Doria, A.; et al. Subclinical Myocardial Fibrosis in Systemic Lupus Erythematosus as Assessed by Pulse-Cancellation Echocardiography: A Pilot Study. J. Clin. Med. 2022, 11, 4788. https://doi.org/10.3390/jcm11164788
Giollo A, Vinco G, Cioffi G, Frizzera F, Quinternetto A, Bergamini C, Dal Porto M, Orsolini G, Zen M, Doria A, et al. Subclinical Myocardial Fibrosis in Systemic Lupus Erythematosus as Assessed by Pulse-Cancellation Echocardiography: A Pilot Study. Journal of Clinical Medicine. 2022; 11(16):4788. https://doi.org/10.3390/jcm11164788
Chicago/Turabian StyleGiollo, Alessandro, Giulia Vinco, Giovanni Cioffi, Francesca Frizzera, Anna Quinternetto, Corinna Bergamini, Marta Dal Porto, Giovanni Orsolini, Margherita Zen, Andrea Doria, and et al. 2022. "Subclinical Myocardial Fibrosis in Systemic Lupus Erythematosus as Assessed by Pulse-Cancellation Echocardiography: A Pilot Study" Journal of Clinical Medicine 11, no. 16: 4788. https://doi.org/10.3390/jcm11164788
APA StyleGiollo, A., Vinco, G., Cioffi, G., Frizzera, F., Quinternetto, A., Bergamini, C., Dal Porto, M., Orsolini, G., Zen, M., Doria, A., Gatti, D., Ribichini, F. L., Targher, G., Rossini, M., & Viapiana, O. (2022). Subclinical Myocardial Fibrosis in Systemic Lupus Erythematosus as Assessed by Pulse-Cancellation Echocardiography: A Pilot Study. Journal of Clinical Medicine, 11(16), 4788. https://doi.org/10.3390/jcm11164788







