Percutaneous Closure of Mitral Paravalvular Leak: Long-Term Results in a Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Baseline and Procedural Variables
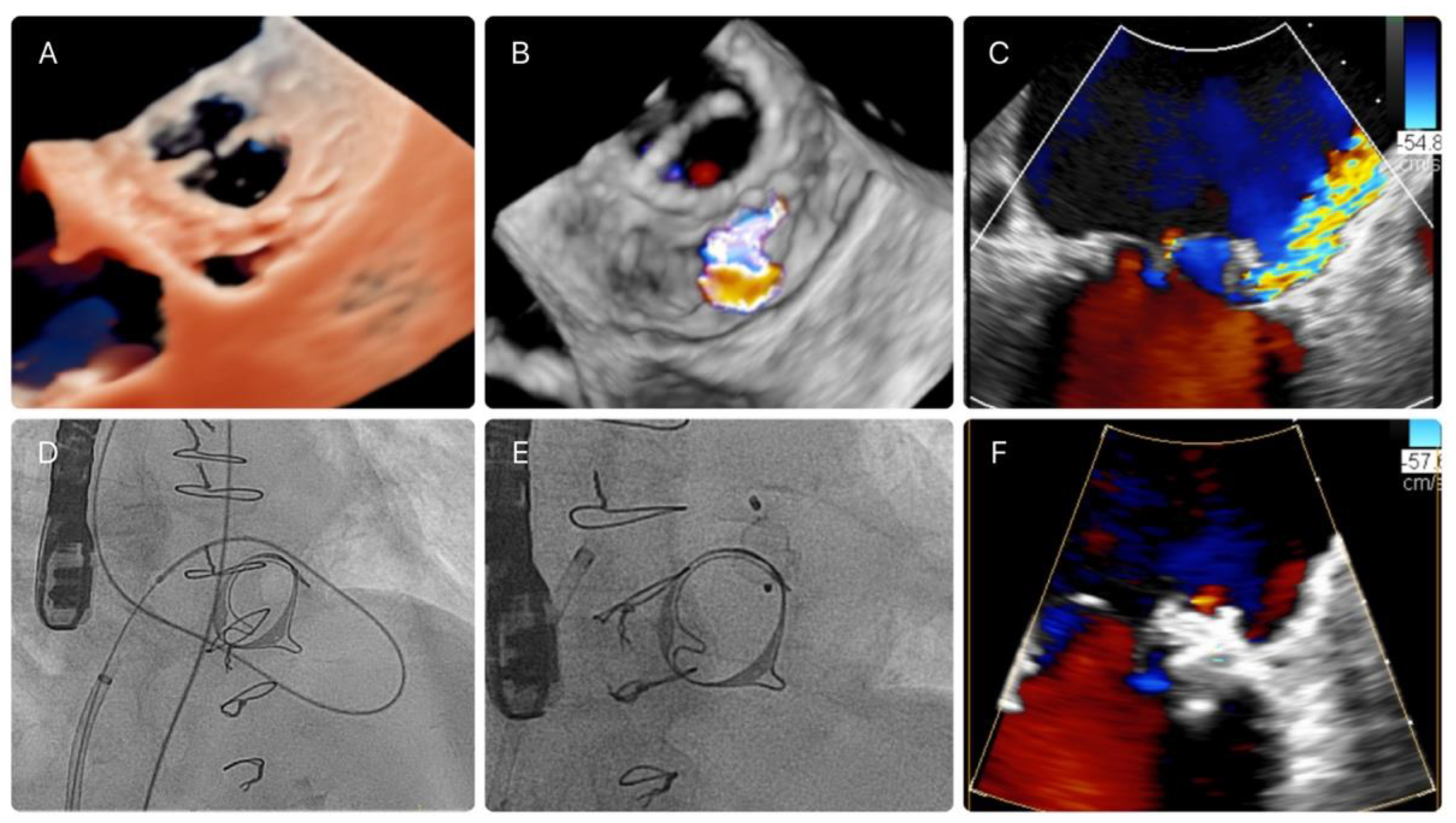
2.3. PVL Closure Technique and Characteristics of the Procedure
2.4. Imaging Data
2.5. Clinical and Echocardiographic Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Procedural Characteristics
3.2. Predictors of Procedural Success
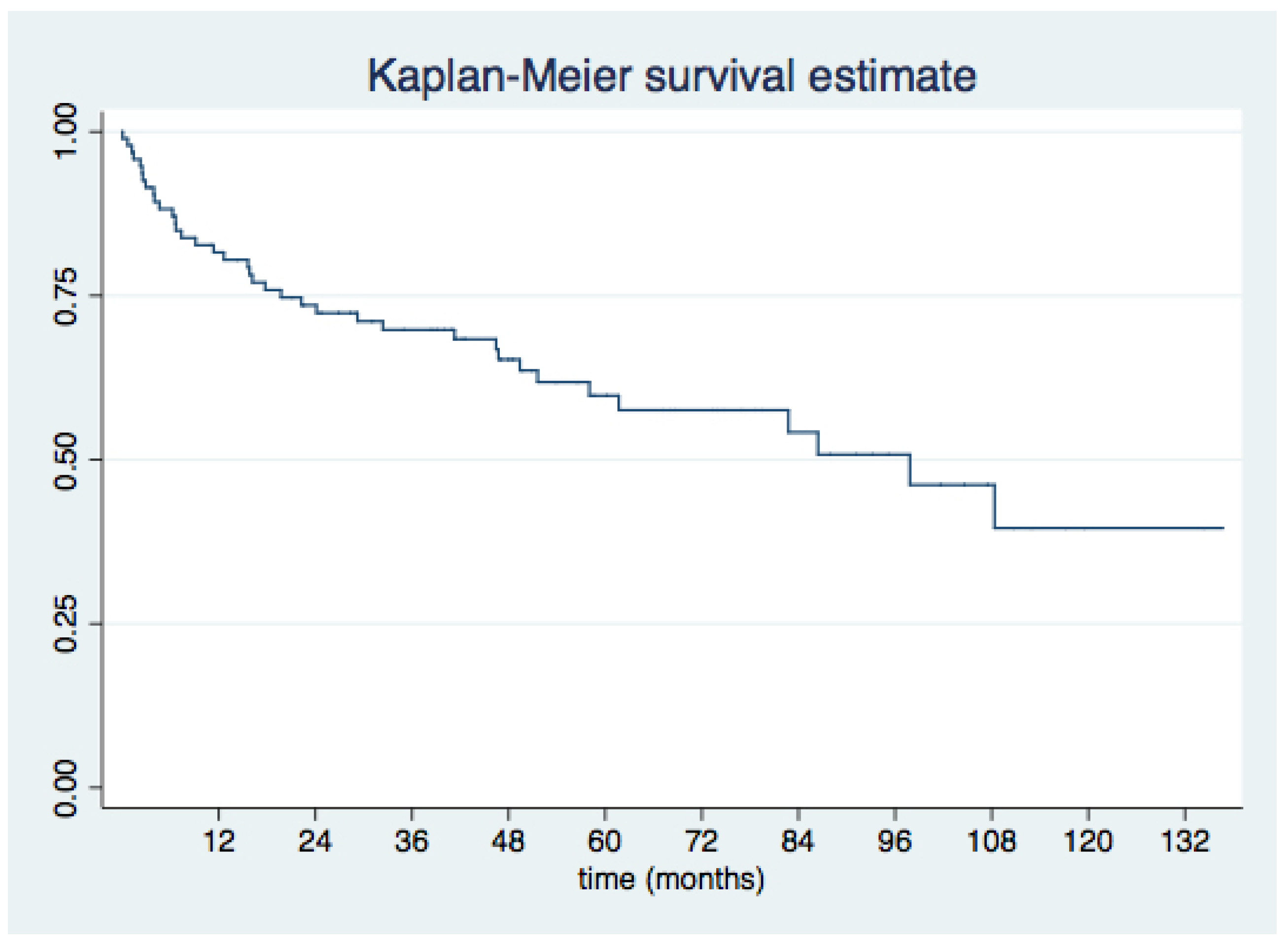
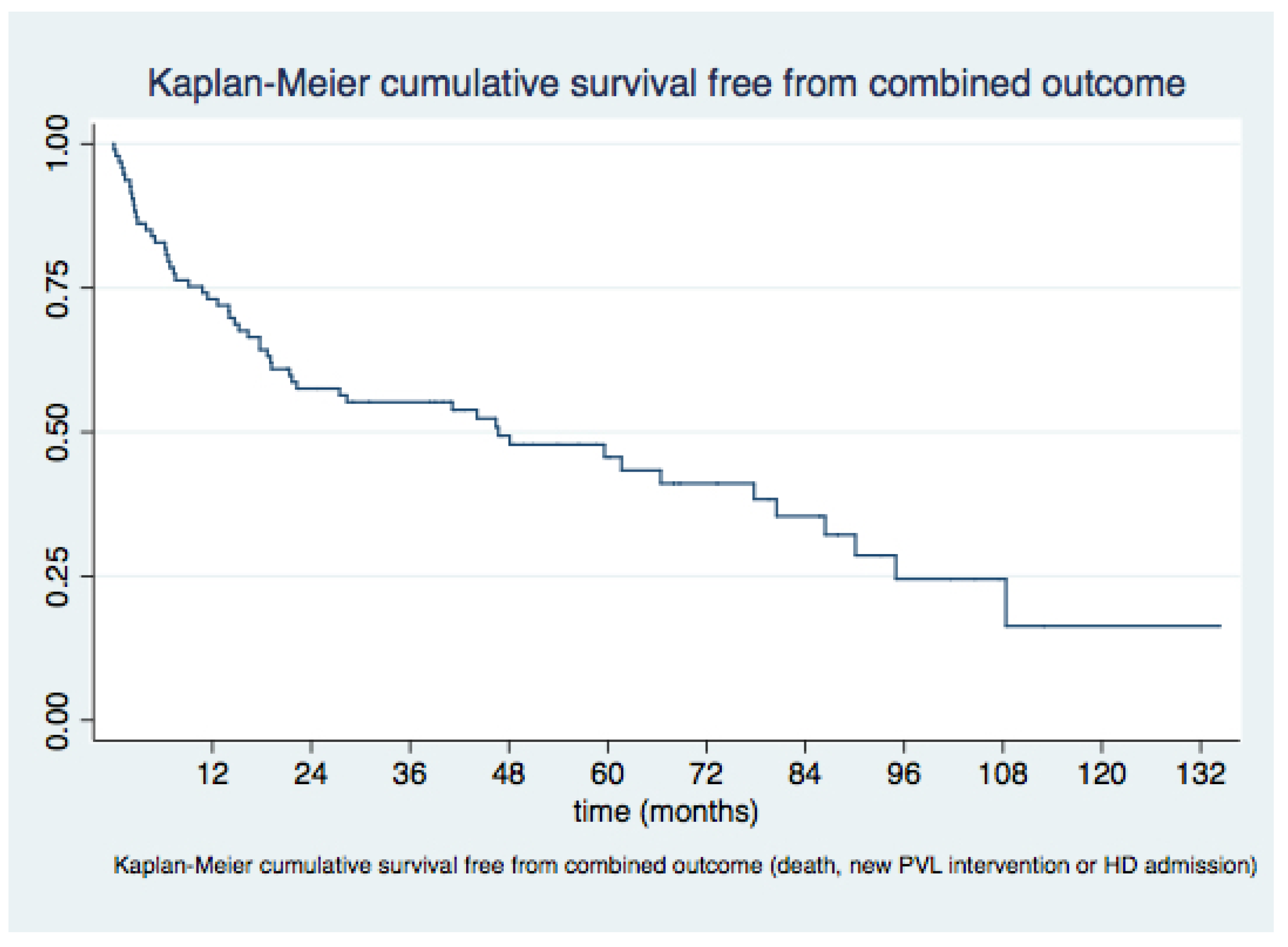
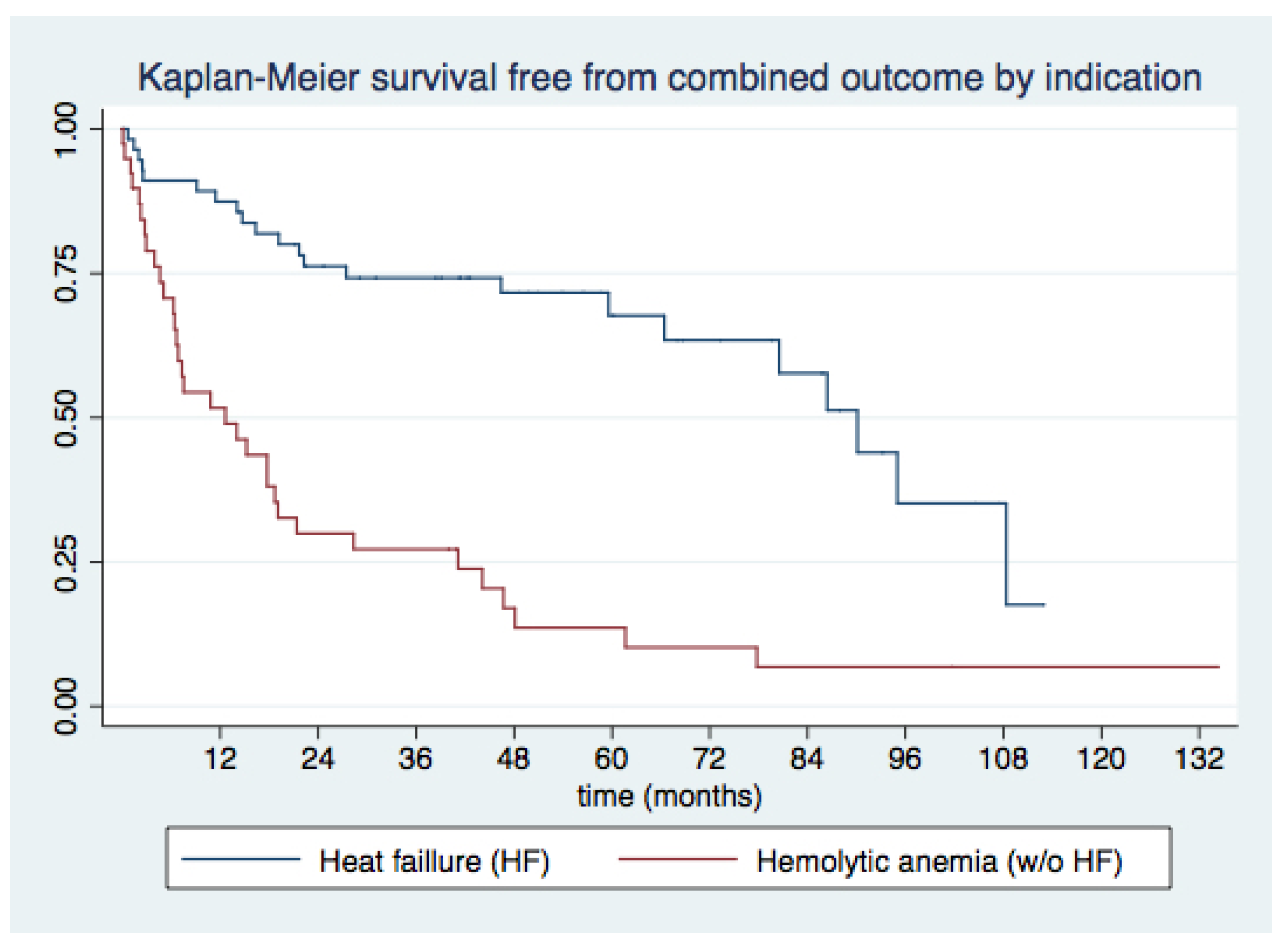
3.3. Medium- and Long-Term Outcomes
4. Discussion
- (1)
- Percutaneous mitral PVL closure procedures can attain high procedural success rates, with an acceptable safety profile, considering the high-risk profile of target patients;
- (2)
- Short- and long-term clinical outcomes after mitral PVL closure are favorable, with sustained improvement in functional class in over 75% of patients during long-term follow-up and 51% survival at 4 years;
- (3)
- The presence of multiple PVLs was the sole independent predictor of procedural failure;
- (4)
- Underlying hemolytic anemia as the indication for PVL closure, recent admissions for decompensated HF, and lack of improvement in functional class emerged as consistent predictors of MACE and all-cause death during long-term follow-up.
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Gonzalez, I.; Rama-Merchan, J.C.; Rodríguez-Collado, J.; Martín-Moreiras, J.; Diego-Nieto, A.; Barreiro-Perez, M.; Sanchez, P.L. Transcatheter closure of paravalvular leaks: State of the art. Neth. Heart J. 2016, 25, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Yucel, E. Paravalvular Leaks—From Diagnosis to Management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, I.; Mazine, A.; Ghoneim, A.; Millàn, X.; El-Hamamsy, I.; Pellerin, M.; Cartier, R.; Demers, P.; Lamarche, Y.; Bouchard, D. Long-term results after surgical treatment of paravalvular leak in the aortic and mitral position. J. Thorac. Cardiovasc. Surg. 2016, 151, 1260.e1–1266.e1. [Google Scholar] [CrossRef] [PubMed]
- Kloster, F.E. Diagnosis and management of complications of prosthetic heart valves. Am. J. Cardiol. 1975, 35, 872–885. [Google Scholar] [CrossRef]
- Ionescu, A.; Fraser, A.G.; Butchart, E.G. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: A prospective study using transoesophageal echocardiography. Heart 2003, 89, 1316–1321. [Google Scholar] [CrossRef]
- Kronzon, I.; Sugeng, L.; Perk, G.; Hirsh, D.; Weinert, L.; Garcia Fernandez, M.A.; Lang, R.M. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehis-cence. J. Am. Coll. Cardiol. 2009, 53, 1543–1547. [Google Scholar] [CrossRef]
- Arribas-Jimenez, A.; Rama-Merchan, J.C.; Barreiro-Pérez, M.; Merchan-Gómez, S.; Iscar-Galán, A.; Martín-García, A.; Nieto-Ballestero, F.; Sánchez-Corral, E.; Rodriguez-Collado, J.; Cruz-González, I.; et al. Utility of Real-Time 3-Dimensional Transesophageal Echocardiography in the Assessment of Mitral Paravalvular Leak. Circ. J. 2016, 80, 738–744. [Google Scholar] [CrossRef]
- Kliger, C.; Eiros, R.; Isasti, G.; Einhorn, B.; Jelnin, V.; Cohen, H.; Kronzon, I.; Perk, G.; Fontana, G.P.; Ruiz, C.E. Review of surgical prosthetic paravalvular leaks: Diagnosis and catheter-based closure. Eur. Heart J. 2012, 34, 638–649. [Google Scholar] [CrossRef]
- Rihal, C.S.; Sorajja, P.; Booker, J.D.; Hagler, D.J.; Cabalka, A.K. Principles of Percutaneous Paravalvular Leak Closure. JACC Cardiovasc. Interv. 2012, 5, 121–130. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Genoni, M.; Franzen, D.; Vogt, P.; Seifert, B.; Jenni, R.; Künzli, A.; Niederhäuser, U.; Turina, M. Paravalvular leakage after mitral valve replacement: Improved long-term survival with aggressive surgery? Eur. J. Cardio Thoracic Surg. 2000, 17, 14–19. [Google Scholar] [CrossRef]
- Echevarria, J.R.; Bernal, J.M.; Rabasa, J.M.; Morales, D.; Revilla, Y.; Revuelta, J.M. Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience. Eur. J. Cardiothorac. Surg. 1991, 5, 523–527. [Google Scholar] [CrossRef]
- Expósito, V.; García-Camarero, T.; Bernal, J.M.; Arnáiz, E.; Sarralde, A.; García, I.; Berrazueta, J.R.; Revuelta, J.M. Repeat Mitral Valve Replacement: 30-Years’ Experience. Rev. Esp. Cardiol. 2009, 62, 929–932. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Jelnin, V.; Kronzon, I.; Dudiy, Y.; Del Valle-Fernandez, R.; Einhorn, B.N.; Chiam, P.T.; Martinez, C.; Eiros, R.; Roubin, G.; et al. Clinical Outcomes in Patients Undergoing Percutaneous Closure of Periprosthetic Paravalvular Leaks. J. Am. Coll. Cardiol. 2011, 58, 2210–2217. [Google Scholar] [CrossRef]
- Sorajja, P. Mitral Paravalvular Leak Closure. Interv. Cardiol. Clin. 2016, 5, 45–54. [Google Scholar] [CrossRef]
- García, E.; Arzamendi, D.; Jimenez-Quevedo, P.; Sarnago, F.; Martí, G.; Sanchez-Recalde, A.; Lasa-Larraya, G.; Sancho, M.; Iñiguez, A.; Goicolea, J.; et al. Outcomes and predictors of success and complications for paravalvular leak closure: An analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE)registry. EuroIntervention 2017, 12, 1962–1968. [Google Scholar] [CrossRef]
- Calvert, P.A.; Northridge, D.B.; Malik, I.S.; Shapiro, L.; Ludman, P.; Qureshi, S.A.; Mullen, M.; Henderson, R.; Turner, M.; Been, M.; et al. Percutaneous Device Closure of Paravalvular Leak. Combined Experience from the United Kingdom and Ireland. Circulation 2016, 134, 934–944. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: A report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echo-cardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1084. [Google Scholar]
- Zoghbi, W.A. New recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound. Methodist DeBakey Cardiovasc. J. 2010, 6, 20–26. [Google Scholar] [CrossRef]
- Cortés, M.; García, E.; García-Fernandez, M.A.; Gomez, J.J.; Perez-David, E.; Fernández-Avilés, F. Usefulness of Transesophageal Echocardiography in Percutaneous Transcatheter Repairs of Paravalvular Mitral Regurgitation. Am. J. Cardiol. 2008, 101, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Millán, X.; Skaf, S.; Joseph, L.; Ruiz, C.; García, E.; Smolka, G.; Noble, S.; Cruz-González, I.; Arzamendi, D.; Serra, A.; et al. Transcatheter Reduction of Paravalvular Leaks: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2015, 31, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Zack, C.J.; Sarraf, M.; Eleid, M.F.; Cabalka, A.K.; Reeder, G.S.; Hagler, D.J.; Maalouf, J.F.; Nkomo, V.T.; Rihal, C.S. Successful Percutaneous Mitral Paravalvular Leak Closure Is Associated with Improved Midterm Survival. Circ. Cardiovasc. Interv. 2017, 10, e005730. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Rihal, C.S.; Zack, C.J.; Eleid, M.F.; Maor, E.; Sarraf, M.; Cabalka, A.K.; Reeder, G.S.; Hagler, D.J.; Maalouf, J.F.; et al. Transcatheter and Surgical Management of Mitral Paravalvular Leak-Term Outcomes. JACC Cardiovasc. Interv. 2017, 10, 1946–1956. [Google Scholar] [CrossRef]
- Taramasso, M.; Maisano, F.; Alfieri, O.; Nietlispach, F.; Pozzoli, A.; Meier, B. Catheter-based treatment of paravalvular leaks. EuroIntervention 2016, 12, X55–X60. [Google Scholar] [CrossRef]
- Choi, J.Y.; Suh, Y.J.; Seo, J.; Choi, K.-U.; Hong, G.-R.; Lee, S.; Lee, S.-H.; Ha, J.-W.; Kim, Y.J.; Shim, C.Y. Structural and Functional Characteristics of Mitral Paravalvular Leakage Identified by Multimodal Imaging and Their Implication on Clinical Presentation. J. Clin. Med. 2021, 10, 222. [Google Scholar] [CrossRef]
- Onorato, E.M.; Alamanni, F.; Muratori, M.; Smolka, G.; Wojakowski, W.; Pysz, P.; Zorinas, A.; Zakarkaite, D.; Eltchaninoff, H.; Litzer, P.-Y.; et al. Safety, Efficacy and Long-Term Outcomes of Patients Treated with the Occlutech Paravalvular Leak Device for Significant Paravalvular Regurgitation. J. Clin. Med. 2022, 11, 1978. [Google Scholar] [CrossRef]
- Perl, L.; Cohen, A.; Dadashev, A.; Shapira, Y.; Vaknin-Assa, H.; Yahalom, V.; Sagie, A.; Kornowski, R.; Hirsch, R. Long-term outcomes of catheter-based intervention for clinically significant paravalvular leak. EuroIntervention 2021, 17, 736–743. [Google Scholar] [CrossRef]
- Vassileva, C.M.; Mishkel, G.; McNeely, C.; Boley, T.; Markwell, S.; Scaife, S.; Hazelrigg, S. Long-term survival of patients undergoing mitral valve repair and replacement: A longitudinal analysis of Medicare fee-for-service beneficiaries. Circulation 2013, 127, 1870–1876. [Google Scholar] [CrossRef]
- Mohty, D.; Orszulak, T.A.; Schaff, H.V.; Avierinos, J.F.; Tajik, J.A.; Enriquez-Sarano, M. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation 2001, 104, I1–I7. [Google Scholar] [CrossRef]
- Tanner, R.; Hassan, S.; Ryan, N.; Murphy, N.F.; Campbell, P.; Margey, R.; Walsh, K.; Byrne, R.; Blake, G.; Casserly, I.P. Trans-catheter paravalvular leak closure: A single-centre experience. Ir. J. Med. Sci. 2018, 188, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Cabalka, A.K.; Hagler, D.J.; Rihal, C.S. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgi-tation. J. Am. Coll. Cardiol. 2011, 58, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
| Patients (n) | 96 |
|---|---|
| Age (years) | 71.1 ± 8.1 |
| Sex, men (n, %) | 46.9% |
| Patients with 1 prosthetic valve (n, %) | 56 (58.3%) |
| Patients with 2 prosthetics valves (n, %) | 40 (41.7%) |
| Patients with ≥2 prior sternotomies (n, %) | 27 (28.1%) |
| Time from last surgery to procedure (months) | 65.3 (IQ 126.3) |
| Mitral valve prostheses type (n, %) | |
| Mechanical prosthesis | 80 (83.3%) |
| Bioprosthesis | 16 (16.7%) |
| Procedure indication (n, %) | |
| Heart failure (HF) | 57 (59.4%) |
| Hemolytic anemia (HA) | 5 (5.2%) |
| HF and HA | 34 (35.4%) |
| Comorbidities (n, %) | |
| Previous CAD | 21 (21.9%) |
| HTA | 54 (56.3%) |
| DM | 20 (20.8%) |
| AF | 82 (85.4%) |
| Pulmonary hypertension (PASP > 60 mmHg by TTE) | 52 (54.2%) |
| CKD (GF < 60 mL/min) | 29 (30.2%) |
| COPD | 9 (9.4%) |
| PAD | 11 (11.5%) |
| NYHA Functional Class (n, %) | |
| I | 3 (3.1%) |
| II | 8 (8.3%) |
| III | 68 (70.8) |
| IV | 17 (17.7%) |
| Ejection fraction (%) | 55.7 ± 9.4 |
| Euroscore I | 20.3 ± 10.1 |
| STS score (mortality) | 5.5 ± 4.0 |
| Basal hemoglobin (mg/dL) | 10.5 ± 1.8 |
| Basal LDH (mg/dL) | 775.5 ± 822.3 |
| Previous HF admission (n, %) | 40 (42.6%) |
| Red blood cells transfusion in last year (n, %) | 34 (37.4%) |
| Percutaneous Mitral PVL Procedures (n,) | 128 |
|---|---|
| PVL location (n, %) | |
| Lateral | 42 (32.8%) |
| Anterior | 37 (28.9%) |
| Septal | 19 (14.8%) |
| Posterior | 8 (6.3%) |
| Multiple | 22 (17.2%) |
| Access (n, %) | |
| Femoral | 125 (97.7%) |
| Transapical | 3 (2.3%) |
| Approach (n, %) | |
| Antegrade | 28 (64.8%) |
| Retrograde | 39 (30.5%) |
| Combined | 6 (4.7%) |
| Loop (n, %) | |
| Arterioarterial | 3 (2.3%) |
| Venoarterial | 111 (86.7%) |
| Nonloop | 13 (10.32%) |
| Both | 1 (0.78%) |
| Complications (n, %) | |
| None | 103 (80.5%) |
| Vascular | 12 (9.4%) |
| Device embolization | 3 (2.3%) |
| Disc interference | 6 (4.7%) |
| Cardiac surgery | 2 (1.6%) |
| Other | 2 (1.6%) |
| Death | 0 |
| Device (n, %) | |
| AVP-III | 110 (90.2%) |
| PDA | 1 (0.82%) |
| VSD | 4 (3.3%) |
| AVP-IV | 1 (0.82%) |
| Others | 4 (3.3%) |
| Procedural timing (n, %) | |
| Elective procedure | 120 (93.7%) |
| Procedure during acute HF admission | 8 (6.3%) |
| Technical success (n, %) | 115 (89.8) |
| Procedural success (n, %) | 102 (79.2) |
| Factor | p Value | |
|---|---|---|
| Univariate Analysis | Multivariate Analysis | |
| NYHA I–II vs. III–IV | 0.046 | 0.093 (NS) |
| HF vs. HA indication | 0.002 | 0.054 |
| Transfusion (yes/no) | 0.000 | 0.085 (NS) |
| Location (multiple vs. no multiple PVL) | 0.009 | 0.030 |
| More than 2 previous surgeries | 0.015 | NS |
| Basal hemoglobin | 0.063 | NS |
| Basal LDH | 0.000 | NS |
| Pulmonary hypertension | 0.135 | - |
| Patients (n) | 96 |
|---|---|
| 90-Day Follow-Up | |
| Improvement in NYHA functional class at 90 days (n, %) | 75 (78.1%) |
| NYHA functional class at 90 days (n, %) | 42 (32.8%) |
| I | 37 (28.9%) |
| II | 19 (14.8%) |
| III | 8 (6.3%) |
| IV | 22 (17.2%) |
| Long-Term Follow-Up | |
| Improvement in NYHA functional class at last follow-up visit (n, %) | 74 (77.4%) |
| NYHA functional class at last follow-up visit (n, %) | |
| I | 26 (27.4%) |
| II | 53 (54.8%) |
| III | 9 (9.5%) |
| IV | 8 (8.3%) |
| Total MACEs at last follow-up visit (n, %) | 55 (57.3%) |
| Death | 38 (39.6%) |
| Cardiovascular death | 65 (67.7%) |
| HF readmission | 30 (31.3%) |
| Repeat PVL closure intervention | 14 (14.6%) |
| Surgical repeat PVL intervention | 11 (11.5%) |
| Percutaneous repeat PVL intervention | 3 (3.1) |
| Stroke | 0 |
| p Value | HR | 95% Confidence Interval | |
|---|---|---|---|
| CKD | 0.043 | 2.1 | 1.0–4.6 |
| 3 months HF previous admission | 0.000 | 5.7 | 2.3–13.8 |
| First procedure success | 0.002 | 0.1 | 0.0–0.5 |
| Improve NYHA class at follow-up | 0.011 | 0.4 | 0.4–0.8 |
| HA vs. HF indication | 0.000 | 5.9 | 2.9–12.2 |
| p Value | HR | 95% Confidence Interval | |
|---|---|---|---|
| 3 months HF previous admission | 0.000 | 7.0 | 2.8–16.5 |
| NYHA IV functional class 90 days after PVL closure | 0.030 | 4.5 | 1.2–17.5 |
| Improve NYHA class at follow-up | 0.004 | 0.2 | 0.1–0.6 |
| HA vs. HF indication | 0.000 | 6.7 | 2.8–16.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-González, I.; Luengo-Mondéjar, P.; Trejo-Velasco, B.; Núñez-García, J.C.; González-Ferreiro, R.; Moreno-Samos, J.C.; Fuertes-Barahona, M.; Rama-Merchán, J.C.; Antúnez-Muiños, P.; López-Tejero, S.; et al. Percutaneous Closure of Mitral Paravalvular Leak: Long-Term Results in a Single-Center Experience. J. Clin. Med. 2022, 11, 4835. https://doi.org/10.3390/jcm11164835
Cruz-González I, Luengo-Mondéjar P, Trejo-Velasco B, Núñez-García JC, González-Ferreiro R, Moreno-Samos JC, Fuertes-Barahona M, Rama-Merchán JC, Antúnez-Muiños P, López-Tejero S, et al. Percutaneous Closure of Mitral Paravalvular Leak: Long-Term Results in a Single-Center Experience. Journal of Clinical Medicine. 2022; 11(16):4835. https://doi.org/10.3390/jcm11164835
Chicago/Turabian StyleCruz-González, Ignacio, Pablo Luengo-Mondéjar, Blanca Trejo-Velasco, Jean C. Núñez-García, Rocío González-Ferreiro, José C. Moreno-Samos, Mónica Fuertes-Barahona, Juan C. Rama-Merchán, Pablo Antúnez-Muiños, Sergio López-Tejero, and et al. 2022. "Percutaneous Closure of Mitral Paravalvular Leak: Long-Term Results in a Single-Center Experience" Journal of Clinical Medicine 11, no. 16: 4835. https://doi.org/10.3390/jcm11164835
APA StyleCruz-González, I., Luengo-Mondéjar, P., Trejo-Velasco, B., Núñez-García, J. C., González-Ferreiro, R., Moreno-Samos, J. C., Fuertes-Barahona, M., Rama-Merchán, J. C., Antúnez-Muiños, P., López-Tejero, S., Barreira de Sousa, G., Rodríguez-Collado, J., Martín-Moreiras, J., Diego-Nieto, A., Herrero-Garibi, J., Barreiro-Pérez, M., Díaz-Peláez, E., & Sánchez Fernández, P. L. (2022). Percutaneous Closure of Mitral Paravalvular Leak: Long-Term Results in a Single-Center Experience. Journal of Clinical Medicine, 11(16), 4835. https://doi.org/10.3390/jcm11164835







