Transcatheter Mitral Valve Repair or Replacement: Competitive or Complementary?
Abstract
1. Introduction
2. Transcatheter Mitral Valve Repair (TMVr)
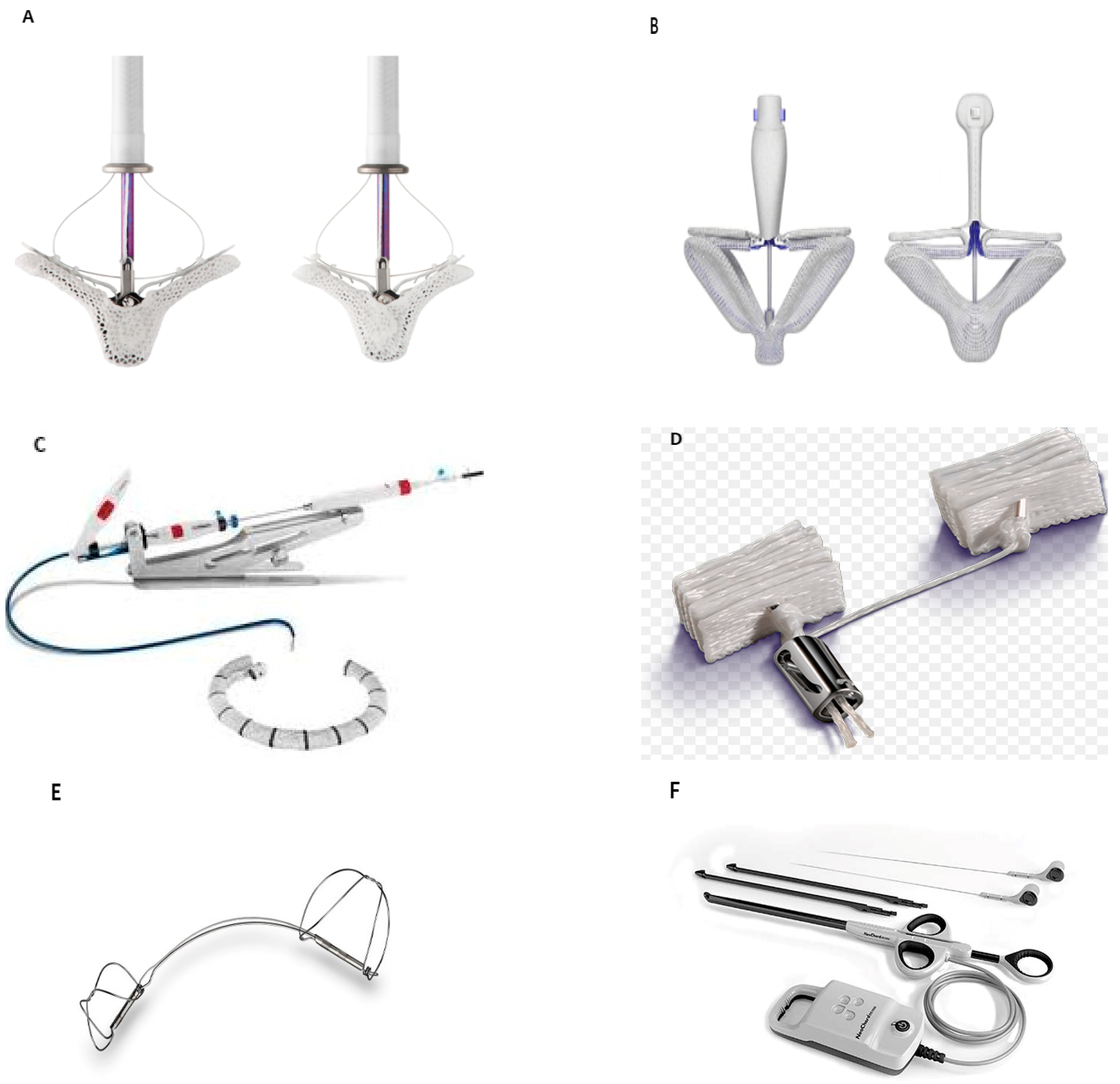
2.1. TEER Devices
- MitraClipTM (Figure 1A)
- PASCAL (Figure 1B)
2.2. Annuloplasty Devices
- Cardioband (Figure 1C)
- Mitralign (Figure 1D)
- Carillon (Figure 1E)
2.3. Chordal Repair
- NeoChord (Figure 1F)
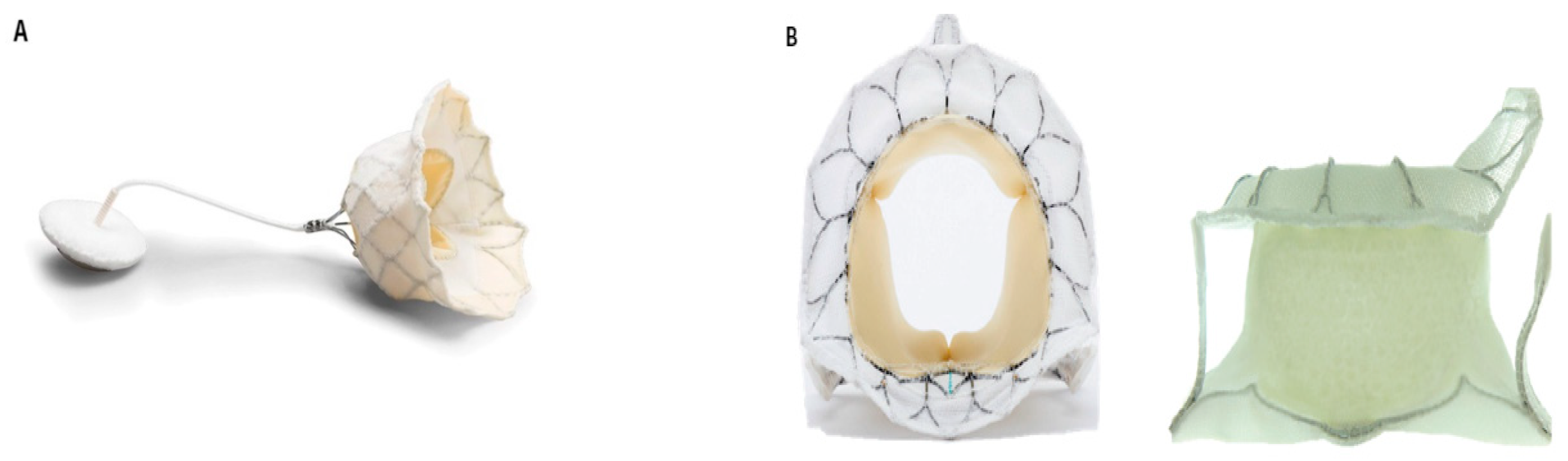
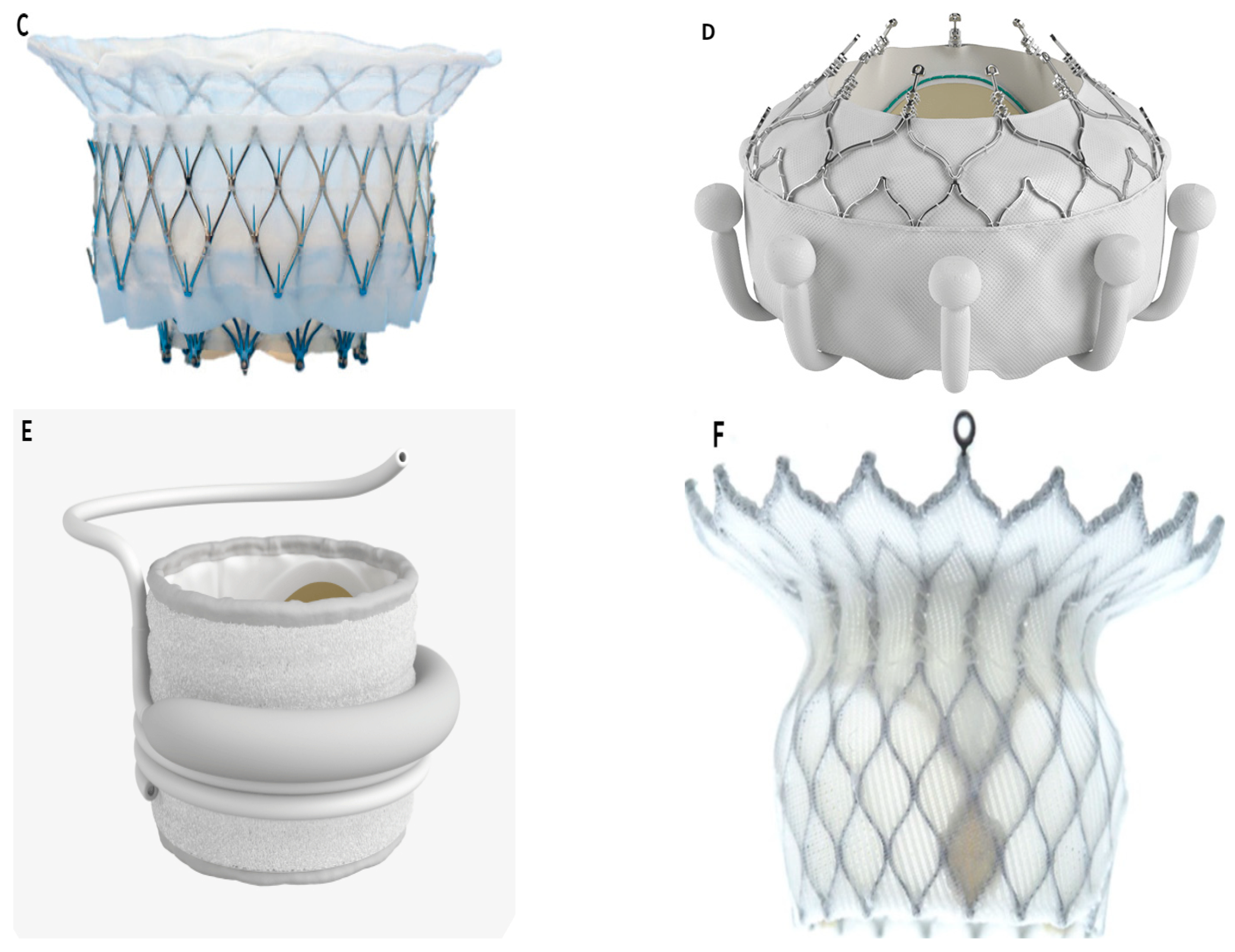
3. Transcatheter Mitral Valve Replacement (TMVR)
TMVR Devices
- Tendyne Mitral Valve System (Abbott Laboratories, IL, USA) (Figure 2A)
- Tiara TMVR System (Neovasc Inc., Richmond, BC, Canada) (Figure 2B)
- Intrepid TMVR System (Medtronic, Minneapolis, MN, USA) (Figure 2C)
- EVOQUE TMVR System (Edwards Lifesciences, Irvine, CA, USA) (Figure 2D)
- SAPIEN M3 System (Edwards Lifesciences, Irvine, CA, USA) (Figure 2E)
- HighLife TMVR system (HighLife Medical, Paris, France) (Figure 2F)
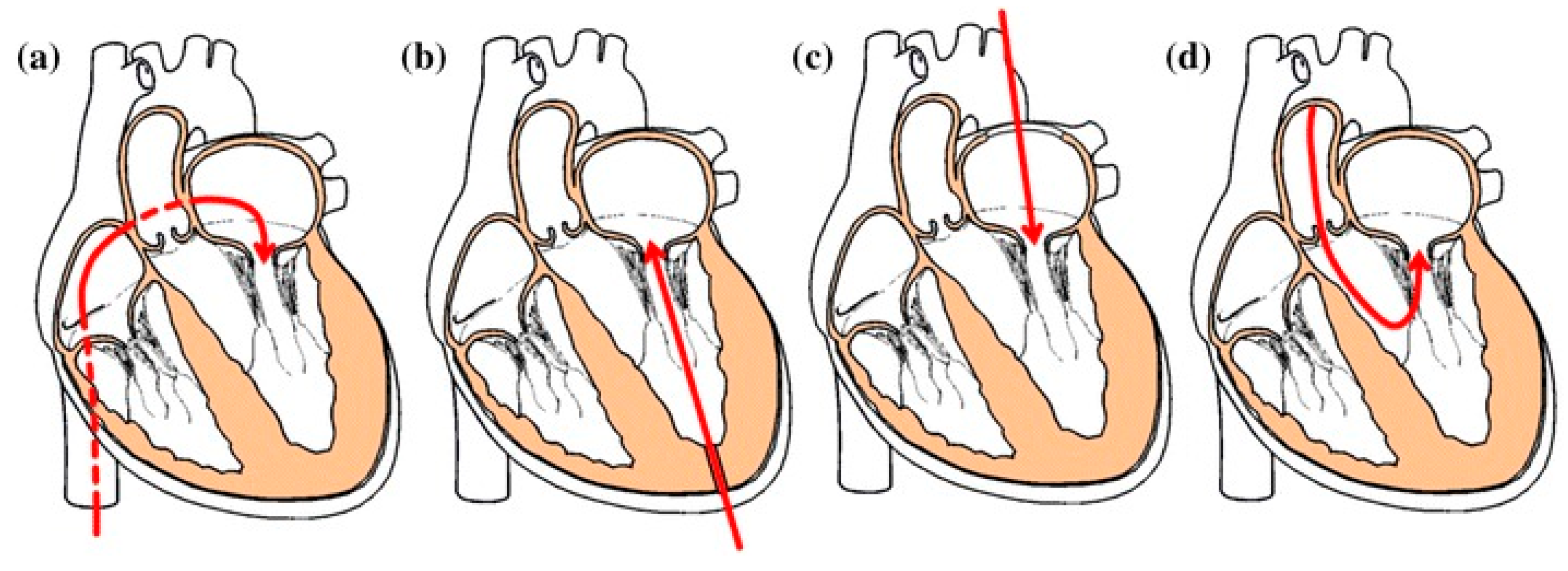
4. Current Challenges and Discussion
- (1)
- The mitral annulus is saddle-shaped and D-shaped, not circular, and is not in the same plane. Even if a skirt is placed on the atrial portion, paravalvular leakage may still occur.
- (2)
- In TAVR, the aortic valve is calcified, rigid, and rounder after pre-dilation, which makes it relatively easy to anchor the circular aortic valved stent to the native annulus in a tube-like area. In contrast, the mitral annulus is compliant, and its shape is constantly changing with the cardiac cycle and underlying pathological process. Thus, it generally does not provide radial support for the new valved stent because the annulus is located between the contracting left atrial and ventricular chamber. Thus, mitral fixation has to be done in a very different way than that for TAVR and engineers face significant challenges when developing new devices.
- (3)
- The intracavitary pressure from the LV contraction can be high (180 mmHg), and the prosthetic valve is at risk of atrial embolization.
- (4)
- There are about 24 chordae in the left ventricular cavity which can interfere with the implantation and fixation of the new prosthetic valve.
- (5)
- The probability of acute LVOTO after TMVR is 8.2% [86], rising to 9.2% if there is calcification of the mitral annulus. In the case of valve in MAC, the 30-day LVOTO rate was 39.7%, and the all-cause mortality reached to 34.5% [55]. LVOTO is related to a variety of factors, including mainly the angle between aortic and mitral annulus, degree of atrial septal hypertrophy, length of anterior mitral leaflet, and the size of the LV [87]. Nevertheless, LVOTO can be predicted by preoperative cardiac 3D-computed tomography (CT).
- (1)
- In the vicinity of the mitral valved stent, where blood flow is maintained at a very low velocity within a relatively small circulatory area, the potential for blood clotting in the left atrium is increased. Indeed, to prevent paravalvular leakage, prosthetic valves are designed to have an atrial skirt and a complex structure aligned towards the atrial portion, where blood flow is slow and therefore prone to thrombus formation. In addition, the peripheral area of the mitral valved stent against the ventricular wall is also blinded to blood flow and is also susceptible to thrombus formation. Clinical studies have shown that thrombosis is, indeed, a problem and a major cause of postoperative death in many patients. Some studies suggest adequate oral anticoagulants as one of the main solutions [89,90,91,92]. Valve leaflet thrombosis has been seen in early TMVR systems, but the optimal antithrombotic strategy has not yet been determined. In the early Tendyne experience, 6% of patients presented thrombus, resulting in patients having to be anticoagulated with warfarin for more than 3 months [59]. With the EVOQUE and SAPIEN M3 systems, all patients underwent anticoagulation after implantation. Further research is needed regarding the optimal duration of anticoagulation and the dose of anticoagulant drugs [71,93]. This is a critical consideration compared to transcatheter repair, which does not require anticoagulation in patients with sinus rhythm. Four-dimensional multilayer spiral CT has a high predictive value for postoperative device thrombosis and may be routinely used after TMVR [94].
- (2)
- The mitral annulus size may easily be underestimated. Nakashima et al. [95] reported modest changes in mitral annulus geometry (7.2–13.9%), resulting in size alignment changes (24.2%) in a significant proportion of patients with the Intrepid TMVR, suggesting that size is essential for TMVR devices. The optimal TMVR valve must balance the need to accommodate a large mitral annulus while minimizing LVOT interactions. Moreover, larger TMVR devices that can accommodate larger mitral annulus come at the cost of a high-profile delivery system, limiting transseptal delivery [96].
- (3)
- The durability of prosthetic valves may also be an issue. The prosthetic aortic valve is implanted in the aortic root where there is little local tissue activity, and therefore valve degeneration is low after TAVR (about 6.6% after 5 years [97]). In contrast, the mitral annulus, chordae and papillary muscles undergo contractile motion in response to the cardiac cycle. The mechanical damage sustained over time can be staggering. Interestingly, in the case of the Tendyne valve, the apical tether is mechanically strained during each cardiac cycle, but this does not appear to be problematic up to seven years after TMVR [59,66,98]. An analogy is the Melody pulmonary valve, which is implanted in the right ventricular outflow tract, which is contractile, resulting in a 1-year fracture rate of 15% after Melody pulmonary valve implantation, with fracture rates of 25% at 2 years after implantation [99].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CABG | coronary artery bypass grafting |
| CMR | cardiovascular magnetic resonance |
| CT | computed tomography |
| FMR | functional mitral regurgitation |
| LV | left ventricle |
| LVEDV | left ventricular end-diastolic volume |
| LVOT | left ventricular outflow tract |
| LVOTO | left ventricular outflow tract obstruction |
| MAC | mitral annular calcification |
| MDCT | multidetector row computed tomography |
| MR | mitral regurgitation |
| MV | mitral valve |
| NYHA | New York Heart Association |
| PET | polyethylene terephthalate |
| STS | Society of Thoracic Surgeons |
| TAVR | transcatheter aortic valve replacement |
| TEE | transesophageal echocardiography |
| TEER | transcatheter edge-to-edge repair |
| TMVr | transcatheter mitral valve repair |
| TMVR | transcatheter mitral valve replacement |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Badhwar, V.; Thourani, V.H.; Ailawadi, G.; Mack, M. Transcatheter mitral valve therapy: The event horizon. J. Thorac. Cardiovasc. Surg. 2016, 152, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Fichtlscherer, S.; Holubec, T.; Vasa-Nicotera, M.; Arsalan, M.; Walther, T. New developments in transcatheter therapy of mitral valve disease. J. Thorac. Dis. 2020, 12, 1728. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Kardiol. Pol. 2018, 76, 1–62. [Google Scholar] [CrossRef]
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365. [Google Scholar] [CrossRef]
- Andalib, A.; Mamane, S.; Schiller, I.; Zakem, A.; Mylotte, D.; Martucci, G.; Lauzier, P.; Alharbi, W.; Cecere, R.; Dorfmeister, M.; et al. A systematic review and meta-analysis of surgical outcomes following mitral valve surgery in octogenarians: Implications for transcatheter mitral valve interventions. EuroIntervention 2014, 9, 1225–1234. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Maisano, F.; Franzen, O.; Baldus, S.; Schäfer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Sievert, H.; Richardt, G.; Widder, J.D.; et al. Percutaneous Mitral Valve Interventions in the Real World: Early and 1-Year Results From the ACCESS-EU, A Prospective, Multicenter, Nonrandomized Post-Approval Study of the MitraClip Therapy in Europe. J. Am. Coll. Cardiol. 2013, 62, 1052–1061. [Google Scholar] [CrossRef]
- Swaans, M.J.; Bakker, A.L.; Alipour, A.; Post, M.C.; Kelder, J.C.; de Kroon, T.L.; Eefting, F.D.; Rensing, B.J.; Van der Heyden, J.A. Survival of transcatheter mitral valve repair compared with surgical and conservative treatment in high-surgical-risk patients. JACC Cardiovasc. Interv. 2014, 7, 875–881. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Chakravarty, T.; Makar, M.; Patel, D.; Oakley, L.; Yoon, S.H.; Stegic, J.; Singh, S.; Skaf, S.; Nakamura, M.; Makkar, R.R. Transcatheter Edge-to-Edge Mitral Valve Repair with the MitraClip G4 System. JACC Cardiovasc. Interv. 2020, 13, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, P.L.; Feldman, T.; Pedersen, W.R.; Lim, D.S.; Kipperman, R.; Smalling, R.; Bajwa, T.; Herrmann, H.C.; Lasala, J.; Maddux, J.T.; et al. Acute and 12-Month Results With Catheter-Based Mitral Valve Leaflet Repair: The EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J. Am. Coll. Cardiol. 2012, 59, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Braun, D.; Unterhuber, M.; Spirito, A.; Orban, M.; Brugger, N.; Brinkmann, I.; Spring, K.; Moschovitis, A.; Nabauer, M.; et al. Edge-to-edge mitral valve repair with extended clip arms: Early experience from a multicenter observational study. Cardiovasc. Interv. 2019, 12, 1356–1365. [Google Scholar]
- Lim, D.S.; Kar, S.; Spargias, K.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.; Fam, N.P.; Walters, D.L.; Webb, J.G.; Smith, R.L.; et al. Transcatheter valve repair for patients with mitral regurgitation: 30-day results of the CLASP study. Cardiovasc. Interv. 2019, 12, 1369–1378. [Google Scholar]
- Mauri, V.; Besler, C.; Riebisch, M.; Al-Hammadi, O.; Ruf, T.; Gerçek, M.; Horn, P.; Grothusen, C.; Mehr, M.; Becher, M.U.; et al. German Multicenter Experience With a New Leaflet-Based Transcatheter Mitral Valve Repair System for Mitral Regurgitation. JACC Cardiovasc. Interv. 2020, 13, 2769–2778. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Nickenig, G.; Latib, A.; Kuck, K.-H.; Baldus, S.; Schueler, R.; La Canna, G.; Agricola, E.; Kreidel, F.; Huntgeburth, M.; et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur. Heart J. 2019, 40, 466–472. [Google Scholar] [CrossRef]
- Nickenig, G.; Hammerstingl, C.; Schueler, R.; Topilsky, Y.; Grayburn, P.A.; Vahanian, A.; Messika-Zeitoun, D.; Urena Alcazar, M.; Baldus, S.; Volker, R.; et al. Transcatheter mitral annuloplasty in chronic functional mitral regurgitation: 6-month results with the cardioband percutaneous mitral repair system. Cardiovasc. Interv. 2016, 9, 2039–2047. [Google Scholar]
- Nickenig, G.; Schueler, R.; Dager, A.; Clark, P.M.; Abizaid, A.; Siminiak, T.; Buszman, P.; Demkow, M.; Ebner, A.; Asch, F.M.; et al. Treatment of Chronic Functional Mitral Valve Regurgitation With a Percutaneous Annuloplasty System. J. Am. Coll. Cardiol. 2016, 67, 2927–2936. [Google Scholar] [CrossRef]
- Witte, K.K.; Lipiecki, J.; Siminiak, T.; Meredith, I.T.; Malkin, C.J.; Goldberg, S.L.; Stark, M.A.; von Bardeleben, R.S.; Cremer, P.C.; Jaber, W.A.; et al. The REDUCE FMR trial: A randomized sham-controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. JACC Heart Fail. 2019, 7, 945–955. [Google Scholar] [CrossRef]
- Schofer, J.; Siminiak, T.; Haude, M.; Herrman, J.P.; Vainer, J.; Wu, J.C.; Levy, W.C.; Mauri, L.; Feldman, T.; Kwong, R.Y.; et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: Results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009, 120, 326–333. [Google Scholar] [CrossRef]
- Lipiecki, J.; Siminiak, T.; Sievert, H.; Müller-Ehmsen, J.; Degen, H.; Wu, J.C.; Schandrin, C.; Kalmucki, P.; Hofmann, I.; Reuter, D.; et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: The TITAN II trial. Open Heart 2016, 3, e000411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siminiak, T.; Wu, J.C.; Haude, M.; Hoppe, U.C.; Sadowski, J.; Lipiecki, J.; Fajadet, J.; Shah, A.M.; Feldman, T.; Kaye, D.M.; et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: Results of the TITAN Trial. Eur. J. Heart Fail. 2012, 14, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Manzan, E.; Aidietis, A.; Rucinskas, K.; Bizzotto, E.; Besola, L.; Pradegan, N.; Pittarello, D.; Janusauskas, V.; Zakarkaite, D.; et al. An early European experience with transapical off-pump mitral valve repair with NeoChord implantation. Eur. J. Cardio-Thorac. Surg. 2018, 54, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Manzan, E.; Rucinskas, K.; Janusauskas, V.; Zucchetta, F.; Zakarkaitė, D.; Aidietis, A.; Gerosa, G. Acute safety and efficacy of the NeoChord procedure. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 575–581. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrie, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and disproportionate functional mitral regurgitation: A new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc. Imaging. 2019, 12, 353–362. [Google Scholar] [CrossRef]
- Pibarot, P.; Delgado, V.; Bax, J.J. MITRA-FR vs. COAPT: Lessons from two trials with diametrically opposed results. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 620–624. [Google Scholar] [CrossRef]
- Mack, M.J.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Grayburn, P.A.; Rinaldi, M.J.; Kapadia, S.R.; et al. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients with Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 1029–1040. [Google Scholar] [CrossRef]
- Kreidel, F.; Alessandrini, H.; Wohlmuth, P.; Schmoeckel, M.; Geidel, S. Is surgical or catheter-based interventions an option after an unsuccessful mitral clip? Semin. Thorac. Cardiovasc. Surg. 2018, 30, 152–157. [Google Scholar] [CrossRef]
- EL-Shurafa, H.; Arafat, A.A.; Albabtain, M.A.; AlFayez, L.A.; AlOtaiby, M.; Algarni, K.D.; Pragliola, C. Reinterventions after transcatheter edge to edge mitral valve repair: Is early clipping warranted? J. Card. Surg. 2020, 35, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, L.; Ielasi, A.; Rensing, B.J.W.M.; Eefting, F.D.; Timmers, L.; Latib, A.; Swaans, M.J. Complications Following Percutaneous Mitral Valve Repair. Front. Cardiovasc. Med. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Gyoten, T.; Schenk, S.; Rochor, K.; Herwig, V.; Harnath, A.; Grimmig, O.; Just, S.; Fritzsche, D.; Messroghli, D. Outcome comparison of mitral valve surgery and MitraClip therapy in patients with severely reduced left ventricular dysfunction. ESC Heart Fail. 2020, 7, 1781–1790. [Google Scholar] [CrossRef]
- Mkalaluh, S.; Szczechowicz, M.; Karck, M.; Weymann, A. Failed MitraClip therapy: Surgical revision in high-risk patients. J. Cardiothorac. Surg. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- El-Shurafa, H.; Arafat, A.A.; Albabtain, M.A.; AlFayez, L.A.; Algarni, K.D.; Pragliola, C.; Alkhushail, A.; Samargandy, S.; AlOtaiby, M. Residual versus recurrent mitral regurgitation after transcatheter mitral valve edge-to-edge repair. J. Card. Surg. 2021, 36, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Hausleiter, J. Transmitral Gradients Following Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2022, 15, 946–949. [Google Scholar] [CrossRef]
- Rottbauer, W.; Kessler, M.; Mathew Williams, P.; Mahoney, R.S.V.B.; Price, M.J.; Grasso, C.; Zamorano, J.L.; Asch, F.M.; Maisano, F.; Kar, S. Contemporary Clinical Outcomes with MitraClip™(NTR/XTR) System: Core-Lab Echo Results from+ 1000 Patient the Global EXPAND Study; PCRonline: Paris, France, 2020. [Google Scholar]
- Maisano, F.; von Bardeleben, R.S.; Lurz, P.; Hausleiter, J.; Rogers, J.; Dur, O.; Sun, L.; Kar, S. Clip Selection Strategy and Outcomes with MitraClip™ (NTR/XTR): Evidence-Based Recommendations from the Global EXPAND Study; PCRonline: Paris, France, 2020. [Google Scholar]
- Grasso, C.; Rubbio, A.P. The PASCAL transcatheter mitral valve repair system for the treatment of mitral regurgitation: Another piece to the puzzle of edge-to-edge technique. J. Thorac. Dis. 2017, 9, 4856. [Google Scholar] [CrossRef] [PubMed]
- Corpataux, N.; Winkel, M.G.; Kassar, M.; Brugger, N.; Windecker, S.; Praz, F. The PASCAL Device—Early Experience with a Leaflet Approximation Device: What Are the Benefits/Limitations Compared with the MitraClip? Curr. Cardiol. Rep. 2020, 22, 1–7. [Google Scholar] [CrossRef]
- Praz, F.; Spargias, K.; Chrissoheris, M.; Büllesfeld, L.; Nickenig, G.; Deuschl, F.; Schueler, R.; Fam, N.P.; Moss, R.; Makar, M.; et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: A multicentre, prospective, observational, first-in-man study. Lancet 2017, 390, 773–780. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Boeder, N.F.; Gaede, L.; Arnold, M.; Vigelius-Rauch, U.; Roth, P.; Sander, M.; Böning, A.; Bayer, M.; Elsässer, A.; et al. Mitral valve leaflet repair with the new PASCAL system: Early real-world data from a German multicentre experience. Clin. Res. Cardiol. 2020, 109, 549–559. [Google Scholar] [CrossRef]
- Szerlip, M.; Spargias, K.S.; Makkar, R.; Kar, S.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.; Smith, R.L.; Fam, N.P.; Rinaldi, M.J.; et al. 2-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc. Interv. 2021, 14, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Smith, R.L.; Zahr, F.; Dhoble, A.; Laham, R.; Lazkani, M.; Kodali, S.; Kliger, C.; Hermiller, J.; Vora, A.; et al. Early outcomes from the CLASP IID trial roll-in cohort for prohibitive risk patients with degenerative mitral regurgitation. Catheter. Cardiovasc. Interv. 2021, 98, E637–E646. [Google Scholar] [CrossRef] [PubMed]
- Seeburger, J.; Rinaldi, M.; Nielsen, S.L.; Salizzoni, S.; Lange, R.; Schoenburg, M.; Alfieri, O.; Borger, M.A.; Mohr, F.W.; Aidietis, A. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: The TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J. Am. Coll. Cardiol. 2014, 63, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Alfieri, O.; Banai, S.; Buchbinder, M.; Colombo, A.; Falk, V.; Feldman, T.; Franzen, O.; Herrmann, H.; Kar, S.; et al. The future of transcatheter mitral valve interventions: Competitive or complementary role of repair vs. replacement? Eur. Heart J. 2015, 36, 1651–1659. [Google Scholar] [CrossRef]
- Seiffert, M.; Franzen, O.; Conradi, L.; Baldus, S.; Schirmer, J.; Meinertz, T.; Reichenspurner, H.; Treede, H. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter. Cardiovasc. Interv. 2010, 76, 608–615. [Google Scholar] [CrossRef]
- Webb, J.G.; Wood, D.A.; Ye, J.; Gurvitch, R.; Masson, J.-B.; Rodés-Cabau, J.; Osten, M.; Horlick, E.; Wendler, O.; Dumont, E.; et al. Transcatheter Valve-in-Valve Implantation for Failed Bioprosthetic Heart Valves. Circulation 2010, 121, 1848–1857. [Google Scholar] [CrossRef]
- Descoutures, F.; Himbert, M.; Maisano, F.; Casselman, F.; De Weger, A.; Bodea, O.; Van Der Kley, F.; Colombo, A.; Giannini, C.; Rein, K.A.; et al. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur. J. Cardio-Thoracic Surg. 2013, 44, e8–e15. [Google Scholar] [CrossRef][Green Version]
- Himbert, D.; Brochet, E.; Radu, C.; Iung, B.; Messika-Zeitoun, D.; Enguerrand, D.; Bougoin, W.; Nataf, P.; Vahanian, A. Transseptal Implantation of a Transcatheter Heart Valve in a Mitral Annuloplasty Ring to Treat Mitral Repair Failure. Circ. Cardiovasc. Interv. 2011, 4, 396–398. [Google Scholar] [CrossRef][Green Version]
- Guerrero, M.; Dvir, D.; Himbert, D.; Urena, M.; Eleid, M.; Wang, D.D.; Greenbaum, A.; Mahadevan, V.S.; Holzhey, D.; O’Hair, D.; et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: Results from the first multicenter global registry. JACC Cardiovasc. Interv. 2016, 9, 1361–1371. [Google Scholar] [CrossRef]
- Guerrero, M.; Urena, M.; Pursnani, A.; Wang, D.D.; Vahanian, A.; O’Neill, W.; Feldman, T.; Himbert, D. Balloon expandable transcatheter heart valves for native mitral valve disease with severe mitral annular calcification. J. Cardiovasc. Surg. 2016, 57, 401–409. [Google Scholar]
- Simonato, M.; Whisenant, B.; Ribeiro, H.B.; Webb, J.G.; Kornowski, R.; Guerrero, M.; Wijeysundera, H.; Søndergaard, L.; De Backer, O.; Villablanca, P.; et al. Transcatheter mitral valve replacement after surgical repair or replacement: Comprehensive midterm evaluation of valve-in-valve and valve-in-ring implantation from the VIVID registry. Circulation 2021, 143, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, B.; Kapadia, S.R.; Eleid, M.F.; Kodali, S.K.; McCabe, J.M.; Krishnaswamy, A.; Morse, M.; Smalling, R.W.; Reisman, M.; Mack, M.; et al. One-Year Outcomes of Mitral Valve-in-Valve Using the SAPIEN 3 Transcatheter Heart Valve. JAMA Cardiol. 2020, 5, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Gennari, M.; Gavazzoni, M.; Pedicino, D.; Pozzoli, A.; Taramasso, M.; Maisano, F. Transcatheter Mitral Valve Implantation: Current Status and Future Perspectives. Circ. Cardiovasc. Interv. 2021, 14, e010628. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, A.; Granada, J.F.; Dagenais, F.; Rodés-Cabau, J. Transcatheter mitral valve replacement: Insights from early clinical experience and future challenges. J. Am. Coll. Cardiol. 2017, 69, 2175–2192. [Google Scholar] [CrossRef]
- De Backer, O.; Piazza, N.; Banai, S.; Lutter, G.; Maisano, F.; Herrmann, H.C.; Franzen, O.W.; Søndergaard, L. Percutaneous transcatheter mitral valve replacement: An overview of devices in preclinical and early clinical evaluation. Circ. Cardiovasc. Interv. 2014, 7, 400–409. [Google Scholar] [CrossRef]
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Initial feasibility study of a new transcatheter mitral prosthesis: The first 100 patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260. [Google Scholar] [CrossRef]
- Cheung, A. The TIARA program: Attributes, challenges, and early clinical data. In Proceedings of the Transcatheter Valve Therapies (TVT) Structural Heart Summit, Chicago, IL, USA, 12–15 June 2019. [Google Scholar]
- Ya’qoub, L.; Eng, M. Transcatheter Mitral Valve Replacement: Evolution and Future Development. In Interventional Treatment for Structural Heart Disease; IntechOpen: London, UK, 2021. [Google Scholar]
- Bapat, V.; Rajagopal, V.; Meduri, C.; Farivar, R.S.; Walton, A.; Duffy, S.J.; Gooley, R.; Almeida, A.; Reardon, M.J.; Kleiman, N.S.; et al. Early Experience with New Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 12–21. [Google Scholar] [CrossRef]
- Webb, J.; Hensey, M.; Fam, N.; Rodes-Cabau, J.; Daniels, D.; Smith, R.; Boone, R.; Ye, J.; Moss, R.; Szeto, W.; et al. Early experience with the EVOQUE mitral valve replacement system. J. Am. Coll. Cardiol. 2020, 75, 1114. [Google Scholar] [CrossRef]
- Makkar, R.; O’Neill, W.; Whisenant, B.; Guerrero, M.; Feldman, T.; Rihal, C.; Gorelick, J.; Webb, J. TCT-8 Updated 30-Day Outcomes for the U.S. Early Feasibility Study of the SAPIEN M3 Transcatheter Mitral Valve Replacement System. J. Am. Coll. Cardiol. 2019, 74, B8. [Google Scholar] [CrossRef]
- Piazza, N. The HIGHLIFE program: Attributes, challenges and clinical data. In Proceedings of the Transcatheter Valve Therapeutics (TVT) 2018, Chicago, IL, USA, 22 June 2018. [Google Scholar]
- Lutter, G.; Lozonschi, L.; Ebner, A.; Gallo, S.; Kall, C.M.Y.; Missov, E.; de Marchena, E. First-in-Human Off-Pump Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2014, 7, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Gössl, M.; Babaliaros, V.; Rizik, D.; Conradi, L.; Bae, R.; Burke, R.F.; Schäfer, U.; Lisko, J.C.; Riley, R.D.; et al. Novel Transcatheter Mitral Valve Prosthesis for Patients with Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2019, 74, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Stub, D.; Moss, R.; Boone, R.H.; Leipsic, J.; Verheye, S.; Banai, S.; Webb, J. Transcatheter mitral valve implantation with Tiara bioprosthesis. EuroIntervention 2014, 10, U115–U119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheung, A.; Webb, J.; Verheye, S.; Moss, R.; Boone, R.; Leipsic, J.; Ree, R.; Banai, S. Short-Term Results of Transapical Transcatheter Mitral Valve Implantation for Mitral Regurgitation. J. Am. Coll. Cardiol. 2014, 64, 1814–1819. [Google Scholar] [CrossRef]
- Sorajja, P.; Bapat, V. Early experience with the Intrepid system for transcatheter mitral valve replacement. Ann. Cardiothorac. Surg. 2018, 7, 792–798. [Google Scholar] [CrossRef]
- Webb, J.G.; Murdoch, D.J.; Boone, R.H.; Moss, R.; Attinger-Toller, A.; Blanke, P.; Cheung, A.; Hensey, M.; Leipsic, J.; Ong, K.; et al. Percutaneous transcatheter mitral valve replacement: First-in-human experience with a new transseptal system. J. Am. Coll. Cardiol. 2019, 73, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Piazza, N.; Mangiafico, S.; Buithieu, J.; Bleiziffer, S.; Ronsivalle, G.; Scandura, S.; Giuffrida, A.; Rubbio, A.P.; Mazzamuto, M.; et al. Transcatheter Mitral Valve Implantation Using the HighLife System. JACC Cardiovasc. Interv. 2017, 10, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.; Rubbio, A.P.; Casenghi, M.; Pero, G.; Latib, A.; Bedogni, F. Transcatheter Mitral Valve Replacement in the Transcatheter Aortic Valve Replacement Era. J. Am. Heart Assoc. 2019, 8, e013352. [Google Scholar] [CrossRef]
- Gertz, Z.M.; Raina, A.; Saghy, L.; Zado, E.; Callans, D.J.; Marchlinski, F.; Keane, M.; Silvestry, F.E. Evidence of Atrial Functional Mitral Regurgitation Due to Atrial Fibrillation: Reversal with Arrhythmia Control. J. Am. Coll. Cardiol. 2011, 58, 1474–1481. [Google Scholar] [CrossRef]
- Levine, R.A.; Hagége, A.A.; Judge, D.P.; Padala, M.; Dal-Bianco, J.P.; Aikawa, E.; Beaudoin, J.; Bischoff, J.; Bouatia-Naji, N.; Bruneval, P.; et al. Mitral valve disease—Morphology and mechanisms. Nat. Rev. Cardiol. 2015, 12, 689–710. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Verhaert, D.; Rega, F.; Thomas, J.D.; Vandervoort, P.M. Atrial functional mitral regurgitation: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Popolo Rubbio, A.; Testa, L.; Grasso, C.; Sisinni, A.; Tusa, M.; De Marco, F.; Petronio, A.S.; Montorfano, M.; Citro, R.; Adamo, M.; et al. Transcatheter edge-to-edge mitral valve repair in atrial functional mitral regurgitation: Insights from the multi-center MITRA-TUNE registry. Int. J. Cardiol. 2022, 349, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rottländer, D.; Golabkesh, M.; Degen, H.; Ögütcü, A.; Saal, M.; Haude, M. Mitral valve edge-to-edge repair versus indirect mitral valve annuloplasty in atrial functional mitral regurgitation. Catheter. Cardiovasc. Interv. 2022, 99, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Acker, M.A.; Parides, M.K.; Perrault, L.P.; Moskowitz, A.; Gelijns, A.C.; Voisine, P.; Smith, P.K.; Hung, J.W.; Blackstone, E.H.; Puskas, J.D.; et al. Mitral-Valve Repair versus Replacement for Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2014, 370, 23–32. [Google Scholar] [CrossRef]
- Braun, D.; Näbauer, M.; Massberg, S.; Hausleiter, J. One-stop shop: Simultaneous direct mitral annuloplasty and percutaneous mitral edge-to-edge repair in a patient with severe mitral regurgitation. Catheter. Cardiovasc. Interv. 2019, 93, E318–E319. [Google Scholar] [CrossRef]
- Mangieri, A.; Colombo, A.; Demir, O.M.; Agricola, E.; Ancona, F.; Regazzoli, D.; Ancona, M.B.; Mitomo, S.; Lanzillo, G.; Del Sole, P.A.; et al. Percutaneous direct annuloplasty with edge-to-edge technique for mitral regurgitation: Replicating a complete surgical mitral repair in a one-step procedure. Can. J. Cardiol. 2018, 34, 1088.e1081–1088.e1082. [Google Scholar] [CrossRef]
- Grasso, C.; Attizzani, G.F.; Ohno, Y.; Dipasqua, F.; Mangiafico, S.; Ministeri, M.; Caggegi, A.; Cannata, S.; Scandura, S.; Tamburino, C. Catheter-Based Edge-to-Edge Mitral Valve Repair After Percutaneous Mitral Valve Annuloplasty Failure. JACC Cardiovasc. Interv. 2014, 7, e85–e86. [Google Scholar] [CrossRef]
- Sugiura, A.; Weber, M.; Charitos, E.I.; Treede, H.; Sinning, J.-M.; Nickenig, G. NeoChord System as an Alternative Option Upon Transmitral Pressure Gradient Elevation in the MitraClip Procedure. JACC Cardiovasc. Interv. 2020, 13, e39–e40. [Google Scholar] [CrossRef]
- Chhatriwalla, A.K.; Vemulapalli, S.; Holmes, D.R., Jr.; Dai, D.; Li, Z.; Ailawadi, G.; Glower, D.; Kar, S.; Mack, M.J.; Rymer, J.; et al. Institutional experience with transcatheter mitral valve repair and clinical outcomes: Insights from the TVT registry. Cardiovasc. Interv. 2019, 12, 1342–1352. [Google Scholar]
- Gavazzoni, M.; Taramasso, M.; Zuber, M.; Russo, G.; Pozzoli, A.; Miura, M.; Maisano, F. Conceiving MitraClip as a tool: Percutaneous edge-to-edge repair in complex mitral valve anatomies. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1059–1067. [Google Scholar] [CrossRef]
- Paradis, J.-M.; Del Trigo, M.; Puri, R.; Rodés-Cabau, J. Transcatheter Valve-in-Valve and Valve-in-Ring for Treating Aortic and Mitral Surgical Prosthetic Dysfunction. J. Am. Coll. Cardiol. 2015, 66, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Naoum, C.; Dvir, D.; Bapat, V.; Ong, K.; Muller, D.; Cheung, A.; Ye, J.; Min, J.K.; Piazza, N.; et al. Predicting LVOT obstruction in transcatheter mitral valve implantation: Concept of the neo-LVOT. JACC Cardiovasc. Imaging 2017, 10, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Foerst, J.R.; Yazdani, S.; McCabe, J.M.; Paone, G.; Eng, M.H.; Leshnower, B.G.; Gleason, P.T.; et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2521–2534. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Daqa, A.; Yeh, J.; Davies, S.; Uebing, A.; Quarto, C.; Moat, N.; Alison, D.; Anan, D.; James, Y.; et al. Transcatheter mitral valve replacement: Long-term outcomes of first-in-man experience with an apically tethered device—A case series from a single centre. EuroIntervention 2017, 13, e1047–e1057. [Google Scholar] [CrossRef]
- Capretti, G.; Urena, M.; Himbert, D.; Brochet, E.; Goublaire, C.; Verdonk, C.; Carrasco, J.L.; Ghodbane, W.; Messika-Zeitoun, D.; Iung, B.; et al. Valve Thrombosis after Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2016, 68, 1814–1815. [Google Scholar] [CrossRef]
- Peppas, A.; Furer, A.; Wilson, J.; Yi, G.; Cheng, Y.; Van Wygerden, K.; Seguin, C.; Shibuya, M.; Kaluza, G.L.; Granada, J.F. Preclinical in vivo long-term evaluation of the novel Mitra-Spacer technology: Experimental validation in the ovine model. Eurointervention 2017, 13, 272–279. [Google Scholar] [CrossRef]
- Silaschi, M.; Nicou, N.; Eskandari, M.; Aldalati, O.; Seguin, C.; Piemonte, T.; McDonagh, T.; Dworakowski, R.; Byrne, J.; MacCarthy, P.; et al. Dynamic transcatheter mitral valve repair: A new concept to treat functional mitral regurgitation using an adjustable spacer. Eurointervention 2017, 13, 280–283. [Google Scholar] [CrossRef]
- Webb, J.; Hensey, M.; Fam, N.; Rodés-Cabau, J.; Daniels, D.; Smith, R.; Szeto, W.; Boone, R.; Ye, J.; Moss, R.; et al. Transcatheter Mitral Valve Replacement With the Transseptal EVOQUE System. JACC Cardiovasc. Interv. 2020, 13, 2418–2426. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Nakashima, M.; Williams, M.; He, Y.; Latson, L.; Saric, M.; Vainrib, A.; Staniloae, C.; Hisamoto, K.; Ibrahim, H.; Querijero, M.; et al. Multiphase Assessment of Mitral Annular Dynamics in Consecutive Patients With Significant Mitral Valve Disease. JACC Cardiovasc. Interv. 2021, 14, 2215–2227. [Google Scholar] [CrossRef]
- Waksman, R.; Medranda, G.A. Transcatheter Mitral Valve Replacement: Size Matters; American College of Cardiology Foundation: Washington, DC, USA, 2021; Volume 14, pp. 2228–2230. [Google Scholar]
- Abdel-Wahab, M.; Landt, M.; Neumann, F.; Massberg, S.; Frerker, C.; Kurz, T.; Kaur, J.; Toelg, R.; Sachse, S.; Jochheim, D.; et al. Investigators CHOICE. 5-year outcomes after TAVR with balloon-expandable versus self-expanding valves: Results from the CHOICE randomized clinical trial. JACC Cardiovasc. Interv. 2020, 13, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.S.; Kapadia, S.R. The Tendyne transcatheter mitral valve replacement system for the treatment of mitral regurgitation. Future Cardiol. 2019, 15, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Nordmeyer, J.; Khambadkone, S.; Coats, L.; Schievano, S.; Lurz, P.; Parenzan, G.; Taylor, A.M.; Lock, J.E.; Bonhoeffer, P. Risk Stratification, Systematic Classification, and Anticipatory Management Strategies for Stent Fracture After Percutaneous Pulmonary Valve Implantation. Circulation 2007, 115, 1392–1397. [Google Scholar] [CrossRef]
- Ruel, M.; Kulik, A.; Lam, B.K.; Rubens, F.D.; Hendry, P.J.; Masters, R.G.; Bédard, P.; Mesana, T.G. Long-term outcomes of valve replacement with modern prostheses in young adults. Eur. J. Cardio-Thoracic Surg. 2005, 27, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Preston-Maher, G.; Torii, R.; Burriesci, G. A Technical Review of Minimally Invasive Mitral Valve Replacements. Cardiovasc. Eng. Technol. 2015, 6, 174–184. [Google Scholar] [CrossRef][Green Version]
- Bax, J.J.; Debonnaire, P.; Lancellotti, P.; Ajmone Marsan, N.; Tops, L.F.; Min, J.K.; Piazza, N.; Leipsic, J.; Hahn, R.T.; Delgado, V. Transcatheter interventions for mitral regurgitation: Multimodality imaging for patient selection and procedural guidance. JACC Cardiovasc. Imaging 2019, 12, 2029–2048. [Google Scholar] [CrossRef]
- Dal-Bianco, J.P.; Levine, R.A. Anatomy of the mitral valve apparatus: Role of 2D and 3D echocardiography. Cardiol. Clin. 2013, 31, 151–164. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J.—Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Brugger, N.; Wustmann, K.; Hürzeler, M.; Wahl, A.; de Marchi, S.F.; Steck, H.; Zürcher, F.; Seiler, C. Comparison of Three-Dimensional Proximal Isovelocity Surface Area to Cardiac Magnetic Resonance Imaging for Quantifying Mitral Regurgitation. Am. J. Cardiol. 2015, 115, 1130–1136. [Google Scholar] [CrossRef]
- Sköldborg, V.; Madsen, P.L.; Dalsgaard, M.; Abdulla, J. Quantification of mitral valve regurgitation by 2D and 3D echocardiography compared with cardiac magnetic resonance a systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2020, 36, 279–289. [Google Scholar] [CrossRef]
- Marsan, N.A.; Westenberg, J.J.; Ypenburg, C.; Delgado, V.; van Bommel, R.J.; Roes, S.D.; Nucifora, G.; van der Geest, R.J.; de Roos, A.; Reiber, J.C.; et al. Quantification of functional mitral regurgitation by real-time 3D echocardiography: Comparison with 3D velocity-encoded cardiac magnetic resonance. JACC Cardiovasc. Imaging 2009, 2, 1245–1252. [Google Scholar] [CrossRef]
- Shanks, M.; Siebelink, H.-M.J.; Delgado, V.; van de Veire, N.R.; Ng, A.C.; Sieders, A.; Schuijf, J.D.; Lamb, H.J.; Ajmone Marsan, N.; Westenberg, J.J.; et al. Quantitative assessment of mitral regurgitation: Comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ. Cardiovasc. Imaging 2010, 3, 694–700. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Rodríguez-Olivares, R.; Ren, B.C.; Van Gils, L.; Maugenest, A.; Geleijnse, M.L.; Budde, R.P.; Vogelaar, J.; Verstraeten, L.; De Jaegere, P.P. Computed tomography optimised fluoroscopy guidance for transcatheter mitral therapies. EuroIntervention 2016, 11, 1428–1431. [Google Scholar] [CrossRef][Green Version]
- Meduri, C.U.; Reardon, M.J.; Lim, D.S.; Howard, E.; Dunnington, G.; Lee, D.P.; Liang, D.; Gooley, R.; O’Hair, D.; Ng, M.K.; et al. Novel multiphase assessment for predicting left ventricular outflow tract obstruction before transcatheter mitral valve replacement. Cardiovasc. Interv. 2019, 12, 2402–2412. [Google Scholar] [CrossRef]
- Namazi, F.; Vo, N.M.; Delgado, V. Imaging of the mitral valve: Role of echocardiography, cardiac magnetic resonance, and cardiac computed tomography. Curr. Opin. Cardiol. 2020, 35, 435–444. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients with Primary Mitral Regurgitation with and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Kusunose, K.; Obuchowski, N.A.; Jellis, C.; Griffin, B.P.; Flamm, S.D.; Kwon, D.H. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. Cardiovasc. Imaging 2020, 13, 1489–1501. [Google Scholar] [CrossRef]

| Device | Repair Method | Approach | Indications | 30-Day Mortality Rate |
|---|---|---|---|---|
| MitraClipTM | TEER | transseptal | Primary/Secondary MR | 0.9–6% [7,8,9,10,11,12,13] |
| PASCAL | TEER | transseptal | Primary/Secondary MR | 1.6–2% [14,15] |
| Cardioband | Direct annuloplasty | transseptal | Secondary MR | 3.3–5% [16,17] |
| Mitralign | Direct annuloplasty | transseptal | Secondary MR | 4.4% [18] |
| Carillon | Indirect annuloplasty | transseptal | Secondary MR | 1.9–2.7% [19,20,21,22] |
| NeoChord * | chordal repair | transapical/transeptal | Primary MR | 0–1.9% [23,24] |
| Trial | Device | Aim |
|---|---|---|
| MITRA-HR RESHAPE-HF2 MATTERHORN REPAIR-MR | MitraClip | Long-term outcomes Risk stratification Patient selection |
| CLASP IID/IIF | PASCAL | Safety and effectiveness compared with MitraClip |
| MiBAND ACTIVE | Cardioband | Post-Market approval safety and efficacy (MiBAND) Identify optimal candidates by comparing with guideline-directed medical therapy in patients with FMR (ACTIVE) |
| Millipede Feasibility | Millipede | Feasibility and safety |
| EMPOWER | Carillon | Safety and efficacy at 5 years of follow-up |
| Rechord | NeoChord | Safety and effectiveness compared with open surgical repair |
| Mitral Valve Anatomy | Clip Selection Recommendations |
|---|---|
| Leaflet length < 9 mm | NTW, NT |
| Leaflet length > 9 mm | XTW, XT |
| Broad jet | NTW, XTW |
| Smaller valve | NT |
| Larger valve | NTW, XTW, XT |
| Device | Anchoring Method | Approach | Indications | 30-Day Mortality Rate |
|---|---|---|---|---|
| Tendyne Mitral Valve System | Apical tether | transapical | Secondary MR | 6% [59] |
| Tiara TMVR System | Native leaflet engagement | transapical | Primary/Secondary MR | 11.3% [60,61] |
| Intrepid TMVR System | Radial forces and sub-annual cleats | transapical | Secondary MR | 14% [62] |
| EVOQUE TMVR System | External anchor | transseptal | Primary/Secondary MR | 7% [63] |
| SAPIEN M3 System | Nitinol dock system | transseptal | Primary/Secondary MR | 2.9% [64] |
| HighLife TMVR system | External anchor mitral annuls capture | transseptal | Secondary MR | 20% [65] |
| Device | Features | Approach | Studies |
|---|---|---|---|
| NAVI System | Nitinal self-expandable system with several annular winglets | Transaptical | No trials ongoing |
| AltaValve TMVR system | Self-expanding supra-annular device, with a bovine tissue valve mounted into a spherical nitinol frame | Transaptical | Early feasibility study protocol (NCT03997305), still recruiting |
| Cephea TMVR System | Self-expanding double-disk and trileaflet bovine pericardium tissue | Transseptal | Cephea Transseptal Mitral Valve System FIH (NCT03988946) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiling, Z.; Puehler, T.; Sondergaard, L.; Frank, D.; Seoudy, H.; Mohammad, B.; Müller, O.J.; Sellers, S.; Meier, D.; Sathananthan, J.; et al. Transcatheter Mitral Valve Repair or Replacement: Competitive or Complementary? J. Clin. Med. 2022, 11, 3377. https://doi.org/10.3390/jcm11123377
Xiling Z, Puehler T, Sondergaard L, Frank D, Seoudy H, Mohammad B, Müller OJ, Sellers S, Meier D, Sathananthan J, et al. Transcatheter Mitral Valve Repair or Replacement: Competitive or Complementary? Journal of Clinical Medicine. 2022; 11(12):3377. https://doi.org/10.3390/jcm11123377
Chicago/Turabian StyleXiling, Zhang, Thomas Puehler, Lars Sondergaard, Derk Frank, Hatim Seoudy, Baland Mohammad, Oliver J. Müller, Stephanie Sellers, David Meier, Janarthanan Sathananthan, and et al. 2022. "Transcatheter Mitral Valve Repair or Replacement: Competitive or Complementary?" Journal of Clinical Medicine 11, no. 12: 3377. https://doi.org/10.3390/jcm11123377
APA StyleXiling, Z., Puehler, T., Sondergaard, L., Frank, D., Seoudy, H., Mohammad, B., Müller, O. J., Sellers, S., Meier, D., Sathananthan, J., & Lutter, G. (2022). Transcatheter Mitral Valve Repair or Replacement: Competitive or Complementary? Journal of Clinical Medicine, 11(12), 3377. https://doi.org/10.3390/jcm11123377






