Controversies and Clinical Applications of Non-Invasive Transspinal Magnetic Stimulation: A Critical Review and Exploratory Trial in Hereditary Spastic Paraplegia
Abstract
1. Introduction
2. Materials and Methods
3. Results
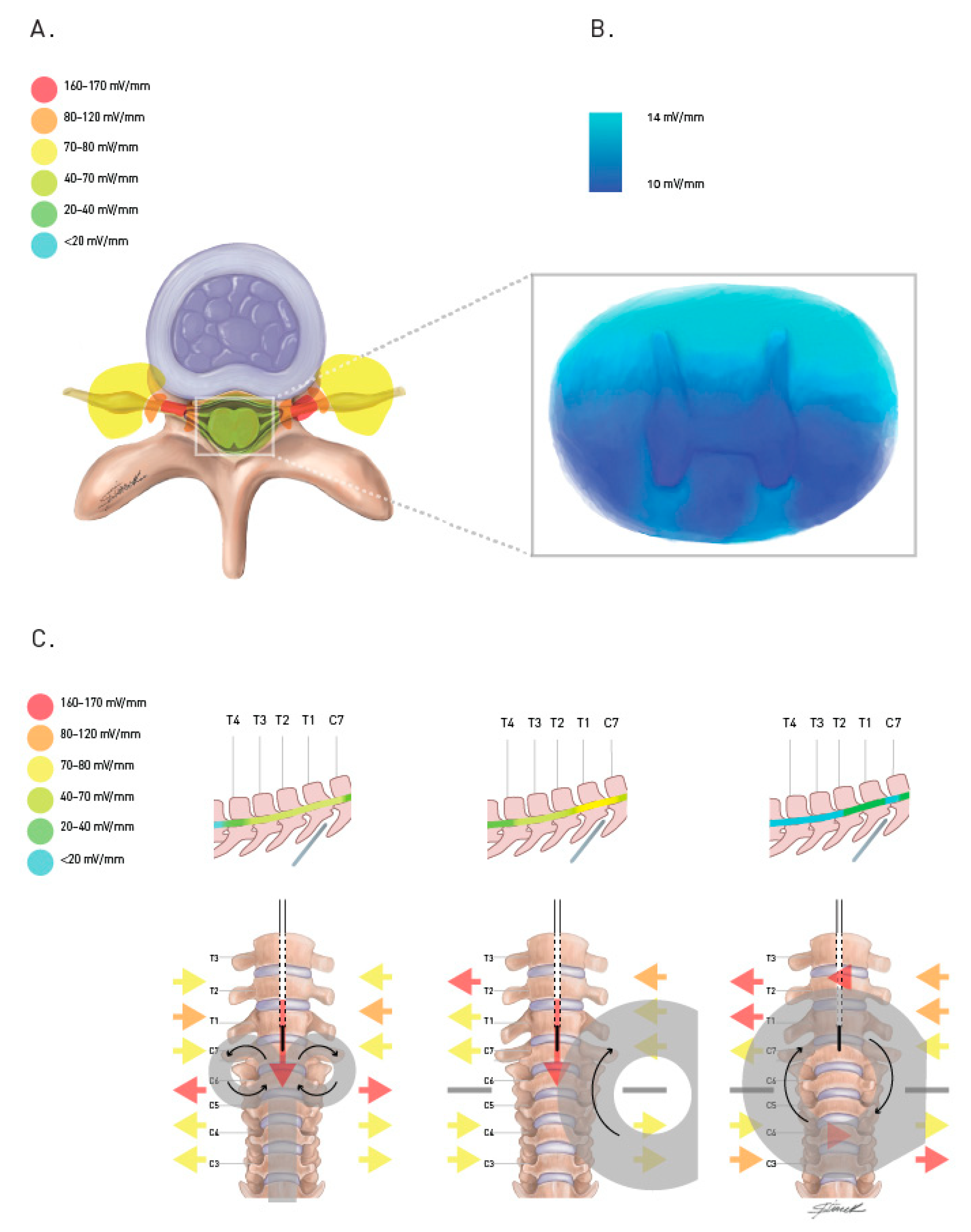
3.1. How Does TsMS Work?
3.2. What Are the Clinical Implications of TsMS?
3.3. Open-Label Pilot Trial
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Polson, M.J.; Barker, A.T.; Freeston, I.L. Stimulation of nerve trunks with time-varying magnetic fields. Med. Biol. Eng. Comput. 1982, 20, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T.; Freeston, I.L.; Jalinous, R.; Jarratt, J.A. Magnetic stimulation of the human brain and peripheral nervous system: An introduction and the results of an initial clinical evaluation. Neurosurgery 1987, 20, 100–109. [Google Scholar] [CrossRef]
- Cai, Y.; Reddy, R.D.; Varshney, V.; Chakravarthy, K.V. Spinal cord stimulation in Parkinson’s disease: A review of the preclinical and clinical data and future prospects. Bioelectron. Med. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Hachmann, J.T.; Yousak, A.; Wallner, J.J.; Gad, P.N.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, 126, 1843–1859. [Google Scholar] [CrossRef] [PubMed]
- Diniz de Lima, F.; Faber, I.; Servelhere, K.R.; Bittar, M.F.R.; Martinez, A.R.M.; Piovesana, L.G.; Martins, M.; Martins, C.; Benaglia, T.; de Sa Carvahlo, M. Randomized trial of botulinum toxin type a in hereditary spastic paraplegia—The SPASTOX trial. Mov. Disord. 2021, 36, 1654–1663. [Google Scholar] [CrossRef]
- Lefaucheur, J.P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar] [CrossRef]
- Masland, W.S. Facilitation during the H-reflex recovery cycle. Arch. Neurol. 1972, 26, 313–319. [Google Scholar] [CrossRef]
- Kumru, H.; Albu, S.; Valls-Sole, J.; Murillo, N.; Tormos, J.M.; Vidal, J. Influence of spinal cord lesion level and severity on H-reflex excitability and recovery curve. Muscle Nerve 2015, 52, 616–622. [Google Scholar] [CrossRef]
- Musienko, P.E.; Bogacheva, I.N.; Savochin, A.A.; Kilimnik, V.A.; Gorskiĭ, O.V.; Nikitin, O.A.; Gerasimenko, P. Non-invasive transcutaneous spinal cord stimulation facilitates locomotor activity in decerebrated and spinal cats. Ross. Fiziol. Zh. Im. I.M. Sechenova 2013, 99, 917–927. [Google Scholar]
- Hunanyan, A.S.; Petrosyan, H.A.; Alessi, V.; Arvanian, V.L. Repetitive spinal electromagnetic stimulation opens a window of synaptic plasticity in damaged spinal cord:role of NMDA receptors. J. Neurophysiol. 2012, 107, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Chalfouh, C.; Guillou, C.; Hardouin, J.; Delarue, Q.; Li, X.; Duclos, C.; Schapman, D.; Marie, J.; Cosette, P.; Guerot, N. The Regenerative Effect of Trans-spinal Magnetic Stimulation after Spinal Cord Injury: Mechanisms and Pathways Underlying the Effect. Neurotherapeutics 2020, 17, 2069–2088. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, Y.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Marsden, C.D. Magnetic stimulation over the spinal enlargements. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Chokroverty, S.; Picone, M.A.; Chokroverty, M. Percutaneous magnetic coil stimulation of human cervical vertebral column: Site of stimulation and clinical application. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1991, 81, 359–365. [Google Scholar] [CrossRef]
- Chokroverty, S.; Deutsch, A.; Guha, C.; Gonzalez, A.; Kwan, P.; Burger, R.; Goldberg, J. Thoracic spinal nerve and root conduction: A magnetic stimulation study. Muscle Nerve 1995, 18, 987–991. [Google Scholar] [CrossRef]
- Chokroverty, S.; Flynn, D.; Picone, M.A.; Chokroverty, M.; Belsh, J. Magnetic coil stimulation of the human lumbosacral vertebral column: Site of stimulation and clinical application. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1993, 89, 54–60. [Google Scholar] [CrossRef]
- Britton, T.C.; Meyer, B.U.; Herdmann, J.; Benecke, R. Clinical use of the magnetic stimulator in the investigation of peripheral conduction time. Muscle Nerve 1990, 13, 396–406. [Google Scholar] [CrossRef]
- Cros, D.; Chiappa, K.H.; Gominak, S.; Fang, J.; Santamaria, J.; King, P.J.; Shahani, B. Cervical magnetic stimulation. Neurology 1990, 40, 1751. [Google Scholar] [CrossRef]
- Tomberg, C. Transcutaneous magnetic stimulation of descending tracts in the cervical spinal cord in humans. Neurosci. Lett. 1995, 188, 199–201. [Google Scholar] [CrossRef]
- Ugawa, Y.; Uesaka, Y.; Terao, Y.; Hanajima, R.; Kanazawa, I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann. Neurol. 1994, 36, 618–624. [Google Scholar] [CrossRef]
- Ugawa, Y.; Uesaka, Y.; Terao, Y.; Suzuki, M.; Sakai, K.; Hanajima, R.; Kanazawa, I. Clinical utility of magnetic corticospinal tract stimulation at the foramen magnum level. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1996, 101, 247–254. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hanajima, R.; Terao, Y.; Ugawa, Y. Magnetic-motor-root stimulation: Review. Clin. Neurophysiol. 2013, 124, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, M.J. Sensory potentials evoked by magnetic stimulation of the cervical spine. Muscle Nerve 1993, 16, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Similowski, T.; Fleury, B.; Launois, S.; Cathala, H.P.; Bouche, P.; Derenne, J.P. Cervical magnetic stimulation: A new painless method for bilateral phrenic nerve stimulation in conscious humans. J. Appl. Physiol. 1989, 67, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Malcolm, M.P.; Newsom, S.A.; Richards, J.C.; Rynn, G.M.; Bell, C. Sympathetic responses to repetitive trans-spinal magnetic stimulation. Clin. Auton. Res. 2011, 21, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, E.; Valls-Sole, J. Excitatory and inhibitory responses to cervical root magnetic stimulation in healthy subjects. Clin. Neurophysiol. Pract. 2021, 6, 265–274. [Google Scholar] [CrossRef]
- Maccabee, P.J.; Amassian, V.E.; Eberle, L.P.; Rudell, A.P.; Cracco, R.Q.; Lai, K.S.; Somasundarum, M. Measurement of the electric field induced into inhomogeneous volume conductors by magnetic coils: Application to human spinal neurogeometry. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1991, 81, 224–237. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Salvador, R.; de Carvalho, M.; Miranda, P.C. Modelling Studies of Non-invasive Electric and Magnetic Stimulation of the Spinal Cord. In Brain and Human Body Modeling 2020: Computational Human Models Presented at EMBC 2019 and the BRAIN Initiative® 2019 Meeting [Internet]; Makarov, S.N., Noetscher, G.M., Nummenmaa, A., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Cretu, M.; Ciupa, R.V.; Darabant, L. Evaluation of Spinal Cord Response During Magnetic Stimulation of the Lumbar Area. Biomed. Tech. 2013, 58 (Suppl. S1A). [Google Scholar] [CrossRef]
- Machida, M.; Kimura, J.; Yamada, T.; Yarita, M. Magnetic coil stimulation of the spinal cord in the dog. Effect of removal of bony structure on eddy current. Spine 1992, 17, 1405–1408. [Google Scholar] [CrossRef]
- Konrad, P.E.; Owen, J.H.; Bridwell, K.H. Magnetic stimulation of the spine to produce lower extremity EMG responses. Significance of coil position and the presence of bone. Spine 1994, 19, 2812–2818. [Google Scholar] [CrossRef]
- Nielsen, J.F.; Sinkjaer, T. Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult. Scler. J. 1997, 3, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M. Neurophysiological characteristics of human leg muscle action potentials evoked by transcutaneous magnetic stimulation of the spine. Bioelectromagnetics 2013, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.F.; Klemar, B.; Hansen, H.J.; Sinkjaer, T. A new treatment of spasticity with repetitive magnetic stimulation in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1995, 58, 254–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nielsen, J.F.; Sinkjaer, T.; Jakobsen, J. Treatment of spasticity with repetitive magnetic stimulation; a double-blind placebo-controlled study. Mult. Scler. J. 1996, 2, 227–232. [Google Scholar] [CrossRef]
- Krause, P.; Straube, A. Repetitive magnetic and functional electrical stimulation reduce spastic tone increase in patients with spinal cord injury. Suppl. Clin. Neurophysiol. 2003, 56, 220–225. [Google Scholar] [CrossRef]
- Krause, P.; Edrich, T.; Straube, A. Lumbar repetitive magnetic stimulation reduces spastic tone increase of the lower limbs. Spinal Cord. 2004, 42, 67–72. [Google Scholar] [CrossRef][Green Version]
- Krause, P.; Straube, A. Reduction of spastic tone increase induced by peripheral repetitive magnetic stimulation is frequency-independent. Neurorehabilitation 2005, 20, 63–65. [Google Scholar] [CrossRef]
- Niu, T.; Bennett, C.J.; Keller, T.L.; Leiter, J.C.; Lu, D.C. A Proof-of-Concept Study of Transcutaneous Magnetic Spinal Cord Stimulation for Neurogenic Bladder. Sci. Rep. 2018, 8, 12549. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Wang, C.P.; Chiu, F.Y.; Tsai, Y.A.; Chang, Y.C.; Chuang, T.Y. Efficacy of functional magnetic stimulation in neurogenic bowel dysfunction after spinal cord injury. J. Rehabil. Med. 2009, 41, 41–47. [Google Scholar] [CrossRef]
- Chiu, C.M.; Wang, C.P.; Sung, W.H.; Huang, S.F.; Chiang, S.C.; Tsai, P.Y. Functional magnetic stimulation in constipation associated with Parkinson’s disease. J. Rehabil. Med. 2009, 41, 1085–1089. [Google Scholar] [CrossRef]
- Arii, Y.; Sawada, Y.; Kawamura, K.; Miyake, S.; Taichi, Y.; Izumi, Y.; Kuroda, Y.; Inui, T.; Kaji, R.; Mitsui, T. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.R.; Carra, R.B.; Nunes, G.A.; Simões, J.S.; Teixeira, M.J.; Duarte, K.P.; de Andrade, D.; Barbosa, E.; Marcolin, M.; Cury, R. Transcutaneous magnetic spinal cord stimulation for freezing of gait in Parkinson’s disease. J. Clin. Neurosci. 2020, 81, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Arii, Y.; Taniguchi, K.; Tsutsumi, S.; Takahara, M.; Mabuchi, M.; Sumitomo, N.; Matsuura, M.; Kuroda, Y. Efficacy of Repetitive Trans-spinal Magnetic Stimulation for Patients with Parkinson’s Disease: A Randomised Controlled Trial. Neurotherapeutics, 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lance, J. Spasticity: Disorders motor control. In Symposium Synopsis; Feldman, R.G., Young, R.P., Koella, W.P., Eds.; Year Book Medical Publishers: Miami, FL, USA, 1980; p. 3. [Google Scholar]
- Fonoff, E.T.; de Lima-Pardini, A.C.; Coelho, D.B.; Monaco, B.A.; Machado, B.; Pinto de Souza, C.; dos Santos Ghilardi, M.; Hamani, C. Spinal Cord Stimulation for Freezing of Gait: From Bench to Bedside. Front. Neurol. 2019, 10, 905. [Google Scholar] [CrossRef]
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018, 18, 1048–1067. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Einhorn, J.; Li, A.; Hazan, R.; Knikou, M. Cervicothoracic multisegmental transpinal evoked potential in humans. PLoS ONE 2013, 8, e76940. [Google Scholar] [CrossRef]
- Zschorlich, V.R.; Hillebrecht, M.; Tanjour, T.; Qi, F.; Behrendt, F.; Kirschstein, T.; Kohling, R. Repetitive Peripheral Magnetic Nerve Stimulation (rPMS) as Adjuvant Therapy Reduces Skeletal Muscle Reflex Activity. Front. Neurol. 2019, 10, 930. [Google Scholar] [CrossRef]
- Marz-Loose, H.; Siemes, H. Repetitive periphere Magnetstimulation. Therapieoption bei Spastik? Repetitive peripheral magnetic stimulation. Treatment option for spasticity? Nervenarzt 2009, 80, 1489–1495. (In German) [Google Scholar] [CrossRef]
- Behrens, M.; Mau-Möller, A.; Zschorlich, V.; Bruhn, S. Repetitive Peripheral Magnetic Stimulation (15 Hz RPMS) of the Human Soleus Muscle did not Affect Spinal Excitability. J. Sports Sci. Med. 2011, 10, 39–44. [Google Scholar]
- Matsumoto, H.; Octaviana, F.; Hanajima, R.; Terao, Y.; Yugeta, A.; Hamada, M.; Inomata, S.; Nakatani, S.; Tsuji, S.; Ugawa, Y. Magnetic lumbosacral motor root stimulation with a flat, large round coil. Clin. Neurophysiol. 2009, 120, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.B.; Halje, P.; Simplício, H.; Richter, U.; Freire, M.A.M.; Petersson, P.; Fuentes, R.; Nicolelis, M. Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron 2014, 84, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, P.; Yin, R.; Xiao, M.; Zhang, Y.; Reinhardt, J.D.; Wang, H.; Xu, G. Combination of repetitive transcranial magnetic stimulation and treadmill training reduces hyperreflexia by rebalancing motoneuron excitability in rats after spinal cord contusion. Neurosci. Lett. 2022, 775, 136536. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Balbi, P.; Russo, G.; Nilsson, J. H-reflex recovery curve and reciprocal inhibition of H-reflex of the upper limbs in patients with spasticity secondary to stroke. Am. J. Phys. Med. Rehabil. 1995, 74, 357–363. [Google Scholar] [CrossRef]
- Phadke, C.P.; Robertson, C.T.; Condliffe, E.G.; Patten, C. Upper extremity H-reflex measurement post-stroke: Reliability and inter-limb differences. Clin. Neurophysiol. 2012, 123, 1606–1615. [Google Scholar] [CrossRef]
- Diamantopoulos, E.; Zander Olsen, P. Excitability of motor neurones in spinal shock in man. J. Neurol. Neurosurg. Psychiatry 1967, 30, 427–431. [Google Scholar] [CrossRef]
- Taylor, S.; Ashby, P.; Verrier, M. Neurophysiological changes following traumatic spinal lesions in man. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1102–1108. [Google Scholar] [CrossRef]

| Study | Population and Trial Design | Device, Location and Intensity | Frequency and Number of Sessions | Main findings |
|---|---|---|---|---|
| Nielsen et al., 1995 [34] | 12 multiple sclerosis patients Open label trial | 13.4 cm circular coil at T8 0.8 to 1.3 Tesla | 12 Hz repetitive (5760 pulses) single session | Significant 28% reduction of stretch reflex amplitude and 50% increase of stretch reflex threshold. Significant reduction of Ashworth spasticity scale and daily activities questionnaire. Side effect of tight feeling, brief dizziness in one patient. |
| Nielsen et al., 1996 [35] | 38 multiple sclerosis patients Double blinded randomized placebo-controlled trial | 13.4 cm circular coil at T8 0.6 to 0.7 Tesla | 25 Hz repetitive (10,000 pulses) or sham Two daily sessions for 7 days | Significant 27% reduction of stretch reflex amplitude in active group. Significant reduction of Ashworth spasticity scale and reflex grading only in the active group. Improvement of daily activities questionnaire in both groups. Significant baseline spasticity score between groups. Two episodes brief dizziness, one palpitation not confirmed by ECG after 2 h of stimulation |
| Nielsen et al., 1997 [32] | 11 multiple sclerosis patients, 9 HC Open label trial | 13.4 cm circular coil at T8 0.4, 0.6 or 0.8 Tesla | 25 Hz (3750) repetitive, Single session Secondary comparison of 1 Hz, 10 Hz and 25 Hz 16 pulses. | Significant H reflex reduction at 1, 10, and 25 Hz in patients and controls after 16 pulses, but only in patients after full 3750 pulses session. No cortical motor evoked potentials changes Minor discomfort. |
| Krause et al., 2003 [36] | 6 spinal cord injury patients (3 complete) Single blinded crossover-controlled trial | 90 mm circular coil L3–L4 2 cm lateral 120% of lower limb contraction | 20 Hz repetitive (2000 pulses) vs. electrical stimulation vs. sham Single session | Significant reduction in spastic tone in the Ashworth scale and modified Wartenberg’s pendulum test of spasticity after TsMS, similar to results produced by electrical stimulation, and superior to sham. No discomfort. |
| Krause et al., 2004 [37] | 16 HC, 15 varied spinal lesion patients Open label trial | 90 mm circular coil L3–L4 2 cm lateral 120% of lower limb contraction | 20 Hz repetitive (2000 pulses) 1 session | Significant reduction in the Ashworth scale between 4 and 24 h after stimulation Significant increase in modified Wartenberg’s pendulum test of spasticity peak velocity in patients between 4 and 24 h but not HC. Effect was more pronounced contralateral to stimulation site. No reported side effects |
| Krause et al., 2005 [38] | Single spinal myelitis patient, single blinded controlled trial | 90 mm circular coil L3–L4 2 cm lateral 120% of lower limb contraction | 20 Hz, 15 Hz, 10 Hz (2000 pulses each), 3 sessions of each plus sham in random order | Significant reduction in relaxation index in all protocols compared to sham; no significant difference between frequency groups. No reported side effects |
| Niu et al., 2018 [39] | 5 spinal cord injury patients (complete) requiring intermittent catheterization Crossover single blinded followed by open label | 75 mm figure of eight coil at T12–L1 80% of paraspinal muscle contraction (0.8 to 1.2 Tesla) | Phase one: 1 Hz (720 pulses) vs. 30 Hz (21,600 pulses) one session Phase two: 1 Hz (720 pulses) in 16 weekly sessions | 1 Hz was superior to 30 Hz in all patients in decreasing urethral pressure and increasing detrusor pressure in urodynamic test after phase one. Significant improvement in spontaneous urine volume, reduction in self catheterization frequency from 6.6 to 2.4 per day and increase in quality of life after phase two. No reported side effects |
| Tsai et al., 2009 [40] | 22 spinal cord injury patients with constipation Open label trial | No coil description. coil At T9 and L3 1.1 to 1.54 Tesla | 20 Hz, 800 pulses, at each site. Two daily sessions for 21 days | Significant improvement of colonic transit time (62.6 to 50.4 h) and bowel symptom questionnaires. Benefit was greater in questionnaires immediately after protocol completion but remained significantly greater than baseline after 3 months. No reported side effects |
| Chiu et al., 2009 [41] | 16 patients with Parkinson’s disease and constipation Open label trial | No coil description. Coil at T9 and L3 1.1 to 1.54 Tesla | 20 Hz, 800 pulses, at each site. Two daily sessions for 21 days | Significant improvement of colonic transit time (64.9 to 53.6 h), reduced residual barium and bowel symptom questionnaires. Sustained benefit in questionnaires after three months No reported side effects |
| Ari et al., 2014 [42] | 37 Parkinson’s disease patients with camptocormia Randomized single blind crossover placebo-controlled trial | 115 mm circular coil Level of worse angulation (thoracic or lumbar) 1 Tesla | 5 Hz repetitive (40 pulses) or sham Single session | Reduction in camptocormia flexion angle by average 10.9°, no change with sham. Benefit duration was reported to be of three days, no difference observed after one week. Two patients reported mild lumbar discomfort lasting less than a day. |
| Menezes et al., 2020 [43] | 5 Parkinson’s disease patients with freezing of gait. Open label trial | 90 mm Circular coil at T5 90% of abdominal motor contraction | intermittent Theta burst (400 pulses) 3 sessions in one day | Significant improvement consisting of mean change of UPDRS of 6.6 points and freezing of gait questionnaire after 7 days of stimulation. No significant change of timed up and go was seen, or in any outcome immediately after stimulation or after 28 days. One patient reported transitory fatigue |
| Mitsui et al., 2022 [44] | 100 Parkinson’s disease patients, Randomized single blinded placebo-controlled trial | Figure of eight coil at T12–L1 Intensity not explicit | 5 Hz repetitive (50 pulses) or sham 8 biweekly sessions. | Significant improvement consisting of mean change of UPDRS of 10.28 points immediately after TsMS, 5.04 after three months and 2.38 at 6 months. Significant improvement in timed up and go test in all endpoints. Side effects were similar in active and sham groups and included self-limited back/arm pain, falls, and dizziness in a total of 11 patients. |
| Clinical and Neurophysiological Data | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Sex | Male | Female | Female |
| Age (years old) | 40 | 37 | 63 |
| Age of onset (years old) | 1 | 26 | 50 |
| Phenotype | Pure | Pure | Pure |
| Genotype | SPG4 | SPG33 | SPG33 |
| Baseline SPRS score | 21 | 25 | 34 |
| Medications in use | none | none | none |
| Pre TsMS | |||
| H2/H1 ratio with paired stimuli at ISI of 50 ms | 1.71 | 1.13 | 1.34 |
| H2/H1 ratio with paired stimuli at ISI of 100 ms | 0.99 | 1.08 | 1.17 |
| Post TsMS | |||
| H2/H1 ratio with paired stimuli at ISI of 50 ms | 0.10 | 0.09 | 0.05 |
| H2/H1 ratio with paired stimuli at ISI of 100 ms | 0.88 | 0.79 | 0.76 |
| Clinical Response | Neurophysiological Response |
|---|---|
Reduction in spasticity:
| Electromyography testing:
|
Improvement in neurogenic bladder:
| Urodynamic testing:
|
Improvement in neurogenic bowel:
| Colonic transit:
|
Improvement in Parkinson’s disease:
| Still under study (unknown) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carra, R.B.; Silva, G.D.; Paraguay, I.B.B.; Diniz de Lima, F.; Menezes, J.R.; Pineda, A.M.; Nunes, G.A.; Simões, J.d.S.; França, M.C., Jr.; Cury, R.G. Controversies and Clinical Applications of Non-Invasive Transspinal Magnetic Stimulation: A Critical Review and Exploratory Trial in Hereditary Spastic Paraplegia. J. Clin. Med. 2022, 11, 4748. https://doi.org/10.3390/jcm11164748
Carra RB, Silva GD, Paraguay IBB, Diniz de Lima F, Menezes JR, Pineda AM, Nunes GA, Simões JdS, França MC Jr., Cury RG. Controversies and Clinical Applications of Non-Invasive Transspinal Magnetic Stimulation: A Critical Review and Exploratory Trial in Hereditary Spastic Paraplegia. Journal of Clinical Medicine. 2022; 11(16):4748. https://doi.org/10.3390/jcm11164748
Chicago/Turabian StyleCarra, Rafael Bernhart, Guilherme Diogo Silva, Isabela Bruzzi Bezerra Paraguay, Fabricio Diniz de Lima, Janaina Reis Menezes, Aruane Mello Pineda, Glaucia Aline Nunes, Juliana da Silva Simões, Marcondes Cavalcante França, Jr., and Rubens Gisbert Cury. 2022. "Controversies and Clinical Applications of Non-Invasive Transspinal Magnetic Stimulation: A Critical Review and Exploratory Trial in Hereditary Spastic Paraplegia" Journal of Clinical Medicine 11, no. 16: 4748. https://doi.org/10.3390/jcm11164748
APA StyleCarra, R. B., Silva, G. D., Paraguay, I. B. B., Diniz de Lima, F., Menezes, J. R., Pineda, A. M., Nunes, G. A., Simões, J. d. S., França, M. C., Jr., & Cury, R. G. (2022). Controversies and Clinical Applications of Non-Invasive Transspinal Magnetic Stimulation: A Critical Review and Exploratory Trial in Hereditary Spastic Paraplegia. Journal of Clinical Medicine, 11(16), 4748. https://doi.org/10.3390/jcm11164748






