Evolving Devices and Material in Transcatheter Aortic Valve Replacement: What to Use and for Whom
Abstract
1. Introduction
2. Types of TAVR Devices
2.1. Balloon-Expandable Valves
2.2. Self-Expanding Valves
2.3. Mechanically-Expandable Valves
2.4. Valves with Active Fixation Mechanisms
3. Adverse Events after TAVR
3.1. Stroke and Subclinical Leaflet Thrombosis
3.2. Prosthesis-Patients Mismatch
3.3. Paravalvular Leak
3.4. Complex Post-TAVR Coronary Access
3.5. Vascular Complications
3.6. Conduction Disturbances
4. Clinical and Anatomic Factors to Consider in Bioprosthesis Selection
4.1. Age and Life Expectancy
4.2. Presence, Severity, and Disposition of Calcification
4.3. Aortic Annulus, LVOT and Septum Characteristics
4.4. Vascular Anatomy
5. Knowledge Gaps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø Study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Schueler, R.; Hammerstingl, C.; Sinning, J.M.; Nickenig, G.; Omran, H. Prognosis of octogenarians with severe aortic valve stenosis at high risk for cardiovascular surgery. Heart 2010, 96, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R. How Transcatheter Aortic Valve Implantation (TAVI) Was Born: The Struggle for a New Invention. Front. Cardiovasc. Med. 2021, 8, 1124. [Google Scholar] [CrossRef]
- Andersen, H.R.; Knudsen, L.L.; Hasenkam, J.M. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur. Heart J. 1992, 13, 704–708. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Pagnesi, M.; Chiarito, M.; Stefanini, G.G.; Testa, L.; Reimers, B.; Colombo, A.; Latib, A. Is Transcatheter Aortic Valve Replacement Superior to Surgical Aortic Valve Replacement?: A Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2017, 10, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, A.; Isogai, T.; Agrawal, A.; Shekhar, S.; Puri, R.; Reed, G.W.; Yun, J.J.; Unai, S.; Burns, D.J.P.; Vargo, P.R.; et al. Feasibility and Safety of Same-Day Discharge Following Transfemoral Transcatheter Aortic Valve Replacement. Cardiovasc. Interv. 2022, 15, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Rai, D.; Tanveer Ud Din, M.; Khan, M.Z.; Ullah, W.; Usman Khan, M.; Thakkar, S.; Hussein, A.; Baibhav, B.; Rao, M.; et al. Same-Day Discharge After Transcatheter Aortic Valve Implantation: Insights from the Nationwide Readmission Database 2015 to 2019. J. Am. Heart Assoc. 2022, 11, e024746. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices 2018, 15, 771–791. [Google Scholar] [CrossRef]
- Cahill, T.J.; Chen, M.; Hayashida, K.; Latib, A.; Modine, T.; Piazza, N.; Redwood, S.; Søndergaard, L.; Prendergast, B.D. Transcatheter aortic valve implantation: Current status and future perspectives. Eur. Heart J. 2018, 39, 2625–2634. [Google Scholar] [CrossRef]
- Claessen, B.E.; Tang, G.H.L.; Kini, A.S.; Sharma, S.K. Considerations for Optimal Device Selection in Transcatheter Aortic Valve Replacement: A Review. JAMA Cardiol. 2021, 6, 102–112. [Google Scholar] [CrossRef]
- Binder, R.K.; Rodés-Cabau, J.; Wood, D.A.; Webb, J.G. Edwards SAPIEN 3 valve. EuroIntervention 2012, 8, Q83–Q87. [Google Scholar] [CrossRef]
- Nazif, T.M.; Cahill, T.J.; Daniels, D.; McCabe, J.M.; Reisman, M.; Chakravarty, T.; Makkar, R.; Krishnaswamy, A.; Kapadia, S.; Chehab, B.M.; et al. Real-World Experience with the SAPIEN 3 Ultra Transcatheter Heart Valve: A Propensity-Matched Analysis from the United States. Circ. Cardiovasc. Interv. 2021, 14, 948–957. [Google Scholar] [CrossRef]
- Kawashima, H.; Soliman, O.; Wang, R.; Ono, M.; Hara, H.; Gao, C.; Zeller, E.; Thakkar, A.; Tamburino, C.; Bedogni, F.; et al. Rationale and design of a randomized clinical trial comparing safety and efficacy of myval transcatheter heart valve versus contemporary transcatheter heart valves in patients with severe symptomatic aortic valve stenosis: The LANDMARK trial. Am. Heart J. 2021, 232, 23–38. [Google Scholar] [CrossRef]
- Sharma, S.K.; Rao, R.S.; Chandra, P.; Goel, P.K.; Bharadwaj, P.; Joseph, G.; Jose, J.; Mahajan, A.U.; Mehrotra, S.; Sengottovelu, G.; et al. First-in-human evaluation of a novel balloon-expandable transcatheter heart valve in patients with severe symptomatic native aortic stenosis: The MyVal-1 study. EuroIntervention 2020, 16, 421–429. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tölg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schäfer, U.; et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA J. Am. Med. Assoc. 2014, 311, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Möllmann, H.; Diemert, P.; Grube, E.; Baldus, S.; Kempfert, J.; Abizaid, A. Symetis ACURATE TFTM aortic bioprosthesis. EuroIntervention 2013, 9, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.; Bieliauskas, G.; De Backer, O.; Søndergaard, L. Technical Considerations for Transcatheter Aortic Valve Replacement with ACURATE neo2. JACC Cardiovasc. Interv. 2021, 14, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, O.; Moreno, R.; Pascual-Tejerina, V.; Toggweiler, S.; Brinkert, M.; Baz, J.; Jimenez, V.; Molina, E.; Sánchez-Gila, J.; Taramasso, M.; et al. The Allegra transcatheter heart valve: European multicentre experience with a novel self-expanding transcatheter aortic valve. EuroIntervention 2019, 15, 71–73. [Google Scholar] [CrossRef]
- Wenaweser, P.; Stortecky, S.; Schütz, T.; Praz, F.; Gloekler, S.; Windecker, S.; Elsässer, A. Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: First-in-human experience with a novel self-expanding transcatheter heart valve. EuroIntervention 2016, 12, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.B.; Zhao, Z.G.; Wei, X.; Xu, Y.N.; Zuo, Z.L.; Li, Y.J.; Zheng, M.X.; Feng, Y.; Chen, M. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: Preliminary Experiences in China. Catheter. Cardiovasc. Interv. 2017, 89, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Ielasi, A.; Pellicano, M.; Latib, A.; Tespili, M.; Donatelli, F. An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. J. Clin. Med. 2022, 11, 499. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, W.; Wang, J.; Wu, Y.; Chen, M.; Modine, T.; Mylotte, D.; Piazza, N.; Ge, J. VitaFlowTM transcatheter valve system in the treatment of severe aortic stenosis: One-year results of a multicenter study. Catheter. Cardiovasc. Interv. 2020, 95, 332–338. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Urena, M.; Kuck, K.H.; Webb, J.G.; Rodés-Cabau, J. Edwards CENTERA valve. EuroIntervention 2012, 8, Q79–Q82. [Google Scholar] [CrossRef]
- Manoharan, G.; Spence, M.S.; Rodés-Cabau, J.; Webb, J.G. St Jude Medical Portico valve. EuroIntervention 2012, 8, Q97–Q101. [Google Scholar] [CrossRef]
- Meredith, I.T.; Hood, K.L.; Haratani, N.; Allocco, D.J.; Dawkins, K.D. Boston Scientific Lotus valve. EuroIntervention 2012, 8, Q70–Q74. [Google Scholar] [CrossRef] [PubMed]
- Treede, H.; Mohr, F.W.; Baldus, S.; Rastan, A.; Ensminger, S.; Arnolde, M.; Kempfert, J.; Figulla, H.R. Transapical transcatheter aortic valve implantation using the JenaValveTM system: Acute and 30-day results of the multicentre CE-mark study. Eur. J. Cardiothorac. Surg. 2012, 41, B4556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hensey, M.; Murdoch, D.J.; Sathananthan, J.; Alenezi, A.; Sathananthan, G.; Moss, R.; Blanke, P.; Leipsic, J.; Wood, D.A.; Cheung, A.; et al. First-in-human experience of a new-generation transfemoral transcatheter aortic valve for the treatment of severe aortic regurgitation: The J-Valve transfemoral system. EuroIntervention 2019, 14, E1553–E1555. [Google Scholar] [CrossRef] [PubMed]
- Huded, C.P.; Tuzcu, E.M.; Krishnaswamy, A.; Mick, S.L.; Kleiman, N.S.; Svensson, L.G.; Carroll, J.; Thourani, V.H.; Kirtane, A.J.; Manandhar, P.; et al. Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke. JAMA 2019, 321, 2306–2315. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchétché, D.; Chandrasekhar, J.; De Brito, F.S.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Predictors, incidence, and outcomes of patients undergoing transfemoral transcatheter aortic valve implantation complicated by stroke from the center-collaboration. Circ. Cardiovasc. Interv. 2019, 12, e007546. [Google Scholar] [CrossRef] [PubMed]
- Krasopoulos, G.; Falconieri, F.; Benedetto, U.; Newton, J.; Sayeed, R.; Kharbanda, R.; Banning, A. European real world trans-catheter aortic valve implantation: Systematic review and meta-analysis of European national registries. J. Cardiothorac. Surg. 2016, 11, 159. [Google Scholar] [CrossRef]
- Vranckx, P.; Windecker, S.; Welsh, R.C.; Valgimigli, M.; Mehran, R.; Dangas, G. Thrombo-embolic prevention after transcatheter aortic valve implantation. Eur. Heart J. 2017, 38, 3341–3350. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Dumont, E.; Boone, R.H.; Larose, E.; Bagur, R.; Gurvitch, R.; Bédard, F.; Doyle, D.; De Larochellière, R.; Jayasuria, C.; et al. Cerebral embolism following transcatheter aortic valve implantation: Comparison of transfemoral and transapical approaches. J. Am. Coll. Cardiol. 2011, 57, 18–28. [Google Scholar] [CrossRef]
- De Carlo, M.; Liga, R.; Migaleddu, G.; Scatturin, M.; Spaccarotella, C.; Fiorina, C.; Orlandi, G.; De Caro, F.; Rossi, M.L.; Chieffo, A.; et al. Evolution, Predictors, and Neurocognitive Effects of Silent Cerebral Embolism During Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 1291–1300. [Google Scholar] [CrossRef]
- Pagnesi, M.; Martino, E.A.; Chiarito, M.; Mangieri, A.; Jabbour, R.J.; Van Mieghem, N.M.; Kodali, S.K.; Godino, C.; Landoni, G.; Colombo, A.; et al. Silent cerebral injury after transcatheter aortic valve implantation and the preventive role of embolic protection devices: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 221, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.L.W.; Gurvitch, R.; Wijesinghe, N.; Nielispach, F.; Wood, D.; Cheung, A.; Ye, J.; Lichtenstein, S.V.; Carere, R.; Thompson, C.; et al. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2011, 4, 1290–1297. [Google Scholar] [CrossRef]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Blanke, P.; Leipsic, J.; Thourani, V.; Chakravarty, T.; Brown, D.; Trento, A.; Guyton, R.; Babaliaros, V.; Williams, M.; et al. Subclinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Valves: PARTNER 3 Cardiac Computed Tomography Substudy. J. Am. Coll. Cardiol. 2020, 75, 3003–3015. [Google Scholar] [CrossRef]
- Blanke, P.; Leipsic, J.A.; Popma, J.J.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.; Ramlawi, B.; Kleiman, N.S.; Sorajja, P.; et al. Bioprosthetic Aortic Valve Leaflet Thickening in the Evolut Low Risk Sub-Study. J. Am. Coll. Cardiol. 2020, 75, 2430–2442. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and clinical impact of prosthesis–patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Pibarot, P.; Suri, R.M.; Kodali, S.; Thourani, V.H.; Szeto, W.Y.; Svensson, L.G.; Dumont, E.; Xu, K.; Hahn, R.T.; et al. Impact of Aortic Annulus Size on Valve Hemodynamics and Clinical Outcomes After Transcatheter and Surgical Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2014, 7, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Deeb, G.M.; Chetcuti, S.J.; Yakubov, S.J.; Patel, H.J.; Grossman, P.M.; Kleiman, N.S.; Heiser, J.; Merhi, W.; Zorn, G.L.; Tadros, P.N.; et al. Impact of Annular Size on Outcomes After Surgical or Transcatheter Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 105, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Webb, J.G.; Pibarot, P.; Altwegg, L.; Dumont, E.; Thompson, C.; De Larochellière, R.; Doyle, D.; Masson, J.-B.; Bergeron, S.; et al. Comparison of the Hemodynamic Performance of Percutaneous and Surgical Bioprostheses for the Treatment of Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2009, 53, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Salaun, E.; Dahou, A.; Avenatti, E.; Guzzetti, E.; Annabi, M.S.; Toubal, O.; Bernier, M.; Beaudoin, J.; Ong, G.; et al. Echocardiographic Results of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients: The PARTNER 3 Trial. Circulation 2020, 141, 1527–1537. [Google Scholar] [CrossRef]
- Sponga, S.; Perron, J.; Dagenais, F.; Mohammadi, S.; Baillot, R.; Doyle, D.; Nalli, C.; Voisine, P. Impact of residual regurgitation after aortic valve replacement. Eur. J. Cardiothorac. Surg. 2012, 42, 486–492. [Google Scholar] [CrossRef]
- Kodali, S.; Pibarot, P.; Douglas, P.S.; Williams, M.; Xu, K.; Thourani, V.; Rihal, C.S.; Zajarias, A.; Doshi, D.; Davidson, M.; et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: Characterizing patients and impact on outcomes. Eur. Heart J. 2015, 36, 449–456. [Google Scholar] [CrossRef]
- Van Belle, E.; Rauch, A.; Vincent, F.; Robin, E.; Kibler, M.; Labreuche, J.; Jeanpierre, E.; Levade, M.; Hurt, C.; Rousse, N.; et al. Von willebrand factor multimers during transcatheter aortic-valve replacement. N. Engl. J. Med. 2016, 375, 335–344. [Google Scholar] [CrossRef]
- Rheude, T.; Pellegrini, C.; Lutz, J.; Alvarez-Covarrubias, H.A.; Lahmann, A.L.; Mayr, N.P.; Michel, J.; Kasel, M.A.; Joner, M.; Xhepa, E. Transcatheter Aortic Valve Replacement with Balloon-Expandable Valves: Comparison of SAPIEN 3 Ultra Versus SAPIEN 3. JACC Cardiovasc. Interv. 2020, 13, 2631–2638. [Google Scholar] [CrossRef]
- Choudhury, T.; Solomonica, A.; Bagur, R. The Evolut R and Evolut PRO transcatheter aortic valve systems. Expert Rev. Med. Devices 2019, 16, 3–9. [Google Scholar] [CrossRef]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Tanaka, A.; Finkelstein, A.; Mack, M.; Tamburino, C.; Van Mieghem, N.; de Backer, O.; Testa, L.; Gatto, P.; Purita, P.; et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Fassa, A.A.; Himbert, D.; Vahanian, A. Mechanisms and management of TAVR-related complications. Nat. Rev. Cardiol. 2013, 10, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chiarito, M.; Pagnotta, P.; Reimers, B.; Stefanini, G.G. Coronary revascularisation in transcatheter aortic valve implantation candidates: Why, who, when? Interv. Cardiol. Rev. 2018, 13, 69–76. [Google Scholar] [CrossRef][Green Version]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.L.; Kini, A. Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.W.; Xiang, K.; Matsouaka, R.; Li, Z.; Vemulapalli, S.; Vora, A.N.; Fanaroff, A.; Harrison, J.K.; Thourani, V.H.; Holmes, D.; et al. Incidence, Temporal Trends, and Associated Outcomes of Vascular and Bleeding Complications in Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Insights from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. Circ. Cardiovasc. Interv. 2020, 13, e008227. [Google Scholar]
- Généreux, P.; Webb, J.G.; Svensson, L.G.; Kodali, S.K.; Satler, L.F.; Fearon, W.F.; Davidson, C.J.; Eisenhauer, A.C.; Makkar, R.R.; Bergman, G.W.; et al. Vascular Complications After Transcatheter Aortic Valve Replacement: Insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial. J. Am. Coll. Cardiol. 2012, 60, 1043–1052. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntané-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jørgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef]
- Van Rosendael, P.J.; Delgado, V.; Bax, J.J. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: A systematic review. Eur. Heart J. 2018, 39, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ou, A.; Xia, P.; Tian, J.; Wang, H.; Cheng, Z. Predictors for the risk of permanent pacemaker implantation after transcatheter aortic valve replacement: A systematic review and meta-analysis. J. Card. Surg. 2022, 37, 377–405. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Zahid, S.; Zaidi, S.R.; Sarvepalli, D.; Haq, S.; Roomi, S.; Mukhtar, M.; Khan, M.A.; Gowda, S.N.; Ruggiero, N.; et al. Predictors of Permanent Pacemaker Implantation in Patients Undergoing Transcatheter Aortic Valve Replacement—A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, 129–140. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: 1-Year Results from the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, L.; Ihlemann, N.; Capodanno, D.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Chang, Y.; Steinbrüchel, D.A.; Olsen, P.S.; Petronio, A.S.; et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J. Am. Coll. Cardiol. 2019, 73, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.M.; Jüni, P.; Pilgrim, T.; Stortecky, S.; Büllesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.; Kim, W.K.; Walther, T.; Burgdorf, C.; Möllmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Tamburino, C.; Bleiziffer, S.; Thiele, H.; Scholtz, S.; Hildick-Smith, D.; Cunnington, M.; Wolf, A.; Barbanti, M.; Tchetchè, D.; Garot, P.; et al. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients with Symptomatic Severe Aortic Stenosis: SCOPE 2 Randomized Clinical Trial. Circulation 2020, 142, 2431–2442. [Google Scholar] [CrossRef]
- Buono, A.; Gorla, R.; Ielasi, A.; Costa, G.; Cozzi, O.; Ancona, M.; Soriano, F.; De Carlo, M.; Ferrara, E.; Giannini, F.; et al. Transcatheter Aortic Valve Replacement with Self-Expanding ACURATE neo2: Postprocedural Hemodynamic and Short-Term Clinical Outcomes. JACC Cardiovasc. Interv. 2022, 15, 1101–1110. [Google Scholar] [CrossRef]
- Nai Fovino, L.; Cipriani, A.; Fabris, T.; Massussi, M.; Scotti, A.; Lorenzoni, G.; Guerra, M.C.; Cardaioli, F.; Rodinò, G.; Matsuda, Y.; et al. Anatomical Predictors of Pacemaker Dependency after Transcatheter Aortic Valve Replacement. Circ. Arrhythmia Electrophysiol. 2021, 14, 86–98. [Google Scholar] [CrossRef]
- Meduri, C.U.; Kereiakes, D.J.; Rajagopal, V.; Makkar, R.R.; O’Hair, D.; Linke, A.; Waksman, R.; Babliaros, V.; Stoler, R.C.; Mishkel, G.J.; et al. Pacemaker Implantation and Dependency After Transcatheter Aortic Valve Replacement in the REPRISE III Trial. J. Am. Heart Assoc. 2019, 8, e012594. [Google Scholar] [CrossRef]
- Barbanti, M.; Yang, T.H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Hooda, A.; Chuang, M.Y.; Zaid, S.; Ahmad, H.A.; Goldberg, J.B.; Sharma, S.K.; Kini, A.S.; Webb, J.G.; Sathananthan, J. Outcomes of SAPIEN 3 Ultra TAVR in Extremely Undersized Versus Equivalent Nominally Sized Anatomies. JACC Cardiovasc. Interv. 2022, 15, 463–466. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Tirado-Conte, G.; Dagenais, F.; Ruel, M.; Al-Atassi, T.; Dumont, E.; Mohammadi, S.; Bernier, M.; Pibarot, P.; Rodés-Cabau, J. Aortic Stenosis and Small Aortic Annulus. Circulation 2019, 139, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Regazzoli, D.; Chiarito, M.; Cannata, F.; Pagnesi, M.; Miura, M.; Ziviello, F.; Picci, A.; Reifart, J.; De Marco, F.; Bedogni, F.; et al. Transcatheter Self-Expandable Valve Implantation for Aortic Stenosis in Small Aortic Annuli: The TAVI-SMALL Registry. JACC Cardiovasc. Interv. 2020, 13, 196–206. [Google Scholar] [CrossRef]
- Abdelghani, M.; Mankerious, N.; Allali, A.; Landt, M.; Kaur, J.; Sulimov, D.S.; Merten, C.; Sachse, S.; Mehilli, J.; Neumann, F.-J.; et al. Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement with Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights from the CHOICE Trial and the CHOICE-Extend Registry. JACC Cardiovasc. Interv. 2018, 11, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Armijo, G.; Tang, G.H.L.; Kooistra, N.; Ferreira-Neto, A.N.; Toggweiler, S.; Amat-Santos, I.J.; Keller, L.S.; Urena, M.; Ahmad, H.; Tafur Soto, J.; et al. Third-generation balloon and self-expandable valves for aortic stenosis in large and extra-large aortic annuli from the TAVR-LARGE registry. Circ. Cardiovasc. Interv. 2020, 13, e009047. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Fujita, T.; Fukushima, S.; Shimahara, Y.; Kume, Y.; Matsumoto, Y.; Kawamoto, N.; Minami, K.; Kabata, D.; Kanzaki, H.; et al. Transcatheter Aortic Valve Replacement for Severe Aortic Stenosis Complicated by Sigmoid Septum. Circ. J. 2018, 82, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Giannini, F.; Montorfano, M.; Romano, V.; Ruparelia, N.; Jabbour, R.J.; Benincasa, S.; Latib, A.; Colombo, A. Valve embolization with a second-generation fully-retrievable and repositionable transcatheter aortic valve. Int. J. Cardiol. 2016, 223, 867–869. [Google Scholar] [CrossRef]
- Khan, A.A.; Tang, G.H.L.; Engstrom, K.; Khan, M.; Patel, N.; Dangas, G.D.; Sharma, S.K.; Kini, A. Aortic Stenosis with Severe Asymmetric Septal Hypertrophy: A Novel Management Strategy to Improve TAVR Outcomes. JACC Cardiovasc. Interv. 2019, 12, 2228–2230. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Tuzcu, E.M.; Svensson, L.G.; Kapadia, S.R. Combined transcatheter aortic valve replacement and emergent alcohol septal ablation. Circulation 2013, 128, e366–e368. [Google Scholar] [CrossRef]
- Alperi Garcia, A.; Muntané-Carol, G.; Junquera, L.; del Val, D.; Faroux, L.; Philippon, F.; Rodés-Cabau, J. Can we reduce conduction disturbances following transcatheter aortic valve replacement? Expert Rev. Med. Devices 2020, 17, 309–322. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, H.H.; Liao, T.W.; Leu, H.B.; Chen, I.M.; Chen, P.L.; Lin, S.M. Membranous septum length predicts conduction disturbances following transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2020, 164, 42–51.e2. [Google Scholar] [CrossRef]
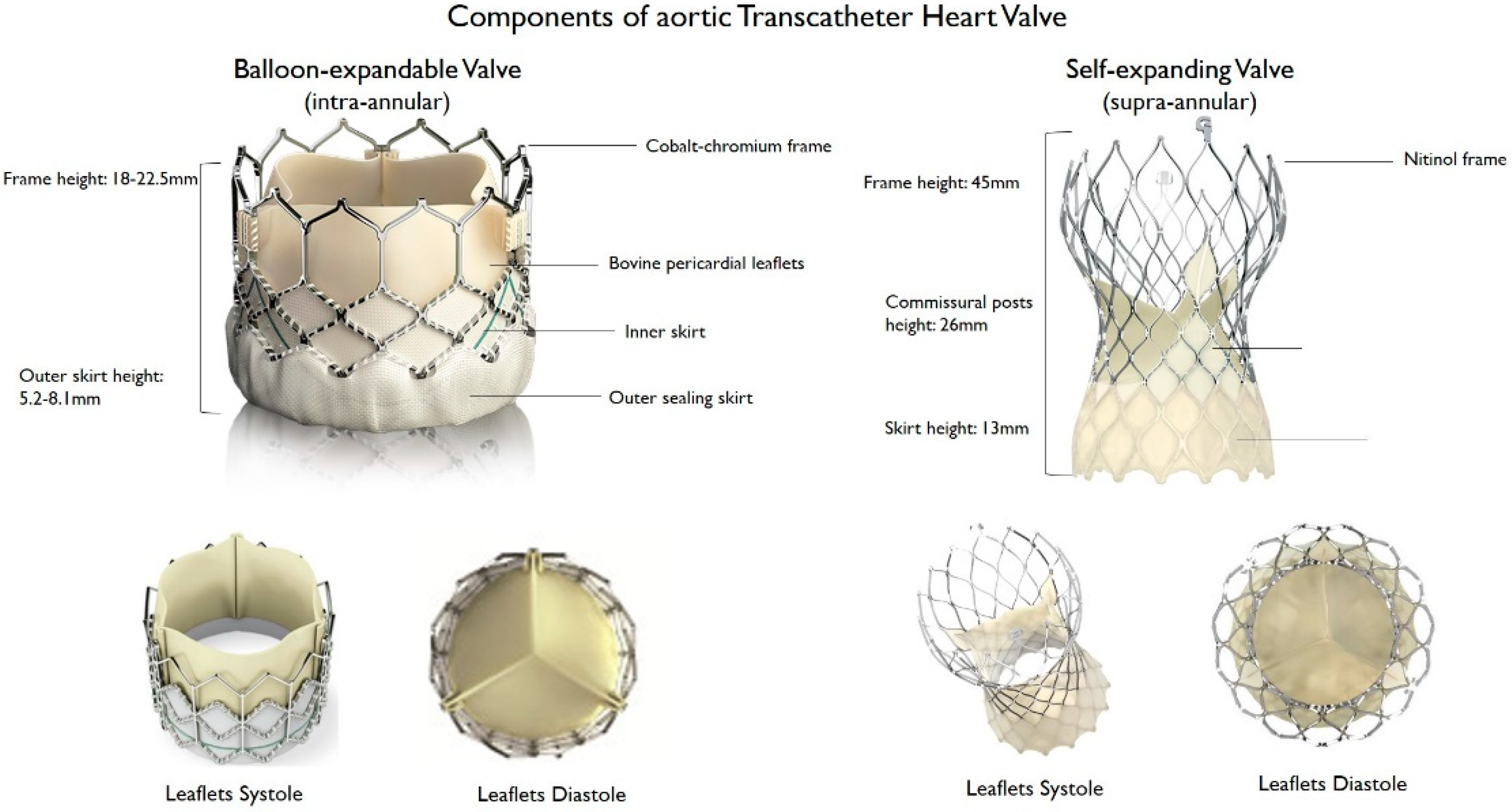
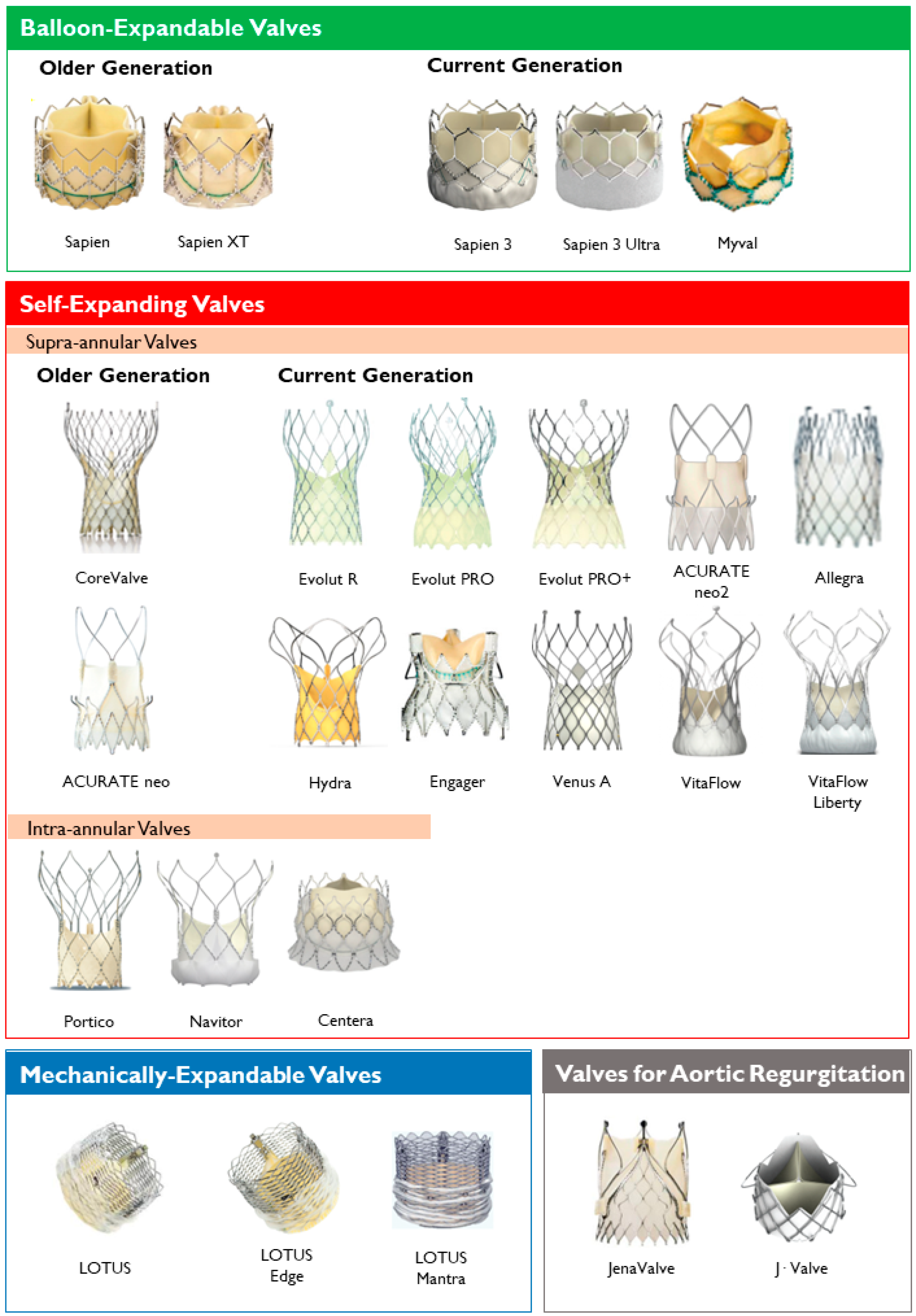
| Prosthesis | Frame Material | Leaflet Material | Valve Sizes (mm) | Sheath Sizes | Supra- or Intra- Annular | Repositionable/Retrievable | Delivery Routes | FDA Approval | CE Mark Approval |
|---|---|---|---|---|---|---|---|---|---|
| Balloon-expandable | |||||||||
| Sapien | Stainless steel | Bovine pericardium | 23, 26 | 22F (23 mm), 24F (26 mm) | Intra-annular | No/No | TF, TA | ✓ | ✓ |
| Sapien XT | Cobalt-chromium | Bovine pericardium | 23, 26, 29 | 16F (23 mm), 18F (26 mm), 20F (29 mm) | Intra-annular | No/No | TF, TA, TAo | ✓ | ✓ |
| Sapien 3 | Cobalt-chromium | Bovine pericardium | 20, 23, 26, 29 | 14F (20, 23, 26 mm), 16F (29 mm) | Intra-annular | No/No | TF, TA, TAo | ✓ | ✓ |
| Sapien 3 Ultra | Cobalt-chromium | Bovine pericardium | 20, 23, 26, 29 | 14F | Intra-annular | No/No | TF | ✓ | ✓ |
| Myval THV | Nickel-cobalt | Bovine pericardium | 20, 23, 26, 29, 21.5, 24.5, 27.5, 30.5, 32 | 14F | Intra-annular | No/No | TF | ✓ | |
| Self-expanding | |||||||||
| CoreValve | Nitinol | Porcine pericardium | 23, 26, 29, 31 | 18F | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut R | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 14F (23, 26, 29 mm), 16F (34 mm) | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut PRO | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 16F | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| Evolut PRO+ | Nitinol | Porcine pericardium | 23, 26, 29, 34 | 14F (23, 26, 29 mm), 16F (34 mm) | Supra-annular | Yes/Yes | TF, TAo, SC | ✓ | ✓ |
| ACURATE neo | Nitinol | Porcine pericardium | 23, 25, 27 | 18F | Supra-annular | No/No | TF, TA | ✓ | |
| ACURATE neo2 | Nitinol | Porcine pericardium | 23, 25, 27 | 14F | Supra-annular | No/No | TF, TA | ✓ | |
| Allegra | Nitinol | Bovine pericardium | 23, 27, 31 | 18F | Supra-annular | Yes/Yes | TF | ||
| Hydra | Nitinol | Bovine pericardium | 22, 26, 30 | 18F | Supra-annular | Yes/Yes | TF | ✓ | |
| Engager | Nitinol | Bovine pericardium | 23, 26 | 30F | Supra-annular | Yes/Yes | TA | ✓ | |
| Venus-A valve | Nitinol | Porcine pericardium | 23, 26, 29, 32 | Supra-annular | Yes/No | TF | |||
| VitaFlow | Nitinol | Bovine pericardium | 21, 24, 27, 30 | 16F (21, 24 mm), 18F (27, 30 mm) | Supra-annular | Yes/No | TF, TAo, CA | ||
| VitaFlow Liberty | Nitinol | Bovine pericardium | 21, 24, 27, 30 | 16F (21, 24 mm), 18F (27, 30 mm) | Supra-annular | Yes/No | TF, TAo, CA | ||
| Centera | Nitinol | Bovine pericardium | 23, 26 29 | 14F | Intra-annular | Yes/Yes | TF | ✓ | |
| Portico | Nitinol | Bovine pericardium | 23, 25, 27, 29 | 18F (23, 25 mm), 19F (27, 29 mm) | Intra-annular | Yes/Yes | TF, TAo, TAx, SC | ✓ | |
| Navitor | Nitinol | Bovine pericardium | 23, 25, 27, 29 | 14F (23, 25 mm), 15F (27, 29 mm) | Intra-annular | Yes/Yes | TF, TAo, TAx | ✓ | |
| Mechanically expandable | |||||||||
| Lotus | Nitinol | Bovine pericardium | 23, 25, 27 | 20F (23, 25 mm), 22F (27 mm) | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Lotus Edge | Nitinol | Bovine pericardium | 23, 25, 27 | 15F | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Lotus Mantra | Nitinol | Bovine pericardium | 23, 25, 27 | 12F | Intra-annular | Yes/Yes | TF, TAo | ✓ | ✓ |
| Aortic regurgitation | |||||||||
| JenaValve | Nitinol | Porcine pericardium | 23, 25, 27 | 19F | Intra-annular | Yes/Yes | TA | ✓ | |
| J·Valve | Nitinol | Bovine pericardium | 22, 25, 28 | 18F | Intra-annular | No/No | TA | ✓ |
| Event | TAVR | SAVR | Follow-Up |
|---|---|---|---|
| Stroke | 0.6–6.7% | 2.4–6.1% | 30 Days |
| 4.1–10.6% | 4.3–8.7% | 1 Year | |
| Subclinical Leaflet Thrombosis | 13% | 5% | 30 Days |
| 28% | 20% | 1 Year | |
| Coronary Obstruction | 0.2–1.7% | 0–0.6% | |
| Severe Prosthesis-Patient Mismatch | 9.3–12% | 27.8% | |
| Clinically Significant Paravalvular Leak | 0.5–13.6% | – | |
| Vascular Complications | 3.8–30.7% | 1.1–11.3% | 30 Days |
| Conduction Disturbances (PPI) | 3.4–34.1% | 1.6–7.1% | 30 Days |
| Balloon-Expandable | Self-Expanding | |
|---|---|---|
| Clinical factors | ||
| Greater life expectancy | ✓ | |
| Heart failure | ✓ | |
| Chronic kidney disease | ✓ | |
| Pre-existing or risk # for conduction disturbances | ✓ | |
| Anatomic features | ||
| Small annulus | ✓ | ✓ (supra-annular) |
| Large annulus | ✓ | |
| Dense annular calcification | ✓ | |
| Need for coronary access | ✓ | |
| Horizontal aorta * | ✓ | |
| Valve-in-Valve | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarito, M.; Spirito, A.; Nicolas, J.; Selberg, A.; Stefanini, G.; Colombo, A.; Reimers, B.; Kini, A.; Sharma, S.K.; Dangas, G.D.; et al. Evolving Devices and Material in Transcatheter Aortic Valve Replacement: What to Use and for Whom. J. Clin. Med. 2022, 11, 4445. https://doi.org/10.3390/jcm11154445
Chiarito M, Spirito A, Nicolas J, Selberg A, Stefanini G, Colombo A, Reimers B, Kini A, Sharma SK, Dangas GD, et al. Evolving Devices and Material in Transcatheter Aortic Valve Replacement: What to Use and for Whom. Journal of Clinical Medicine. 2022; 11(15):4445. https://doi.org/10.3390/jcm11154445
Chicago/Turabian StyleChiarito, Mauro, Alessandro Spirito, Johny Nicolas, Alexandra Selberg, Giulio Stefanini, Antonio Colombo, Bernhard Reimers, Annapoorna Kini, Samin K. Sharma, George D. Dangas, and et al. 2022. "Evolving Devices and Material in Transcatheter Aortic Valve Replacement: What to Use and for Whom" Journal of Clinical Medicine 11, no. 15: 4445. https://doi.org/10.3390/jcm11154445
APA StyleChiarito, M., Spirito, A., Nicolas, J., Selberg, A., Stefanini, G., Colombo, A., Reimers, B., Kini, A., Sharma, S. K., Dangas, G. D., & Mehran, R. (2022). Evolving Devices and Material in Transcatheter Aortic Valve Replacement: What to Use and for Whom. Journal of Clinical Medicine, 11(15), 4445. https://doi.org/10.3390/jcm11154445






