Evolving Indications of Transcatheter Aortic Valve Replacement—Where Are We Now, and Where Are We Going
Abstract
:1. Introduction
2. Current Recommendations
3. Where Are We Going?
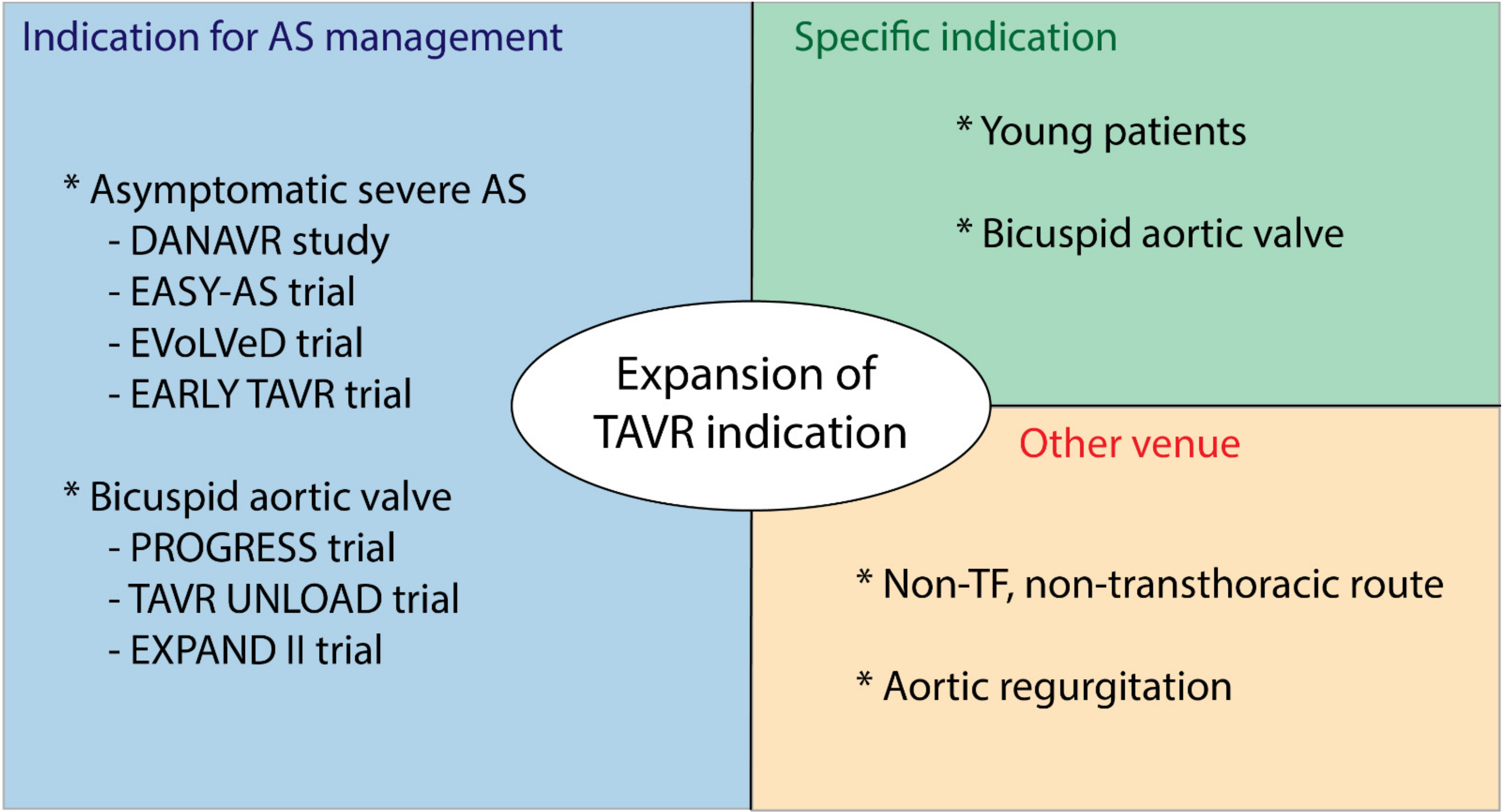
3.1. Expanding the Indications of Interventional Management of AS
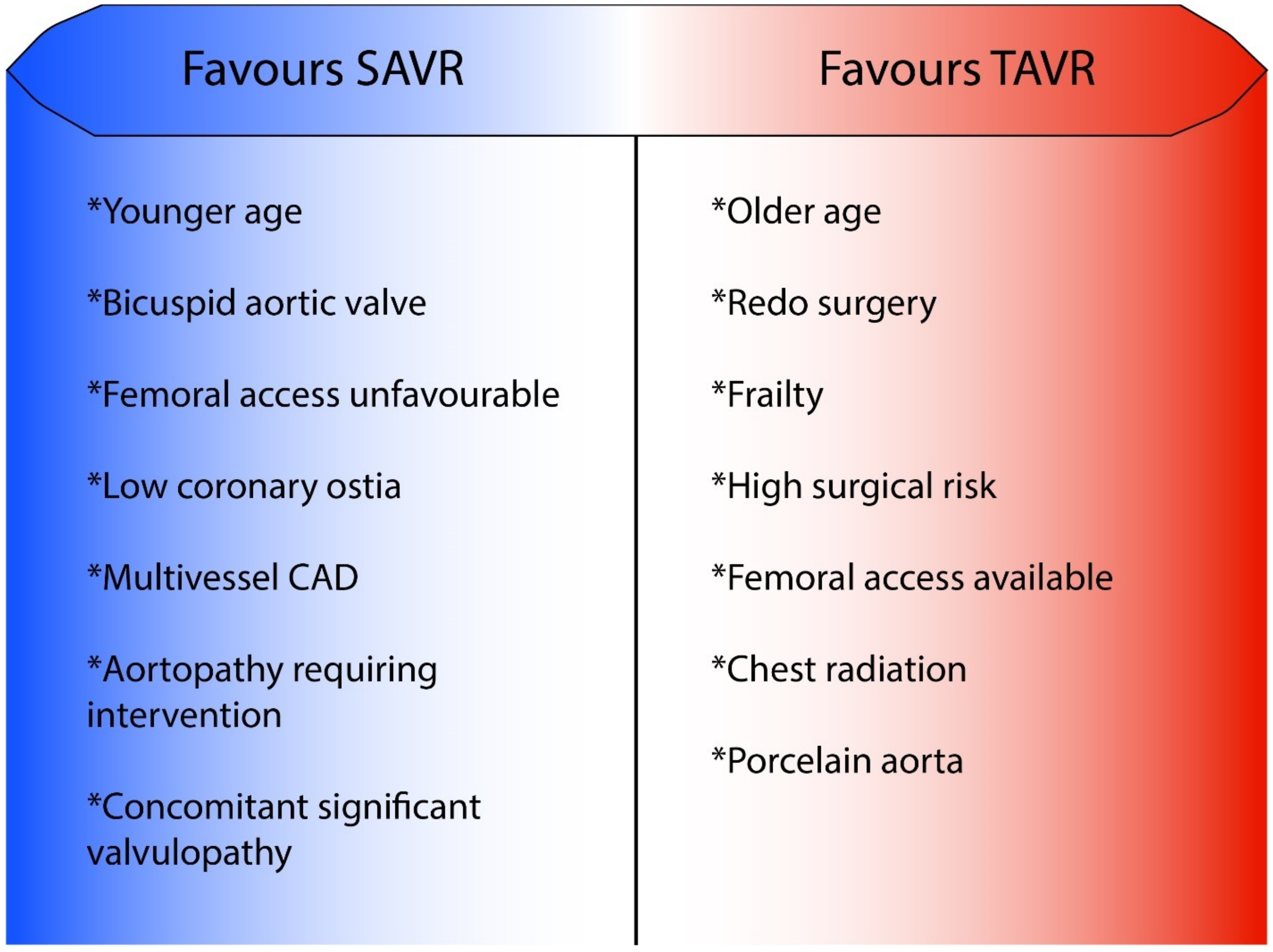
3.2. Choosing TAVR over SAVR When Aortic Valve Replacement with a Bioprosthesis Is Indicated
3.3. Other Venues to Expand TAVR Indication
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| AS | aortic stenosis |
| BAV | bicuspid aortic valve |
| LVEF | left ventricular ejection fraction |
| SAVR | surgical aortic valve replacement |
| TAVR | transcatheter aortic valve replacement |
References
- Cribier, A.; Eltchaninoff, H.; Tron, C.; Bauer, F.; Agatiello, C.; Sebagh, L.; Bash, A.; Nusimovici, D.; Litzler, P.Y.; Bessou, J.-P.; et al. Early experience with percutaneous transcatheter implantation of heart valve pros-thesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J. Am. Coll. Cardiol. 2004, 43, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Bowdish, M.E.; D’Agostino, R.S.; Thourani, V.H.; Schwann, T.A.; Krohn, C.; Desai, N.; Shahian, D.M.; Fernandez, F.G.; Badhwar, V. STS Adult Cardiac Surgery Database: 2021 Update on Outcomes, Quality, and Research. Ann. Thorac. Surg. 2021, 111, 1770–1780. [Google Scholar] [CrossRef]
- Thourani, V.H.; Yadav, P.K.; Prendergast, B. TAVR Sustains Its Promise in Low-Risk Patients, But the Journey Is Far from Over. J. Am. Coll. Cardiol. 2022, 79, 897–899. [Google Scholar] [CrossRef]
- Sá, M.P.B.; Simonato, M.; Eynde, J.V.D.; Cavalcanti, L.R.P.; Roever, L.; Bisleri, G.; Dokollari, A.; Dvir, D.; Zhigalov, K.; Ruhparwar, A.; et al. Asymptomatic severe aortic stenosis, bicuspid aortic valves and moderate aortic stenosis in heart failure: New indications for transcatheter aortic valve implantation. Trends Cardiovasc. Med. 2021, 31, 435–445. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. College Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- Maréchaux, S.; Hachicha, Z.; Bellouin, A.; Dumesnil, J.G.; Meimoun, P.; Pasquet, A.; Bergeron, S.; Arsenault, M.; Le Tourneau, T.; Ennezat, P.V.; et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur. Heart J. 2010, 31, 1390–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garry, J.D.; Goldman, M.; Kohlwes, J.; Sidebotham, D.; Morrow, C.D.; Drake, D.H.; Kang, D.-H.; Park, S.-W. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 383, 91–93. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; et al. B-type natriuretic peptide in patients with asymptomatic severe aortic stenosis. Heart 2018, 105, 384–390. [Google Scholar] [CrossRef]
- Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; Miyake, M.; et al. Initial Surgical Versus Conservative Strategies in Patients with Asymptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2015, 66, 2827–2838. [Google Scholar] [CrossRef] [Green Version]
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; De Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EU-RObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef]
- Pai, R.G.; Kapoor, N.; Bansal, R.C.; Varadarajan, P. Malignant Natural History of Asymptomatic Severe Aortic Stenosis: Benefit of Aortic Valve Replacement. Ann. Thorac. Surg. 2006, 82, 2116–2122. [Google Scholar] [CrossRef]
- Redfors, B.; Pibarot, P.; Gillam, L.D.; Burkhoff, D.; Bax, J.J.; Lindman, B.; Bonow, R.O.; O’Gara, P.T.; Leon, M.B.; Généreux, P. Stress Testing in Asymptomatic Aortic Stenosis. Circulation 2017, 135, 1956–1976. [Google Scholar] [CrossRef]
- Pierard, L.A.; Dulgheru, R. Exercise Testing and Stress Imaging in Aortic Valve Disease. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 54. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 Adults with Asymptomatic, Hemodynamically Significant Aortic Stenosis During Prolonged Follow-Up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef] [Green Version]
- Généreux, P.; Stone, G.W.; O’Gara, P.T.; Marquis-Gravel, G.; Redfors, B.; Giustino, G.; Pibarot, P.; Bax, J.J.; Bonow, R.O.; Leon, M.B. Natural History, Diagnostic Approaches, and Therapeutic Strategies for Patients with Asymptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2016, 67, 2263–2288. [Google Scholar] [CrossRef]
- Banovic, M.; Putnik, S.; Penicka, M.; Doros, G.; Deja, M.A.; Kockova, R.; Kotrc, M.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: The AVATAR Trial. Circulation 2022, 145, 648–658. [Google Scholar] [CrossRef]
- Bing, R.; Everett, R.J.; Tuck, C.; Semple, S.; Lewis, S.; Harkess, R.; Mills, N.; Treibel, T.; Prasad, S.; Greenwood, J.P.; et al. Rationale and design of the randomized, controlled Early Valve Replacement Guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis (EVOLVED) trial. Am. Heart J. 2019, 212, 91–100. [Google Scholar] [CrossRef]
- Genereux, P. Rationale and Status Update of the EARLY TAVR Trial Asymptomatic Severe AS Patients. In Proceedings of the TCT 2017, Denver, CO, USA, 16 June 2017. [Google Scholar]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Van Gils, L.; Clavel, M.-A.; Vollema, E.M.; Hahn, R.T.; Spitzer, E.; Delgado, V.; Nazif, T.; De Jaegere, P.P.; Geleijnse, M.L.; Ben-Yehuda, O.; et al. Prognostic implications of moderate aortic stenosis in patients with left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2383–2392. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D. Poor Long-Term Survival in Patients with Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef]
- Samad, Z.; Vora, A.N.; Dunning, A.; Schulte, P.J.; Shaw, L.K.; Al-Enezi, F.; Ersboll, M.; McGarrah, R.W.; Vavalle, J.P.; Shah, S.H.; et al. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur. Heart J. 2016, 37, 2276–2286. [Google Scholar] [CrossRef] [Green Version]
- Jean, G.; Van Mieghem, N.M.; Gegenava, T.; van Gils, L.; Bernard, J.; Geleijnse, M.L.; Vollema, E.M.; El Azzouzi, I.; Spitzer, E.; Delgado, V.; et al. Moderate Aortic Stenosis in Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 2796–2803. [Google Scholar] [CrossRef]
- Spitzer, E.; Van Mieghem, N.M.; Pibarot, P.; Hahn, R.T.; Kodali, S.; Maurer, M.S.; Nazif, T.; Rodés-Cabau, J.; Paradis, J.-M.; Kappetein, A.-P.; et al. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am. Heart J. 2016, 182, 80–88. [Google Scholar] [CrossRef]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F.; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-Term Durability of Bioprosthetic Aortic Valves: Implications From 12,569 Implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Mille, C.D.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re-placement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Barbanti, M.; Petronio, A.S.; Ettori, F.; Latib, A.; Bedogni, F.; De Marco, F.; Poli, A.; Boschetti, C.; De Carlo, M.; Fiorina, C.; et al. 5-Year Outcomes After Transcatheter Aortic Valve Implantation with CoreValve Prosthesis. JACC Cardiovasc. Interv. 2015, 8, 1084–1091. [Google Scholar] [CrossRef]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef]
- Blackman, D.J.; Saraf, S.; MacCarthy, P.A.; Myat, A.; Anderson, S.G.; Malkin, C.J.; Cunnington, M.S.; Somers, K.; Brennan, P.; Manoharan, G.; et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. J. Am. Coll. Cardiol. 2019, 73, 537–545. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Van Rosendael, P.J.; Delgado, V.; Bax, J.J. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: A systematic review. Eur. Heart J. 2018, 39, 2003–2013. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntané-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jørgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef]
- Pascual, I.; Hernández-Vaquero, D.; Alperi, A.; Almendarez, M.; Avanzas, P.; Kalavrouziotis, D.; Lorca, R.; Mesnier, J.; Arboine, L.; Mohammadi, S.; et al. Permanent Pacemaker Reduction Using Cusp-Overlapping Projection in TAVR. JACC Cardiovasc. Interv. 2022, 15, 150–161. [Google Scholar] [CrossRef]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.-M.; Rodés-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices 2018, 15, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.; Kini, A. Coronary Angiography and Percutaneous Coronary Intervention after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Pellegrini, C.; Ludwig, S.; Möllmann, H.; Leuschner, F.; Makkar, R.; Leick, J.; Amat-Santos, I.J.; Dörr, O.; Breitbart, P.; et al. Feasibility of Coronary Access in Patients with Acute Coronary Syndrome and Previous TAVR. JACC Cardiovasc. Interv. 2021, 14, 1578–1590. [Google Scholar] [CrossRef]
- Faroux, L.; Lhermusier, T.; Vincent, F.; Nombela-Franco, L.; Tchétché, D.; Barbanti, M.; Abdel-Wahab, M.; Windecker, S.; Auffret, V.; Campanha-Borges, D.C.; et al. ST-Segment Elevation Myocardial Infarction Following Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 77, 2187–2199. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G.; Picci, A.; Criscione, E.; Reddavid, C.; Valvo, R.; Todaro, D.; Deste, W.; Condorelli, A.; Scalia, M.; et al. Coronary Cannulation after Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc. Interv. 2020, 13, 2542–2555. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Verardi, R.; Visconti, M.; Conrotto, F.; Scacciatella, P.; Dziewierz, A.; Stefanini, G.G.; Paradis, J.-M.; Omedè, P.; Kodali, S.; et al. Independent impact of extent of coronary artery disease and percutaneous revas-cularisation on 30-day and one-year mortality after TAVI: A meta-analysis of adjusted observational results. EuroIntervention 2018, 14, e1169–e1177. [Google Scholar] [CrossRef] [Green Version]
- Sankaramangalam, K.; Banerjee, K.; Kandregula, K.; Mohananey, D.; Parashar, A.; Jones, B.M.; Jobanputra, Y.; Mick, S.; Krishnaswamy, A.; Svensson, L.G.; et al. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006092. [Google Scholar] [CrossRef] [Green Version]
- Van den Boogert, T.P.W.; Vendrik, J.; Claessen, B.E.P.M.; Baan, J.; Beijk, M.A.; Limpens, J.; Boekholdt, S.A.M.; Hoek, R.; Planken, R.N.; Henriques, J.P. CTCA for detection of significant coronary artery disease in routine TAVI work-up: A systematic review and meta-analysis. Neth. Heart J. 2018, 26, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Mejía-Rentería, H.; Nombela-Franco, L.; Paradis, J.-M.; Lunardi, M.; Lee, J.M.; Amat-Santos, I.J.; Veiga Fernandez, G.; Kalra, A.; Bansal, E.J.; dela Tore Hernandez, J.M.; et al. Angiography-based quantitative flow ratio versus fractional flow reserve in patients with coronary artery disease and severe aortic stenosis. EuroIntervention 2020, 16, e285–e292. [Google Scholar] [CrossRef]
- Gohmann, R.F.; Pawelka, K.; Seitz, P.; Majunke, N.; Heiser, L.; Renatus, K.; Desch, S.; Lauten, P.; Holzhey, D.; Noack, T.; et al. Combined cCTA and TAVR Planning for Ruling Out Significant CAD. JACC Cardiovasc. Imaging 2021, 15, 476–486. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.-M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2017, 391, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Chaitman, B.R.; Alexander, K.P.; Cyr, D.D.; Berger, J.S.; Reynolds, H.R.; Bangalore, S.; Boden, W.E.; Lopes, R.D.; Demkow, W.; Perna, G.P.; et al. Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons. Circulation 2021, 143, 790–804. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Roberts, W.C.; Janning, K.G.; Ko, J.M.; Filardo, G.; Matter, G.J. Frequency of Congenitally Bicuspid Aortic Valves in Patients ≥80 Years of Age Undergoing Aortic Valve Replacement for Aortic Stenosis (With or Without Aortic Regurgitation) and Implications for Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2012, 109, 1632–1636. [Google Scholar] [CrossRef]
- Vincent, F.; Ternacle, J.; Denimal, T.; Shen, M.; Redfors, B.; Delhaye, C.; Simonato, M.; Debry, N.; Verdier, B.; Shahim, B.; et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis. Circulation 2021, 143, 1043–1061. [Google Scholar] [CrossRef]
- Tchetche, D.; de Biase, C.; van Gils, L.; Parma, R.; Ochala, A.; Lefevre, T.; Hovasse, T.; De Backer, O.; Sondergaard, L.; Bleiziffer, S.; et al. Bicuspid Aortic Valve Anatomy and Relationship with Devices: The BAVARD Mul-ticenter Registry: A European Picture of Contemporary Multidetector Computed Tomography Sizing for Bicuspid Valves. Circ. Cardiovasc. Interv. 2019, 12, e007107. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, W.-K.; Dhoble, A.; Pio, S.M.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Abbasi, M.; Azadani, A.N. Leaflet stress and strain distributions following incomplete transcatheter aortic valve expansion. J. Biomech. 2015, 48, 3663–3671. [Google Scholar] [CrossRef]
- Tzemos, N.; Therrien, J.; Yip, J.; Thanassoulis, G.; Tremblay, S.; Jamorski, M.T.; Webb, G.D.; Siu, S.C. Outcomes in Adults with Bicuspid Aortic Valves. JAMA 2008, 300, 1317–1325. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, N.; Ye, J.; Rodés-Cabau, J.; Cheung, A.; Velianou, J.L.; Natarajan, M.K.; Dumont, E.; Nietlispach, F.; Gurvitch, R.; Wood, D.A.; et al. Transcatheter Aortic Valve Implantation in Patients with Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2010, 3, 1122–1125. [Google Scholar] [CrossRef] [Green Version]
- Forrest, J.K.; Kaple, R.K.; Ramlawi, B.; Gleason, T.G.; Meduri, C.U.; Yakubov, S.J.; Jilaihawi, H.; Liu, F.; Reardon, M.J. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valves From the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2020, 13, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Yoon, S.-H.; Leon, M.B.; Chakravarty, T.; Rinaldi, M.; Shah, P.B.; Skipper, E.R.; Thourani, V.H.; Babaliaros, V.; Cheng, W.; et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA 2019, 321, 2193–2202. [Google Scholar] [CrossRef]
- Fan, J.; Fang, X.; Liu, C.; Zhu, G.; Hou, C.R.; Jiang, J.; Lin, X.; Wang, L.; He, Y.; Zhu, Q.; et al. Brain Injury After Transcatheter Replacement of Bicuspid Versus Tricuspid Aortic Valves. J. Am. Coll. Cardiol. 2020, 76, 2579–2590. [Google Scholar] [CrossRef]
- Kalra, A.; Raza, S.; Hussain, M.; Shorbaji, K.; Delozier, S.; Deo, S.V.; Khera, S.; Kleiman, N.S.; Reardon, M.J.; Kolte, D.; et al. Aortic Valve Replacement in Bioprosthetic Failure: Insights from The Society of Thoracic Surgeons National Database. Ann. Thorac. Surg. 2019, 110, 1637–1642. [Google Scholar] [CrossRef]
- Webb, J.G.; Mack, M.J.; White, J.M.; Dvir, D.; Blanke, P.; Herrmann, C.H.; Leipsic, J.; Kodali, S.K.; Makkar, R.; Miller, D.C.; et al. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Biopros-theses: PARTNER 2 Valve-in-Valve Registry. J. Am. Coll. Cardiol. 2017, 69, 2253–2262. [Google Scholar] [CrossRef]
- De Freitas Campos Guimarães, L.; Urena, M.; Wijeysundera, H.C.; Munoz-Garcia, A.; Serra, C.; Benitez, L.M.; Auffret, V.; Cheema, A.N.; Amat-Santos, I.J.; Fisher, Q.; et al. Long-Term Outcomes after Transcatheter Aortic Valve-in-Valve Replacement. Circ. Cardiovasc. Interv. 2018, 11, e007038. [Google Scholar] [CrossRef]
- Tuzcu, E.M.; Kapadia, S.R.; Vemulapalli, S.; Carroll, J.D.; Holmes, D.R.; Mack, M.J.; Thourani, V.H.; Grover, F.L.; Brennan, J.M.; Suri, R.M.; et al. Transcatheter Aortic Valve Replacement of Failed Surgically Implanted Bio-prostheses: The STS/ACC Registry. J. Am. Coll. Cardiol. 2018, 72, 370–382. [Google Scholar] [CrossRef]
- Sá, M.P.B.O.; Van den Eynde, J.; Simonato, M.; Cavalcanti, L.R.P.; Doulamis, I.P.; Weixler, V.; Kampaktsis, P.N.; Gallo, M.; Laforgia, P.L.; Zhigalov, K.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement: An Updated Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 211–220. [Google Scholar] [CrossRef]
- Bleiziffer, S.; Simonato, M.; Webb, J.G.; Rodés-Cabau, J.; Pibarot, P.; Kornowski, R.; Windecker, S.; Erlebach, M.; Duncan, A.; Seiffert, M.; et al. Long-term outcomes after transcatheter aortic valve implantation in failed biopros-thetic valves. Eur. Heart J. 2020, 41, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Park, J.K.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2017, 39, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Transcatheter Aortic Valve Implantation in Failed Bioprosthetic Surgical Valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Saxon, J.T.; Allen, K.B.; Cohen, D.J.; Chhatriwalla, A.K. Bioprosthetic Valve Fracture during Valve-in-valve TAVR: Bench to Bedside. Interv. Cardiol. Rev. Res. Resour. 2017, 13, 20–26. [Google Scholar] [CrossRef]
- Allen, K.B.; Chhatriwalla, A.K.; Saxon, J.T.; Sathananthan, J.; Dvir, D.; Webb, J.G. Bioprosthetic valve fracture to facilitate valve-in-valve transcatheter aortic valve repair. Ann. Cardiothorac. Surg. 2020, 9, 528–530. [Google Scholar] [CrossRef]
- Salem, S.A.; Foerst, J.R. Valve-in-Valve Transcatheter Aortic Valve Replacement, with Present-Day Innovations and Up-to-Date Techniques. Interv. Cardiol. Clin. 2021, 10, 491–504. [Google Scholar] [CrossRef]
- Khan, J.M.; Dvir, D.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Aldea, G.; Reisman, M.; Mackensen, G.B.; Eng, M.H.K.; Paone, G.; et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc. Interv. 2018, 11, 677–689. [Google Scholar] [CrossRef]
- Khan, J.M.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Eng, M.H.; Paone, G.; Leshnower, B.G.; Reisman, M.; Satler, L.; Waksman, R.; et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc. Interv. 2019, 12, 1240–1252. [Google Scholar] [CrossRef]
- De Backer, O.; Landes, U.; Fuchs, A.; Yoon, S.-H.; Mathiassen, O.N.; Sedaghat, A.; Kim, W.-K.; Pilgrim, T.; Buzzatti, N.; Ruile, P.; et al. Coronary Access After TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2528–2538. [Google Scholar] [CrossRef]
- Landes, U.; Webb, J.G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Crusius, L.; Kim, W.-K.; Hamm, C.; Buzzatti, N.; Montorfano, M.; et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dys-function. J. Am. Coll. Cardiol. 2020, 75, 1882–1893. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Kamioka, N.; Vavalle, J.P.; Lisko, J.C.; Gleason, P.T.; Paone, G.; Grubb, K.J.; Bruce, C.G.; Lederman, R.J.; Babaliaros, V.C. Balloon-Assisted BASILICA to Facilitate Redo TAVR. JACC Cardiovasc. Interv. 2021, 14, 578–580. [Google Scholar] [CrossRef]
- Dallan, L.A.P.; Forrest, J.K.; Reardon, M.J.; Szeto, W.Y.; George, I.; Kodali, S.; Kleiman, N.S.; Yakubov, S.J.; Grubb, K.J.; Liu, F.; et al. Transcatheter Aortic Valve Replacement with Self-Expandable Supra-Annular Valves for Degenerated Surgical Bioprostheses: Insights from Transcatheter Valve Therapy Registry. J. Am. Heart Assoc. 2021, 10, e021871. [Google Scholar] [CrossRef]
- Auffret, V.; Lefevre, T.; VAN Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef]
- Grover, F.L.; Vemulapalli, S.; Carroll, J.D.; Edwards, F.H.; Mack, M.J.; Thourani, V.H.; Brindis, R.G.; Shahian, D.M.; Ruiz, C.E.; Jacobs, J.P.; et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J. Am. Coll. Cardiol. 2016, 69, 1215–1230. [Google Scholar] [CrossRef]
- Dahle, T.G.; Kaneko, T.; McCabe, J.M. Outcomes Following Subclavian and Axillary Artery Access for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 662–669. [Google Scholar] [CrossRef]
- Chamandi, C.; Akar, R.A.; Rodés-Cabau, J.; Blanchard, D.; Dumont, E.; Spaulding, C.; Doyle, D.; Pagny, J.-Y.; DeLarochellière, R.; Lafont, A.; et al. Transcarotid Compared with Other Alternative Access Routes for Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2018, 11, e006388. [Google Scholar] [CrossRef]
- Blackstone, E.H.; Suri, R.M.; Rajeswaran, J.; Babaliaros, V.; Douglas, P.S.; Fearon, W.F.; Miller, D.C.; Hahn, R.T.; Kapadia, S.; Kirtane, A.J.; et al. Propensity-Matched Comparisons of Clinical Outcomes After Transapical or Transfemoral Transcatheter Aortic Valve Replacement: A Placement of Aortic Transcatheter Valves (PARTNER)-I Trial Substudy. Circulation 2015, 131, 1989–2000. [Google Scholar] [CrossRef]
- Beurtheret, S.; Karam, N.; Resseguier, N.; Houel, R.; Modine, T.; Folliguet, T.; Chamandi, C.; Com, O.; Gelisse, R.; Bille, J.; et al. Femoral Versus Nonfemoral Peripheral Access for Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2728–2739. [Google Scholar] [CrossRef]
- Overtchouk, P.; Folliguet, T.; Pinaud, F.; Fouquet, O.; Pernot, M.; Bonnet, G.; Hubert, M.; Lapeze, J.; Claudel, J.P.; Ghostine, S.; et al. Transcarotid Approach for Transcatheter Aortic Valve Replacement with the Sapien 3 Prosthesis. JACC Cardiovasc. Interv. 2019, 12, 413–419. [Google Scholar] [CrossRef]
- Gleason, T.G.; Schindler, J.T.; Hagberg, R.C.; Deeb, M.; Adams, D.H.; Conte, J.V.; Zorn, G.L.; Hughes, G.C.; Guo, J.; Popma, J.J.; et al. Subclavian/Axillary Access for Self-Expanding Transcatheter Aortic Valve Re-placement Renders Equivalent Outcomes as Transfemoral. Ann. Thorac. Surg. 2018, 105, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Franzone, A.; Piccolo, R.; Siontis, G.C.; Lanz, J.; Stortecky, S.; Praz, F.; Roost, E.; Vollenbroich, R.; Windecker, S.; Pilgrim, T. Transcatheter Aortic Valve Replacement for the Treatment of Pure Native Aortic Valve Regurgitation. JACC Cardiovasc. Interv. 2016, 9, 2308–2317. [Google Scholar] [CrossRef] [PubMed]
- Poschner, T.; Werner, P.; Kocher, A.; Laufer, G.; Musumeci, F.; Andreas, M.; Russo, M. The JenaValve pericardial transcatheter aortic valve replacement system to treat aortic valve disease. Future Cardiol. 2022, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Gogia, S.; Vahl, T.; Khalique, O.; Hamid, N.; Borden, S.; Chung, C.; Ng, V.; Patel, A.; George, I.; Hahn, R.; et al. TCT CONNECT-92 Initial Single-Center Experience with Transfemoral Transcatheter Aortic Valve Replacement in Patients with Symptomatic Severe Aortic Regurgitation. J. Am. Coll. Cardiol. 2020, 76, B41. [Google Scholar] [CrossRef]
- Hensey, M.; Murdoch, D.J.; Sathananthan, J.; Alenezi, A.; Sathananthan, G.; Moss, R.; Blanke, P.; Leipsic, J.; Wood, D.A.; Cheung, A.; et al. First-in-human experience of a new-generation transfemoral transcatheter aortic valve for the treatment of severe aortic regurgitation: The J-Valve transfemoral system. EuroIntervention 2019, 14, e1553–e1555. [Google Scholar] [CrossRef]
- Barbanti, M.; Ye, J.; Pasupati, S.; El-Gamel, A.; Webb, J.G. The Helio transcatheter aortic dock for patients with aortic regurgitation. EuroIntervention 2013, 9, S91–S94. [Google Scholar] [CrossRef]
| AHA Guidelines | ESC Guidelines | |||
|---|---|---|---|---|
| Indication for Intervention | Class | Level of Evidence | Class | Level of Evidence |
| Symptomatic patients | ||||
| High-gradient severe AS | 1 | A | I | B |
| Low-flow low-gradient severe AS with reduced LVEF (<50%) | 1 | B | ||
| With contractile reserve | - | - | I | B |
| Without contractile reserve | - | - | IIa | C |
| Low-flow low-gradient severe AS with preserved LVEF if symptoms related to AS | 1 | B | IIa | C |
| Asymptomatic patients | ||||
| Severe AS and reduced LVEF (<50%) | 1 | B | I | B |
| Severe high-gradient AS with exertional symptoms | - | - | I | C |
| Severe AS with sustain fall in BP | 2a | B | IIa | C |
| Severe AS with decreased exercice tolerance | 2a | B | ||
| Very severe AS | 2a | B | IIa | B |
Severe AS with low procedural risk and:
| 2a | B | IIa | B |
| Severe high-gradient AS with a progressive decrease in LVEF on at least 3 serial TTE < 60% | 2b | B | - | - |
| Other cardiac surgery | ||||
| Severe AS | 1 | B | I | C |
| Moderate AS | 2b | C | IIa | C |
| Maximum Velocity | Mean Transaortic Gradient | Aortic Valve Area | LVEF | Stroke Volume Indexed | Other | |
|---|---|---|---|---|---|---|
| Severe high-gradient AS | ≥4 m/s | ≥40 mmH | Irrespective | Irrespective | - | Eliminate high flow status |
| Very severe AS | ≥5 m/s | ≥60 mmHg | Irrespective | Irrespective | - | Eliminate high flow status |
| Low-flow low-gradient severe AS with reduced LVEF | <4 m/s | <40 mmHg | ≤1 cm2 ≤0.6 cm2/m2 | LVEF < 50% | ≤35 mL/m2 | |
| Low-flow low-gradient severe AS with persevered LVEF | <4 m/s | <40 mmHg | ≤1 cm2 ≤0.6 cm2/m2 | LVEF ≥ 50% | ≤35 mL/m2 | Measured in normotensive patients (SBP < 140 mmHg) |
| Moderate AS | - | 20 to 40 mmHg | 1.0–1.5 cm2 * | Irrespective | - | In normal flow condition |
| Trial Name | NCT | Design | Population | Inclusion Criteria | Primary Outcome | Estimated Completion Date |
|---|---|---|---|---|---|---|
| Asymptomatic severe aortic stenosis | ||||||
| DANAVR (Danish National Randomized Study on Early Aortic Valve Replacement in Patients With Asymptomatic Severe Aortic Stenosis) | NCT03972644 | Open randomized trial; Watchful waiting vs. SAVR or TAVR | 1700 patients | Asymptomatic severe AS with preserved LVEF but subclinical sign of LV dysfunction | All-cause mortality (5-year time frame) | September 2029 |
| EASY-AS (The Early Valve Replacement in Severe ASYmptomatic Aortic Stenosis Study) | NCT04204915 | Open randomized trial; Watchful waiting vs. TAVR or SAVR | 2844 patients | Asymptomatic severe AS with preserved LVEF | Composite outcome of all-cause death and hospitalization for heart failure after 663 events | October 2029 |
| EVoLVeD (Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients with Severe AS) | NCT03094143 | Associated with EASY-AS Randomized early intervention according to presence of mid-LV fibrosis in MRI | 1000 patients | Asymptomatic severe AS with preserved LVEF | Composite of all-cause mortality or unplanned aortic stenosis-related hospitalization up until study completion (estimated 2.75 years of follow-up) | October 2024 |
| EARLY-TAVR (Evaluation of TAVR Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis) | NCT03042104 | Open randomized trial; TAVR vs. watchful waiting | 901 patients | Asymptomatic severe AS with preserved LVEF and age ≥ 65 years old | All-cause death, all stroke, and unplanned cardiovascular hospitalization at 2 years | March 2024 |
| EXPAND I—Feasibility study | NCT04639258 | Single group trial | 75 patients | Asymptomatic severe AS with preserved LVEF over 65 years old | All-cause and cardiovascular mortality at 30 days | July 2022 |
| Moderate aortic stenosis | ||||||
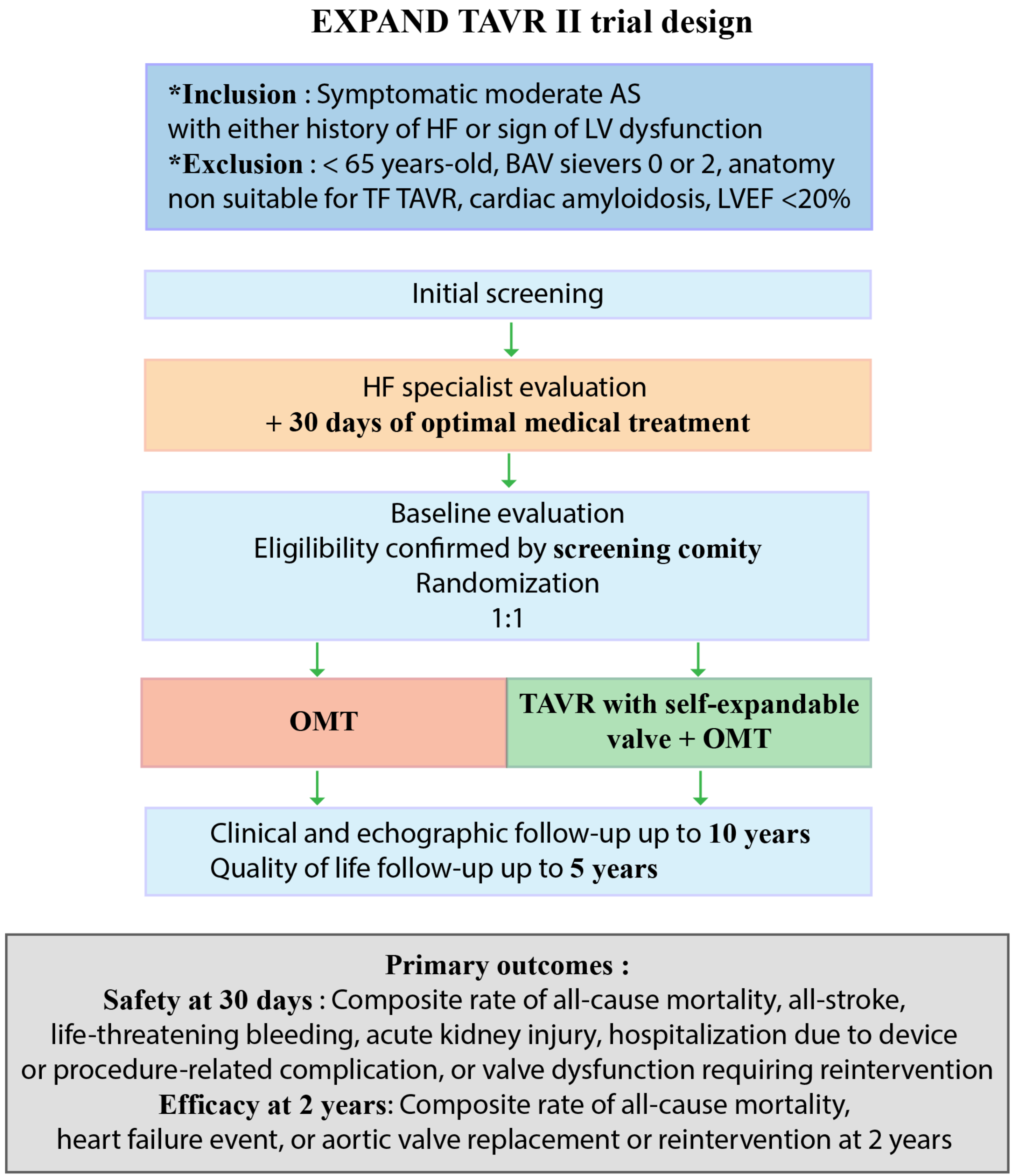
| Evolut™ EXPAND TAVR II Pivotal Trial | NCT05149755 | Open randomized trial; TAVR and OMT vs. OMT | 650 patients | Symptomatic moderate AS with either HF in the past year or elevated cardiac biomarkers or reduced longitudinal strain (≤15%) or elevated LV filling pressures. Age ≥ 65 years old. | Composite of all-cause mortality, all-stroke, life-threatening bleeding, acute kidney injury, hospitalization due to device or procedure-related complication, or valve dysfunction requiring reintervention at 30 days | February 2026 |
| PROGRESS (A Prospective, Randomized, Controlled Trial to Assess the Management of Moderate Aortic Stenosis by Clinical Surveillance or Transcatheter Aortic Valve Replacement) | NCT04889872 | Open randomized trial; TAVR and OMT vs. OMT | 750 patients | Moderate AS with evidence of cardiac dysfunction or symptoms and age ≥ 65 years old | Composite of death, stroke, and unplanned cardiovascular hospitalization at 2 years | June 2029 |
| TAVR UNLOAD (Transcatheter Aortic Valve Replacement to UNload the Left Ventricle in Patients With ADvanced Heart Failure) | NCT02661451 | Open randomized trial; TAVR and OMT vs. OMT | 300 patients | Symptomatic moderate AS with LVEF < 50% | All-cause death at 1 year | March 2023 |
| Other trials | ||||||
| NOTION-II (Comparison of Transcatheter Versus Surgical Aortic Valve Replacement in Younger Low Surgical Risk Patients With Severe Aortic Stenosis) | NCT02825134 | Open randomized trial; TAVR vs. SAVR | 372 patients | Symptomatic severe AS low-risk (STS-PROM < 4%) patients suitable for transfemoral TAVR and <75 years old | Composite of all-cause mortality, stroke and device-related rehospitalization at 1 year | December 2029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesnier, J.; Panagides, V.; Nuche, J.; Rodés-Cabau, J. Evolving Indications of Transcatheter Aortic Valve Replacement—Where Are We Now, and Where Are We Going. J. Clin. Med. 2022, 11, 3090. https://doi.org/10.3390/jcm11113090
Mesnier J, Panagides V, Nuche J, Rodés-Cabau J. Evolving Indications of Transcatheter Aortic Valve Replacement—Where Are We Now, and Where Are We Going. Journal of Clinical Medicine. 2022; 11(11):3090. https://doi.org/10.3390/jcm11113090
Chicago/Turabian StyleMesnier, Jules, Vassili Panagides, Jorge Nuche, and Josep Rodés-Cabau. 2022. "Evolving Indications of Transcatheter Aortic Valve Replacement—Where Are We Now, and Where Are We Going" Journal of Clinical Medicine 11, no. 11: 3090. https://doi.org/10.3390/jcm11113090
APA StyleMesnier, J., Panagides, V., Nuche, J., & Rodés-Cabau, J. (2022). Evolving Indications of Transcatheter Aortic Valve Replacement—Where Are We Now, and Where Are We Going. Journal of Clinical Medicine, 11(11), 3090. https://doi.org/10.3390/jcm11113090






