Clinical Efficacy of Melon GliSODin® for the Treatment of Aging-Related Dysfunction in Motor Organs—A Double Blind, Randomized Placebo-Controlled Study
Abstract
:1. Introduction
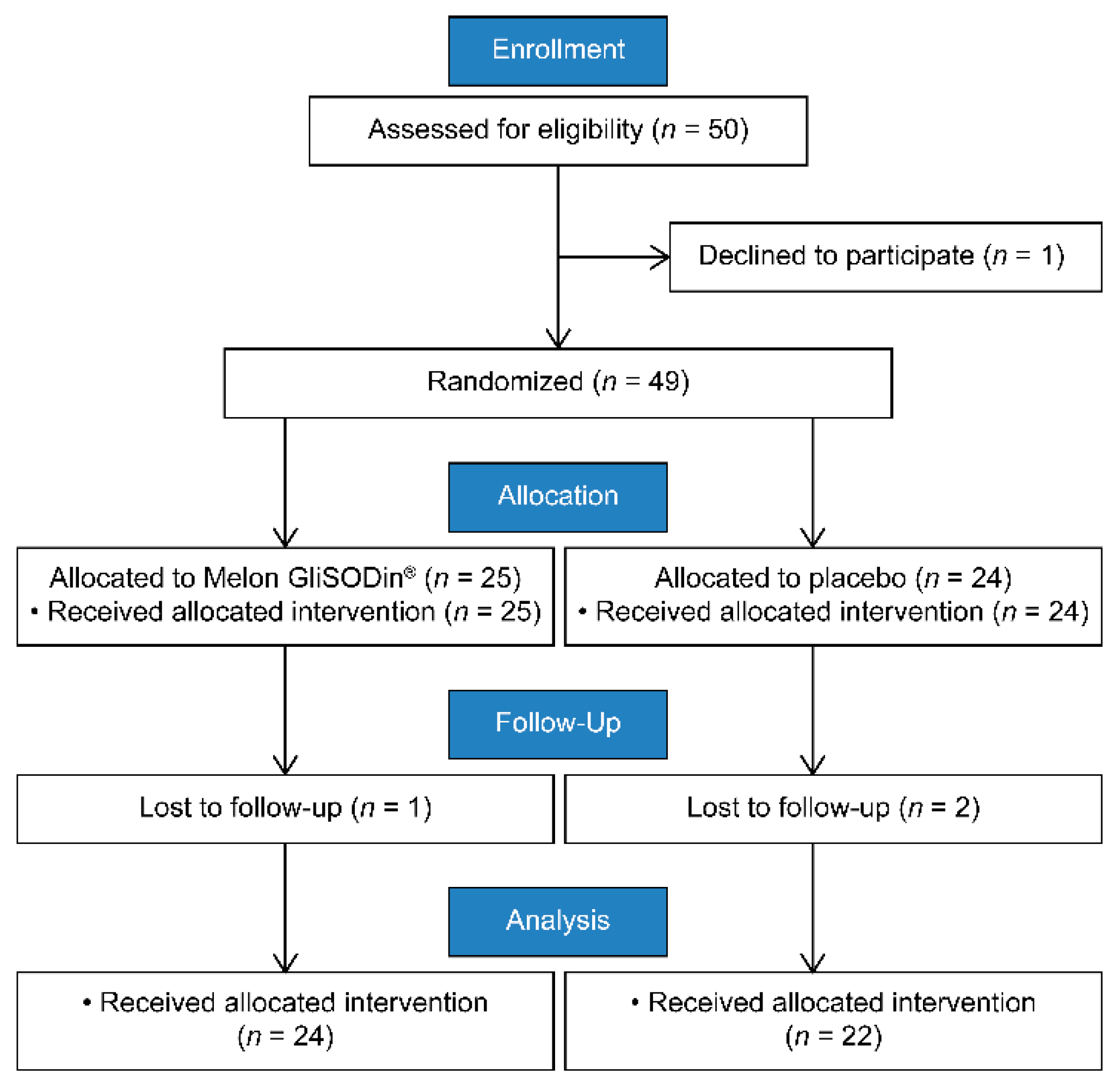
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Outcomes
2.5. Subjective Symptoms
2.5.1. Oxidative Markers, Antioxidants, and Inflammatory Markers
2.5.2. Biochemical Tests, Body Composition, and Motor Function
2.6. Sample Size Analysis
2.7. Statistical Analysis
3. Results
4. Effects of Melon GliSODin® on Symptom Severity
5. Effects of Melon GliSODin® on Oxidative Markers, Antioxidants, and Inflammatory Markers
6. Effect of Melon GliSODin® on Blood Biochemistry, Bone Metabolism, Body Composition, and Motor Function
7. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
References
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty among Community-Dwelling Older Adults. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Ogata, T. Locomotive Syndrome: Definition and Management. Clin. Rev. Bone Miner. Metab. 2016, 14, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, E.; Ishibashi, Y.; Tsuda, E.; Ono, A.; Yamamoto, Y.; Inoue, R.; Takahashi, I.; Umeda, T.; Nakaji, S. Evaluation of locomotive disability using loco-check: A cross-sectional study in the Japanese general population. J. Orthop. Sci. 2013, 18, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hame, S.L.; Alexander, R.A. Knee osteoarthritis in women. Curr. Rev. Musculoskelet. Med. 2013, 6, 182–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, M.; Ikezoe, T.; Tsuboyama, T.; Tabara, Y.; Matsuda, F.; Ichihashi, N.; on behalf of the Nagahama Study group. Prevalence and physical characteristics of locomotive syndrome stages as classified by the new criteria 2020 in older Japanese people: Results from the Nagahama study. BMC Geriatr. 2021, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Aigner, T.; Fundel, K.; Saas, J.; Gebhard, P.M.; Haag, J.; Weiss, T.; Zien, A.; Obermayr, F.; Zimmer, R.; Bartnik, E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006, 54, 3533–3544. [Google Scholar] [CrossRef]
- Ruiz-Romero, C.; Calamia, V.; Mateos, J.; Carreira, V.; Martinez-Gomariz, M.; Fernandez, M.; Blanco, F.J. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. 2009, 8, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, 11722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Nojiri, H.; Saita, Y.; Morikawa, D.; Ozawa, Y.; Watanabe, K.; Koike, M.; Asou, Y.; Shirasawa, T.; Yokote, K.; et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci. Rep. 2015, 5, 9148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwahara, H.; Horie, T.; Ishikawa, S.; Tsuda, C.; Kawakami, S.; Noda, Y.; Kaneko, T.; Tahara, S.; Tachibana, T.; Okabe, M.; et al. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic. Biol. Med. 2010, 48, 1252–1262. [Google Scholar] [CrossRef]
- Koike, M.; Nojiri, H.; Kanazawa, H.; Yamaguchi, H.; Miyagawa, K.; Nagura, N.; Banno, S.; Iwase, Y.; Kurosawa, H.; Kaneko, K. Superoxide dismutase activity is significantly lower in end-stage osteoarthritic cartilage than non-osteoarthritic cartilage. PLoS ONE 2018, 13, e0203944. [Google Scholar] [CrossRef]
- Romao, S. Therapeutic value of oral supplementation with melon superoxide dismutase and wheat gliadin combination. Nutrition 2015, 31, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, N.; Muraki, S.; Oka, H.; Tanaka, S.; Ogata, T.; Kawaguchi, H.; Akune, T.; Nakamura, K. Association between new indices in the locomotive syndrome risk test and decline in mobility: Third survey of the ROAD study. J. Orthop. Sci. 2015, 20, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Akai, M.; Doi, T.; Fujino, K.; Iwaya, T.; Kurosawa, H.; Nasu, T. An outcome measure for Japanese people with knee osteoarthritis. J. Rheumatol. 2005, 32, 1524–1532. [Google Scholar]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Aicher, B.; Peil, H.; Peil, B.; Diener, H.C. Pain measurement: Visual Analogue Scale (VAS) and Verbal Rating Scale (VRS) in clinical trials with OTC analgesics in headache. Cephalalgi Int. J. Headache 2012, 32, 185–197. [Google Scholar] [CrossRef]
- Yoh, K.; Yoshizawa, M.; Kuwabara, A.; Tanaka, K. Multi-Component Structure of Roland-Morris Disability Questionnaire (RDQ), a Lumbago-Specific QOL (Quality of Life) Measure. Jpn. Clin. Med. 2011, 2, 9–14. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Matsunaga, H.; Fujisawa, S. Relationship between Leg Extension Torque by StrengthErgo and Knee Flexion Strength: Action of Knee Flexion Muscles in a Closed Kinetic Chain. Rigakuryoho Kagaku 2010, 25, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.D.; Chiu, V.; Stewart, A.L. Mobility-related function in older adults: Assessment with a 6-minute walk test. Arch. Phys. Med. Rehabil. 1999, 80, 837–841. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H. Locomotive syndrome in Japan. Osteoporos Sarcopenia 2018, 4, 86–94. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tomonaga, A.; Fukagawa, M.; Mitsui, Y.; Sato, M.; Fujita, S. Effect of a Dietary Supplement Containing Yeast SM-10 on Joint Functions of Individuals with Knee Joint Pain. Funct. Food Res. 2018, 14, 48–56. [Google Scholar] [CrossRef]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielecka, E.; Woitas-Slubowska, D.; Adach, Z. Effects of oral supplementation with plant superoxide dismutase extract on selected redox parameters and an inflammatory marker in a 2000-m rowing-ergometer test. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 124–134. [Google Scholar] [CrossRef]
- Suantawee, T.; Tantavisut, S.; Adisakwattana, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Deepaisarnsakul, B.; Honsawek, S. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. J. Clin. Diagn. Res. 2013, 7, 1855–1859. [Google Scholar] [CrossRef]
- Sarban, S.; Kocyigit, A.; Yazar, M.; Isikan, U.E. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 2005, 38, 981–986. [Google Scholar] [CrossRef]
- Kubo, Y.; Ikeya, M.; Sugiyama, S.; Takachu, R.; Tanaka, M.; Sugiura, T.; Kobori, K.; Kobori, M. Association between Preoperative Long-Chain Polyunsaturated Fatty Acids and Oxidative Stress Immediately after Total Knee Arthroplasty: A Pilot Study. Nutrients 2021, 13, 2093. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-α and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Schiltenwolf, M.; Buchner, M. The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin. J. Pain 2008, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Muth, C.M.; Glenz, Y.; Klaus, M.; Radermacher, P.; Speit, G.; Leverve, X. Influence of an orally effective SOD on hyperbaric oxygen-related cell damage. Free Radic. Res. 2004, 38, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Takesue, F.; Inutsuka, S.; Honda, M.; Nozoe, T.; Korenaga, D. Prognostic significance of serum superoxide dismutase activity in patients with gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2002, 5, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, M.; Caillard, P.; Provost, J.C.; Dever, J.M.; Elbeze, Y.; Zamaria, N. GliSODin, a vegetal sod with gliadin, as preventative agent vs. atherosclerosis, as confirmed with carotid ultrasound-B imaging. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 45–50. [Google Scholar]
| Melon GliSODin® | Placebo | p-Value | |
|---|---|---|---|
| Age | 68.25 (6.96) | 65.95 (7.83) | 0.30 |
| Height, cm | 154.16 (5.05) | 153.63 (5.18) | 0.71 |
| Body weight, kg | 54.22 (7.48) | 53.33 (5.47) | 0.96 # |
| BMI, kg/m2 | 22.40 (2.32) | 22.70 (3.15) | 0.84 # |
| Locomo Grade, n | |||
| Pre-Locomo, n | 1 | 6 | |
| Grade 1, n | 10 | 5 | |
| Grade 2, n | 13 | 11 | |
| Locomo 25 | 15.63 (11.89) | 10.82 (12.11) | 0.06 # |
| JKOM | 46.71 (13.37) | 40.18 (13.03) | 0.04 #,* |
| Chalder Fatigue Scale | 18.75 (7.28) | 14.55 (6.70) | 0.05 * |
| VRS | 2.75 (0.94) | 2.23 (1.15) | 0.05 # |
| RDQ | 4.33 (4.17) | 3.00 (4.05) | 0.21 # |
| Outcomes | 6-Month Outcome | Baseline to 6-Month Change | ||||
|---|---|---|---|---|---|---|
| Melon GliSODin® | Placebo | p-Value | Melon GliSODin® | Placebo | p-Value | |
| Locomo 25 | 13.38 (10.2) | 11.91 (16.08) | 0.39 | −2.25 (9.35) | 1.09 (7.25) | 0.19 |
| JKOM | 41.42 (13.16) | 39.85 (16.57) | 0.49 | −5.29 (12.53) | −0.32 (10.01) | 0.15 |
| Chalder Fatigue Scale | 15.54 (6.30) | 13.95 (7.72) | 0.45 | −3.21 (7.02) | −0.59 (4.63) | 0.15 |
| VRS | 2.33 (1.05) | 2.14 (1.17) | 0.75 | −0.42 (1.25) | −0.09 (0.87) | 0.31 |
| RDQ | 2.67 (2.65) | 2.32 (4.11) | 0.31 | −1.67 (3.40) | −0.68 (2.61) | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, M.; Nagao, M.; Iwase, Y.; Kaneko, K.; Ishijima, M.; Nojiri, H. Clinical Efficacy of Melon GliSODin® for the Treatment of Aging-Related Dysfunction in Motor Organs—A Double Blind, Randomized Placebo-Controlled Study. J. Clin. Med. 2022, 11, 2747. https://doi.org/10.3390/jcm11102747
Koike M, Nagao M, Iwase Y, Kaneko K, Ishijima M, Nojiri H. Clinical Efficacy of Melon GliSODin® for the Treatment of Aging-Related Dysfunction in Motor Organs—A Double Blind, Randomized Placebo-Controlled Study. Journal of Clinical Medicine. 2022; 11(10):2747. https://doi.org/10.3390/jcm11102747
Chicago/Turabian StyleKoike, Masato, Masashi Nagao, Yoshiyuki Iwase, Kazuo Kaneko, Muneaki Ishijima, and Hidetoshi Nojiri. 2022. "Clinical Efficacy of Melon GliSODin® for the Treatment of Aging-Related Dysfunction in Motor Organs—A Double Blind, Randomized Placebo-Controlled Study" Journal of Clinical Medicine 11, no. 10: 2747. https://doi.org/10.3390/jcm11102747
APA StyleKoike, M., Nagao, M., Iwase, Y., Kaneko, K., Ishijima, M., & Nojiri, H. (2022). Clinical Efficacy of Melon GliSODin® for the Treatment of Aging-Related Dysfunction in Motor Organs—A Double Blind, Randomized Placebo-Controlled Study. Journal of Clinical Medicine, 11(10), 2747. https://doi.org/10.3390/jcm11102747







