Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions
Abstract
:1. Introduction
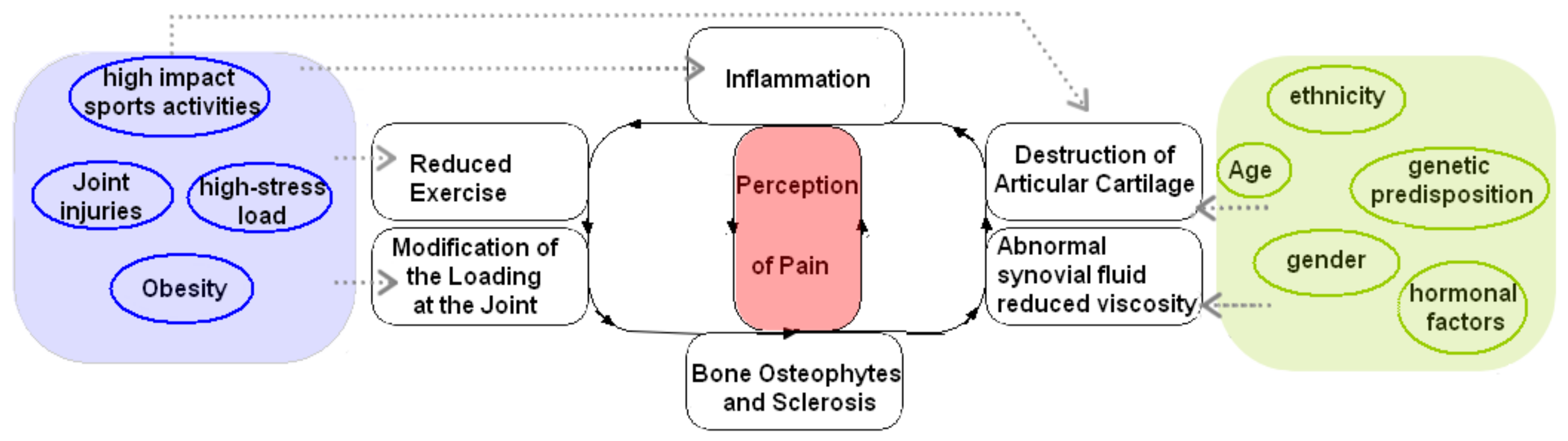
2. Pathogenesis and Risk Factors
2.1. Pathogenesis
2.2. Risk Factors
2.2.1. Age
2.2.2. Obesity
2.2.3. Biomechanical Load
3. Physical Therapy Interventions
3.1. Diathermy
3.2. Exercise Therapy
3.3. Ultrasound Therapy
3.4. Knee Brace
3.5. Electrical Stimulation
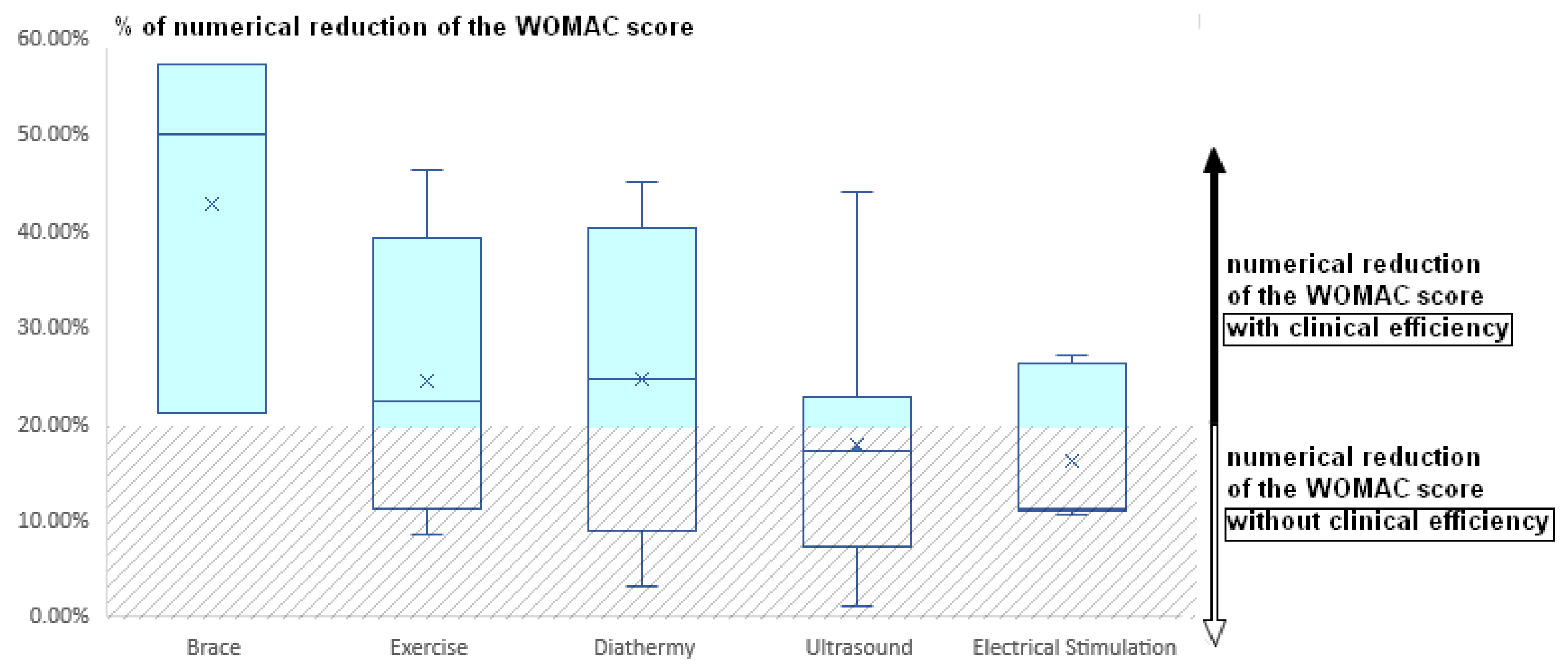
4. Data Analysis
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. Vol. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [Green Version]
- Palsis, J.A.; Brehmer, T.S.; Pellegrini, V.D.; Drew, J.M.; Sachs, B.L. The Cost of Joint Replacement: Comparing Two Approaches to Evaluating Costs of Total Hip and Knee Arthroplasty. J. Bone Jt. Surg. 2018, 100, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lespasio, M.J.; Piuzzi, N.S.; Husni, M.E.; Muschler, G.F.; Guarino, A.; Mont, M.A. Knee Osteoarthritis: A Primer. TPJ Perm. J. 2017, 21, 16–183. [Google Scholar] [CrossRef] [Green Version]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and Osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Brandt, K.D.; Dieppe, P.; Radin, E. Etiopathogenesis of Osteoarthritis. Med. Clin. N. Am. 2009, 93, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wieland, H.A.; Michaelis, M.; Kirschbaum, B.J.; Rudolphi, K.A. Osteoarthritis—An untreatable disease? Nat. Rev. Drug Discov. 2005, 4, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Poulet, B.; Hamilton, R.W.; Shefelbine, S.; Pitsillides, A.A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011, 63, 137–147. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. IJMS 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nevitt, M.; Niu, J.; Lewis, C.; Torner, J.; Guermazi, A.; Roemer, F.; McCulloch, C.; Felson, D. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011, 63, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Adatia, A.; Rainsford, K.D.; Kean, W.F. Osteoarthritis of the knee and hip. Part I: Aetiology and pathogenesis as a basis for pharmacotherapy. J. Pharm. Pharmacol. 2012, 64, 617–625. [Google Scholar] [CrossRef]
- Neogi, T. Clinical significance of bone changes in Osteoarthritis. Ther. Adv. Musculoskelet. 2012, 4, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in Osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargrave-Thomas, E.; van Sloun, F.; Dickinson, M.; Broom, N.; Thambyah, A. Multi-scalar mechanical testing of the calcified cartilage and subchondral bone comparing healthy vs early degenerative states. Osteoarthr. Cartil. 2015, 23, 1755–1762. [Google Scholar] [CrossRef] [Green Version]
- Botter, S.; Glasson, S.; Hopkins, B.; Clockaerts, S.; Weinans, H.; van Leeuwen, J.; van Osch, G. ADAMTS5−/− mice have less subchondral bone changes after induction of Osteoarthritis through surgical instability: Implications for a link between cartilage and subchondral bone changes. Osteoarthr. Cartil. 2009, 17, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Huang, L.; Welch, I.; Norley, C.; Holdsworth, D.W.; Beier, F.; Cai, D. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci. Rep. 2018, 8, 2855. [Google Scholar] [CrossRef]
- Amini, M.; Nazemi, S.M.; Lanovaz, J.L.; Kontulainen, S.; Masri, B.A.; Wilson, D.R.; Szyszkowski, W.; Johnston, J.D. Individual and combined effects of OA-related subchondral bone alterations on proximal tibial surface stiffness: A parametric finite element modeling study. Med. Eng. Phys. 2015, 37, 783–791. [Google Scholar] [CrossRef]
- Castañeda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol. 2012, 83, 315–323. [Google Scholar] [CrossRef]
- Goldring, M.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of Osteoarthritis: Articular cartilage and subchondral bone. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Cisternas, M.G.; Murphy, L.; Sacks, J.J.; Solomon, D.H.; Pasta, D.J.; Helmick, C.G. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey: OA Prevalence in a Population-Based Survey. Arthritis Care Res. 2016, 68, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caine, D.J.; Golightly, Y. Osteoarthritis as an outcome of paediatric sport: An epidemiological perspective. Br. J. Sports Med. 2011, 45, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Helmick, C.G. The Impact of Osteoarthritis in the United States: A Population-Health Perspective A population-based review of the fourth most common cause of hospitalization in US adults. Orthop. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Felson, D.T. The epidemiology of knee osteoarthritis: Results from the framingham osteoarthritis study. Semin. Arthritis Rheum. 1990, 20, 42–50. [Google Scholar] [CrossRef]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Goggins, J.; Niu, J.; Zhang, Y.; Hunter, D.J. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004, 50, 3904–3909. [Google Scholar] [CrossRef]
- Wang, P.; Guan, P.; Guo, C.; Zhu, F.; Konstantopoulos, K.; Wang, Z. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. 2013, 27, 4664–4677. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, A.M.; Li, L.C. Occupational Physical Loading Tasks and Knee Osteoarthritis: A Review of the Evidence. Physiother. Can. 2014, 66, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caine, D.; Meyers, R.; Nguyen, J.; Schöffl, V.; Maffulli, N. Primary Periphyseal Stress Injuries in Young Athletes: A Systematic Review. Sports Med. 2022, 52, 741–772. [Google Scholar] [CrossRef] [PubMed]
- Nagura, T.; Matsumoto, H.; Kiriyama, Y.; Chaudhari, A.; Andriacchi, T.P. Tibiofemoral Joint Contact Force in Deep Knee Flexion and Its Consideration in Knee Osteoarthritis and Joint Replacement. J. Appl. Biomech. 2006, 22, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Sharma, L.; Song, J.; Felson, D.T.; Cahue, S.; Shamiyeh, E.; Dunlop, D.D. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001, 286, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The Long-term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [Green Version]
- Novak, S.; Guerron, G.; Zou, Z.; Cheung, G.; Berteau, J.-P. New Guidelines for Electrical Stimulation Parameters in Adult Patients With Knee Osteoarthritis Based on a Systematic Review of the Current Literature. Am. J. Phys. Med. Rehabil. 2020, 99, 682–688. [Google Scholar] [CrossRef]
- Heddon, S.; Saulnier, N.; Mercado, J.; Shalmiyev, M.; Berteau, J.-P. Systematic review shows no strong evidence regarding the use of elastic taping for pain improvement in patients with primary knee osteoarthritis. Medicine 2021, 100, e25382. [Google Scholar] [CrossRef]
- Latif-Zade, T.; Tucci, B.; Verbovetskaya, D.; Bialkin, E.; Ng, B.; Heddon, S.; Berteau, J.-P. Systematic Review Shows Tele-Rehabilitation Might Achieve Comparable Results to Office-Based Rehabilitation for Decreasing Pain in Patients with Knee Osteoarthritis. Medicina 2021, 57, 764. [Google Scholar] [CrossRef]
- Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. What is the Minimum Clinically Important Difference for the WOMAC Index After TKA? Clin. Orthop. Relat. Res. 2018, 476, 2005–2014. [Google Scholar] [CrossRef]
- Sarıfakıoğlu, B.; Yıldırım Güzelant, A.; Özduran, E. Gonartroz Tedavisinde Kısa Dalga Diatermi ve Ultrason Tedavi Etkinliğinin Karşılaştırılması. Türk Osteoporoz Derg. 2014, 20, 16–20. [Google Scholar] [CrossRef]
- Boyaci, A.; Tutoğlu, A.; Boyaci, N.; Aridici, R.; Koca, I. Comparison of the efficacy of ketoprofen phonophoresis, ultrasound, and short-wave diathermy in knee osteoarthritis. Rheumatol. Int. 2013, 33, 2811–2818. [Google Scholar] [CrossRef]
- Rabini, A.; Piazzini, D.B.; Tancredi, G.; Foti, C.; Milano, G.; Ronconi, G.; Specchia, A.; E Ferrara, P.; Maggi, L.; Amabile, E.; et al. Deep heating therapy via microwave diathermy relieves pain and improves physical function in patients with knee osteoarthritis: A double-blind randomized clinical trial. Eur. J. Phys. Rehabil. Med. 2012, 48, 549–559. [Google Scholar] [PubMed]
- Giombini, A.; Di Cesare, A.; Di Cesare, M.; Ripani, M.; Maffulli, N. Localized hyperthermia induced by microwave diathermy in Osteoarthritis of the knee: A randomized placebo-controlled double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 980–987. [Google Scholar] [CrossRef]
- Laufer, Y.; Zilberman, R.; Porat, R.; Nahir, A.M. Effect of pulsed short-wave diathermy on pain and function of subjects with Osteoarthritis of the knee: A placebo-controlled double-blind clinical trial. Clin. Rehabil. 2005, 19, 255–263. [Google Scholar] [CrossRef]
- Rattanachaiyanont, M.; Kuptniratsaikul, V. No additional benefit of short-wave diathermy over-exercise program for knee osteoarthritis in peri-/post-menopausal women: An equivalence trial. Osteoarthr. Cartil. 2008, 16, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Ozen, S.; Doganci, E.B.; Ozyuvali, A.; Yalcin, A.P. Effectiveness Of Continuous Versus Pulsed Short-Wave Diathermy In The Management Of Knee Osteoarthritis: A Randomised Pilot Study. Caspian J. Intern. Med. 2019, 10, 431. [Google Scholar] [CrossRef]
- Onwunzo, C.N.; Igwe, S.E.; Umunnah, J.O.; Uchenwoke, C.I.; Ezugwu, U.A. Effects of Isometric Strengthening Exercises on Pain and Disability Among Patients With Knee Osteoarthritis. Cureus 2021, 13, e18972. [Google Scholar] [CrossRef] [PubMed]
- Knoop, J.; Dekker, J.; van der Leeden, M.; van der Esch, M.; Thorstensson, C.; Gerritsen, M.; Voorneman, R.; Peter, W.; de Rooij, M.; Romviel, S.; et al. Knee joint stabilization therapy in patients with Osteoarthritis of the knee: A randomized, controlled trial. Osteoarthr. Cartil. 2013, 21, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Vincent, K.R.; Vasilopoulos, T.; Montero, C.; Vincent, H.K. Eccentric and Concentric Resistance Exercise Comparison for Knee Osteoarthritis. Med. Sci. Sports Exerc. 2019, 51, 1977–1986. [Google Scholar] [CrossRef]
- Hall, M.; Hinman, R.S.; Wrigley, T.V.; Kasza, J.; Lim, B.-W.; Bennell, K.L. Knee extensor strength gains mediate symptom improvement in knee osteoarthritis: Secondary analysis of a randomised controlled trial. Osteoarthr. Cartil. 2018, 26, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Hafez, A.; Al-Johani, A.H.; Zakaria, A.R.; Al-Ahaideb, A.; Buragadda, S.; Melam, G.; Kachanathu, S.J. Treatment of Knee Osteoarthritis in Relation to Hamstring and Quadriceps Strength. J. Phys. Ther. Sci. 2013, 25, 1401–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Johani, A.H.; Kachanathu, S.J.; Hafez, A.R.; Al-Ahaideb, A.; Algarni, A.D.; Alroumi, A.M.; Alenazi, A.M. Comparative Study of Hamstring and Quadriceps Strengthening Treatments in the Management of Knee Osteoarthritis. J. Phys. Ther. Sci. 2014, 26, 817–820. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, A.M.I.; Peccin, M.S.; da Silva, K.N.G.; de Teixeira, L.E.P.P.; Trevisani, V.F.M. Impact of exercise on the functional capacity and pain of patients with knee osteoarthritis: A randomized clinical trial. Rev. Bras. Reumatol. 2012, 52, 876–882. [Google Scholar]
- Lin, D.-H.; Lin, C.-H.J.; Lin, Y.-F.; Jan, M.-H. Efficacy of 2 non-weight-bearing Interventions, Proprioception Training Versus Strength Training, for Patients With Knee Osteoarthritis: A Randomized Clinical Trial. J. Orthop. Sports Phys. Ther. 2009, 39, 450–457. [Google Scholar] [CrossRef]
- O’Reilly, S.C.; Muir, K.R.; Doherty, M. Effectiveness of home exercise on pain and disability from Osteoarthritis of the knee: A randomised controlled trial. Ann. Rheum. Dis. 1999, 58, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Luksurapan, W.; Boonhong, J. Effects of Phonophoresis of Piroxicam and Ultrasound on Symptomatic Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2013, 94, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.O.; Klyve, D.; Ortiz, R.; Best, T.M. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: A randomized, placebo-controlled double-blind study. J. Orthop. Surg. Res. 2018, 13, 257. [Google Scholar] [CrossRef] [Green Version]
- Özgönenel, L.; Okur, S.; Dogan, Y.P.; Çaglar, N.S. Effectiveness of therapeutic ultrasound on clinical parameters and ultrasonographic cartilage thickness in knee osteoarthritis: A double-blind trial. J. Med. Ultrasound 2018, 26, 194. [Google Scholar] [CrossRef]
- Karakaş, A.; Dilek, B.; Şahin, M.A.; Ellidokuz, H.; Şenocak, Ö. The effectiveness of pulsed ultrasound treatment on pain, function, synovial sac thickness and femoral cartilage thickness in patients with knee osteoarthritis: A randomized, double-blind clinical, controlled study. Clin. Rehabil. 2020, 34, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Alfredo, P.P.; Junior, W.S.; Casarotto, R.A. Efficacy of continuous and pulsed therapeutic ultrasound combined with exercises for knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2020, 34, 480–490. [Google Scholar] [CrossRef]
- Tascioglu, F.; Kuzgun, S.; Armagan, O.; Ogutler, G. Short-Term Effectiveness of Ultrasound Therapy in Knee Osteoarthritis. J. Int. Med. Res. 2010, 38, 1233–1242. [Google Scholar] [CrossRef]
- Loyola-Sánchez, A.; Richardson, J.; Beattie, K.A.; Otero-Fuentes, C.; Adachi, J.D.; MacIntyre, N.J. Effect of Low-Intensity Pulsed Ultrasound on the Cartilage Repair in People With Mild to Moderate Knee Osteoarthritis: A Double-Blinded, Randomized, Placebo-Controlled Pilot Study. Arch. Phys. Med. Rehabil. 2012, 93, 35–42. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Chen, J.; Chen, W. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2016, 6, 35453. [Google Scholar] [CrossRef] [Green Version]
- Kozanoglu, E.; Basaran, S.; Guzel, R.; Guler-Uysal, F. Short term efficacy of ibuprofen phonophoresis versus continuous ultrasound therapy in knee osteoarthritis. Swiss Med. Wkly 2003, 133, 333–338. [Google Scholar]
- Külcü, D.G.; Gül, G. Short-Term Efficacy of Pulsed Electromagnetic Field Therapy on Pain and Functional Level in Knee Osteoarthritis: A Randomized Controlled Study. Arch. Rheumatol. 2009, 24, 144–148. [Google Scholar]
- Petersen, W.; Ellermann, A.; Zantop, T.; Rembitzki, I.V.; Semsch, H.; Liebau, C.; Best, R. Biomechanical effect of unloader braces for medial Osteoarthritis of the knee: A systematic review (CRD 42015026136). Arch. Orthop. Trauma. Surg. 2016, 136, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, D.K.; Russell, M.E. Unloader Braces for Medial Compartment Knee Osteoarthritis: Implications on Mediating Progression. Sports Health 2009, 1, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Hurley, S.T.; Murdock, G.L.H.; Stanish, W.D.; Hubley-Kozey, C.L. Is There a Dose-Response for Valgus Unloader Brace Usage on Knee Pain, Function, and Muscle Strength? Arch. Phys. Med. Rehabil. 2012, 93, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.; Matheny, L.; Steadman, J. Improvement in Quality of Life with Use of an Unloader Knee Brace in Active Patients with OA: A Prospective Cohort Study. J. Knee Surg. 2012, 25, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Pollo, F.E.; Otis, J.C.; Backus, S.; Warren, R.F.; Wickiewicz, T.L. Reduction of Medial Compartment Loads with Valgus Bracing of the Osteoarthritic Knee. Am. J. Sports Med. 2002, 30, 414–421. [Google Scholar] [CrossRef]
- Pagani, C.H.F.; Böhle, C.; Potthast, W.; Brüggemann, G.-P. Short-Term Effects of a Dedicated Knee Orthosis on Knee Adduction Moment, Pain, and Function in Patients With Osteoarthritis. Arch. Phys. Med. Rehabil. 2010, 91, 1936–1941. [Google Scholar] [CrossRef]
- Richards, J.D.; Sanchez-Ballester, J.; Jones, R.; Darke, N.; Livingstone, B.N. A comparison of knee braces during walking for the treatment of Osteoarthritis of the medial compartment of the knee. J. Bone Jt. Surg. Br. Vol. 2005, 87, 937–939. [Google Scholar] [CrossRef] [Green Version]
- Brandon, S.C.; Brown, M.J.; Clouthier, A.L.; Campbell, A.; Richards, J.; Deluzio, K.J. Contributions of muscles and external forces to medial knee load reduction due to osteoarthritis braces. Knee 2019, 26, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Li, H.; Yang, T.; Deng, Z.-H.; Yang, Y.; Zhang, Y.; Lei, G.-H. Electrical stimulation for pain relief in knee osteoarthritis: Systematic review and network meta-analysis. Osteoarthr. Cartil. 2015, 23, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrosimone, B.; Luc-Harkey, B.A.; Harkey, M.S.; Davis-Wilson, H.C.; Pfeiffer, S.J.; Schwartz, T.A.; Nissman, D.; Padua, D.A.; Blackburn, J.T.; Spang, J.T. Using TENS to Enhance Therapeutic Exercise in Individuals with Knee Osteoarthritis. Med. Sci. Sports Exerc. 2020, 52, 2086–2095. [Google Scholar] [CrossRef]
- Atamaz, F.C.; Durmaz, B.; Baydar, M.; Demircioglu, O.Y.; Iyiyapici, A.; Kuran, B.; Oncel, S.; Sendur, O.F. Comparison of the Efficacy of Transcutaneous Electrical Nerve Stimulation, Interferential Currents, and Shortwave Diathermy in Knee Osteoarthritis: A Double-Blind, Randomized, Controlled, Multicenter Study. Arch. Phys. Med. Rehabil. 2012, 93, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Fary, R.E.; Carroll, G.J.; Briffa, T.G.; Briffa, N.K. The effectiveness of pulsed electrical stimulation in the management of Osteoarthritis of the knee: Results of a double-blind, randomized, placebo-controlled, repeated-measures trial. Arthritis Rheum. 2011, 63, 1333–1342. [Google Scholar] [CrossRef] [Green Version]
- Adedoyin, R.A.; Olaogun, M.O.; Oyeyemi, A.L. Transcutaneous Electrical Nerve Stimulation and Interferential Current Combined with Exercise for the Treatment of Knee Osteoarthritis: A Randomised Controlled Trial. Hong Kong Physiother. J. 2005, 23, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Garland, D.; Holt, P.; Harrington, J.T.; Caldwell, J.; Zizic, T.; Cholewczynski, J. A 3-month, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a highly optimized, capacitively coupled, pulsed electrical stimulator in patients with Osteoarthritis of the knee. Osteoarthr. Cartil. 2007, 15, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Shimoura, K.; Iijima, H.; Suzuki, Y.; Aoyama, T. Immediate Effects of Transcutaneous Electrical Nerve Stimulation on Pain and Physical Performance in Individuals With Preradiographic Knee Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2019, 100, 300–306.e1. [Google Scholar] [CrossRef] [Green Version]
| Authors | Interventions | Sample Size | Relative Risk Reduction of Pain WOMAC |
|---|---|---|---|
| Laufer et al., (2005) [41] | H-PSWD | n (H-PSWD) = 32 | 9% |
| L-PSWD | n (L-PSWD) = 38 | 3% | |
| Giombini et al., (2011) [40] | MD | n (MD) = 30 | 44% |
| Rattanachaiyanont et al., (2008) [42] | SWD | n (SWD) = 50 | 27% |
| Rabini et al., (2012) [39] | SHT | n (SHT) = 27 | 8% |
| DHT | n (DHT) = 27 | 45% | |
| Ozen et al., (2019) [43] | CSWD | n (CSWD) = 14 | 26% |
| PSWD | n(PSWD) = 20 | 23% | |
| Boyaci et al., (2013) [38] | SWD | n (SWD) = 35 | 22% |
| Sarifakioglu et al., (2014) [37] | SWD | n (SWD) = 63 | 39% |
| Authors | Interventions | Sample Size | Relative Risk Reduction of Pain WOMAC |
|---|---|---|---|
| Vincent et al., (2019) [46] | Concentric | n = 28 | 11.3% |
| Eccentric | n = 30 | 16.9% | |
| Hall et al., (2018) [47] | Isometric | n = 49 | 8.4% |
| No Intervention | n = 48 | ||
| Hafez et al., (2013) [48] | Pre strengthening exercises | n = 20 | 46.1% |
| Post strengthening exercises | |||
| Al-Johani et al., (2014) [49] | Conservative PT+ strengthening exercises | n = 20 | 27.7% |
| Versus conservative PT | n = 20 | ||
| Oliveira et al., (2012) [50] | Instructions | n = 50 | 11% |
| Versus quadricep strengthening | n = 50 | ||
| Lin et al., (2009) [51] | Strengthening exercises | n = 36 | 42.1% |
| No intervention | n = 36 | ||
| O’Reilly et al., (1999) [52] | Strengthening exercises | n = 108 | 30.3% |
| No intervention | n = 72 |
| Authors | Interventions | Sample Size | Relative Risk Reduction of Pain WOMAC |
|---|---|---|---|
| Boyaci et al., (2013) [38] | Continuous Ultrasound (CU) | n = 33 | 16% |
| Phonophoresis (PhP) | n = 33 | ||
| Alfredo et al., (2020) [57] | CU | n = 20 | 6% |
| Control Group (C) | n = 20 | ||
| Özgönenel et al., (2008) [55] | CU | n = 34 | 18% |
| Sham Ultrasound Group (SU) | n = 33 | ||
| Luksurapan & Boonhong (2013) [53] | CU | n = 23 | 108% |
| PhP | n = 23 | ||
| Loyola-Sánchez et al., (2012) [59] | Pulsed Ultrasound Group (PU) | n = 14 | 23% |
| Sham Ultrasound Group (SU) | n = 13 | ||
| Kozanoglu et al., (2003) [61] | CU | n = 30 | 22% |
| Ibuprofen Phonophoresis (PH) | n = 30 | ||
| Külcü et al., (2009) [62] | CU | n = 15 | 44% |
| SU | n = 15 | ||
| Karakaş et al., (2020 [56]) | CU | n = 39 | 11% |
| C | n = 36 |
| Authors | Interventions | Sample Size | Relative Risk Reduction of Pain WOMAC |
|---|---|---|---|
| Hurley et al., (2012) [65] | Valgus Unloader Knee Brace | n = 24 | 21% |
| Briggs et al., (2012) [66] | Valgus Unloader Knee Brace | n = 39 | 57.1% |
| Pollo et al., (2002) [67] | Valgus Unloader Knee Brace | n = 11 | 44.4% (VAS) |
| Fatani-Pagani et al., (2010) [68] | Valgus Unloader Knee Brace | n = 11 | 50%% |
| Richards et al., (2005) [69] | Valgus Unloader Knee Brace | n = 30 | 41% (VAS) |
| Authors | Interventions | Sample Size | Relative Risk Reduction of Pain WOMAC |
|---|---|---|---|
| Atamaz et al., (2012) [73] | TENS | n = 29 | 11% |
| IFC | n = 27 | 11% | |
| Pietrosimone et al., (2020) [72] | TENS + TE | n = 30 | 10.57% |
| sham TENS + TE | n = 30 | 3.3% | |
| TE only | n = 30 | 12% | |
| Adedoyin et al., (2005) [75] | exercise+ electrical stimulation | n = 16 | 27% |
| exercise | n = 11 | 7% | |
| Garland et al., (2007) [76] | Active device | n = 38 | 26%% |
| Placebo device | n = 20 | 7% | |
| Fary et al., (2011) [74] | Pulse Electrical Stimulation | n = 34 | 11% |
| placebo | n = 36 | ||
| Shimoura et al., (2019) [77] | TENS Stair climb | n = 50 | 33% (VAS) |
| TENS Timed up and go | 26% (VAS) | ||
| TENS 6 mi walk test | 55% (VAS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berteau, J.-P. Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions. J. Clin. Med. 2022, 11, 3252. https://doi.org/10.3390/jcm11123252
Berteau J-P. Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions. Journal of Clinical Medicine. 2022; 11(12):3252. https://doi.org/10.3390/jcm11123252
Chicago/Turabian StyleBerteau, Jean-Philippe. 2022. "Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions" Journal of Clinical Medicine 11, no. 12: 3252. https://doi.org/10.3390/jcm11123252
APA StyleBerteau, J.-P. (2022). Knee Pain from Osteoarthritis: Pathogenesis, Risk Factors, and Recent Evidence on Physical Therapy Interventions. Journal of Clinical Medicine, 11(12), 3252. https://doi.org/10.3390/jcm11123252






